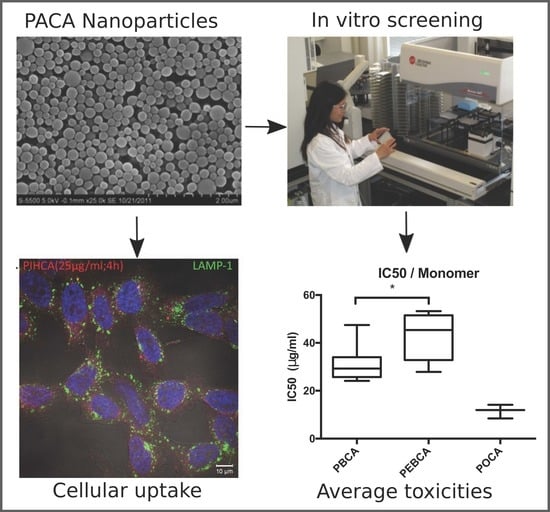
Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Properties of PACA NPs
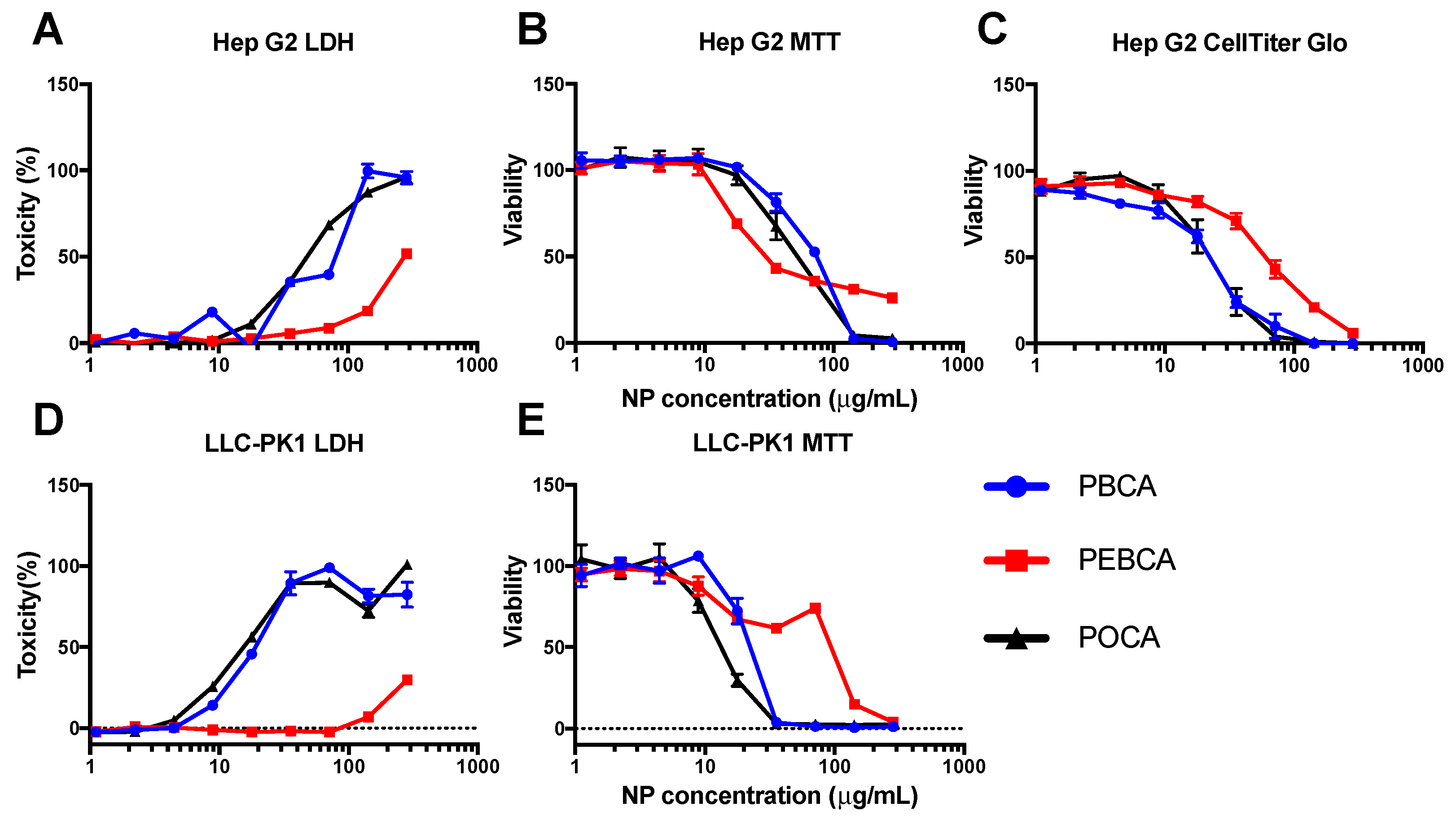
2.2. High-Throughput Cytotoxicity Screening
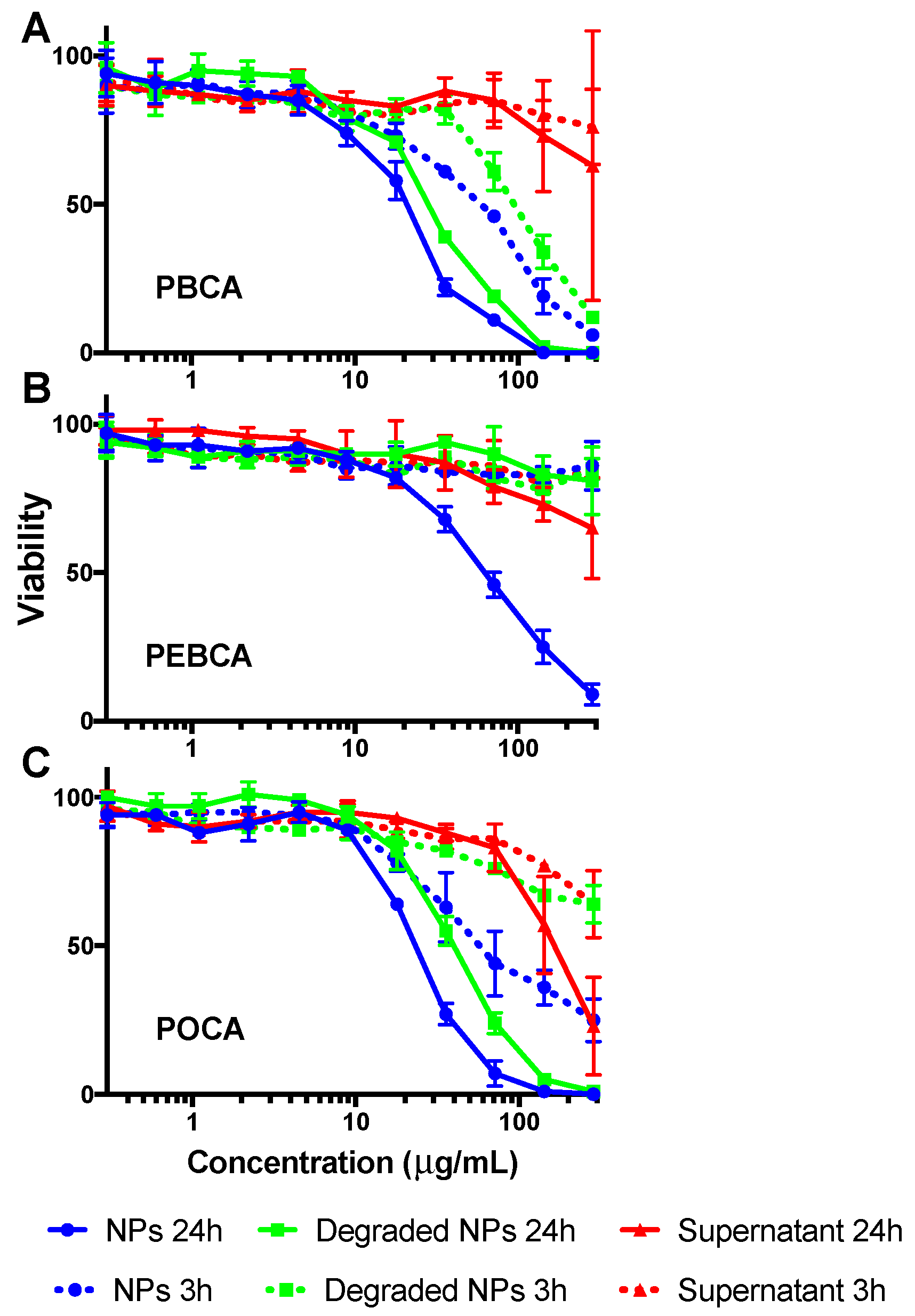
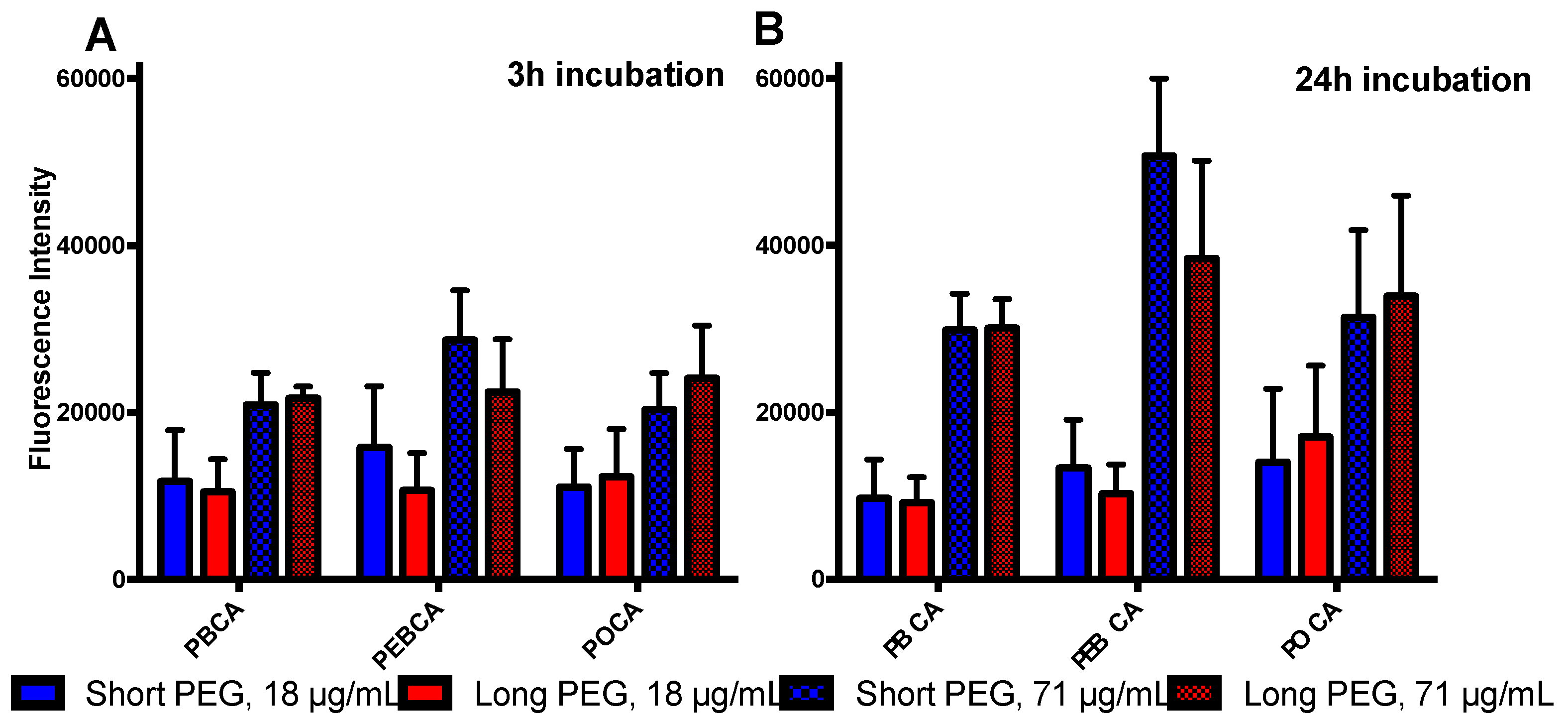
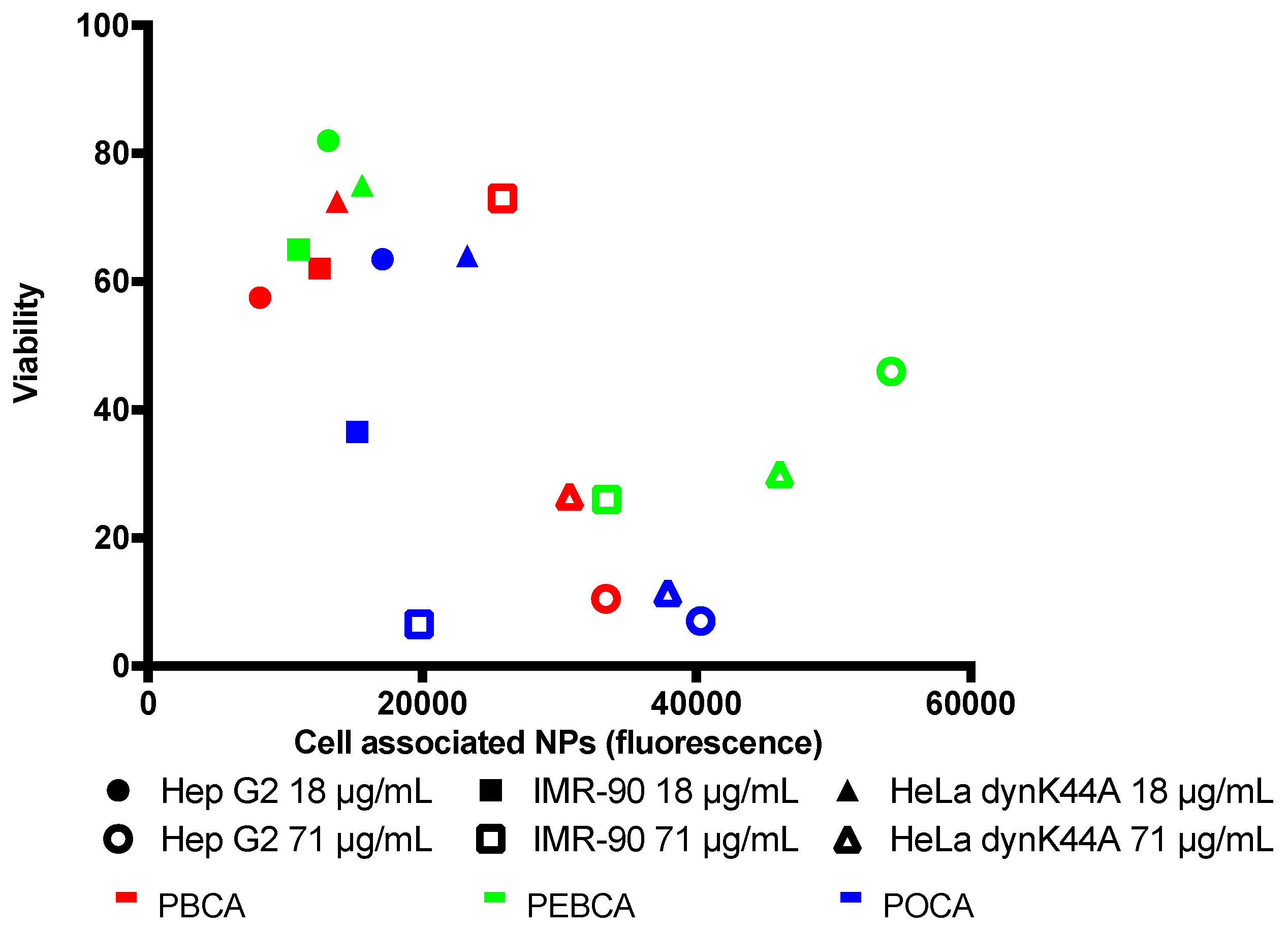
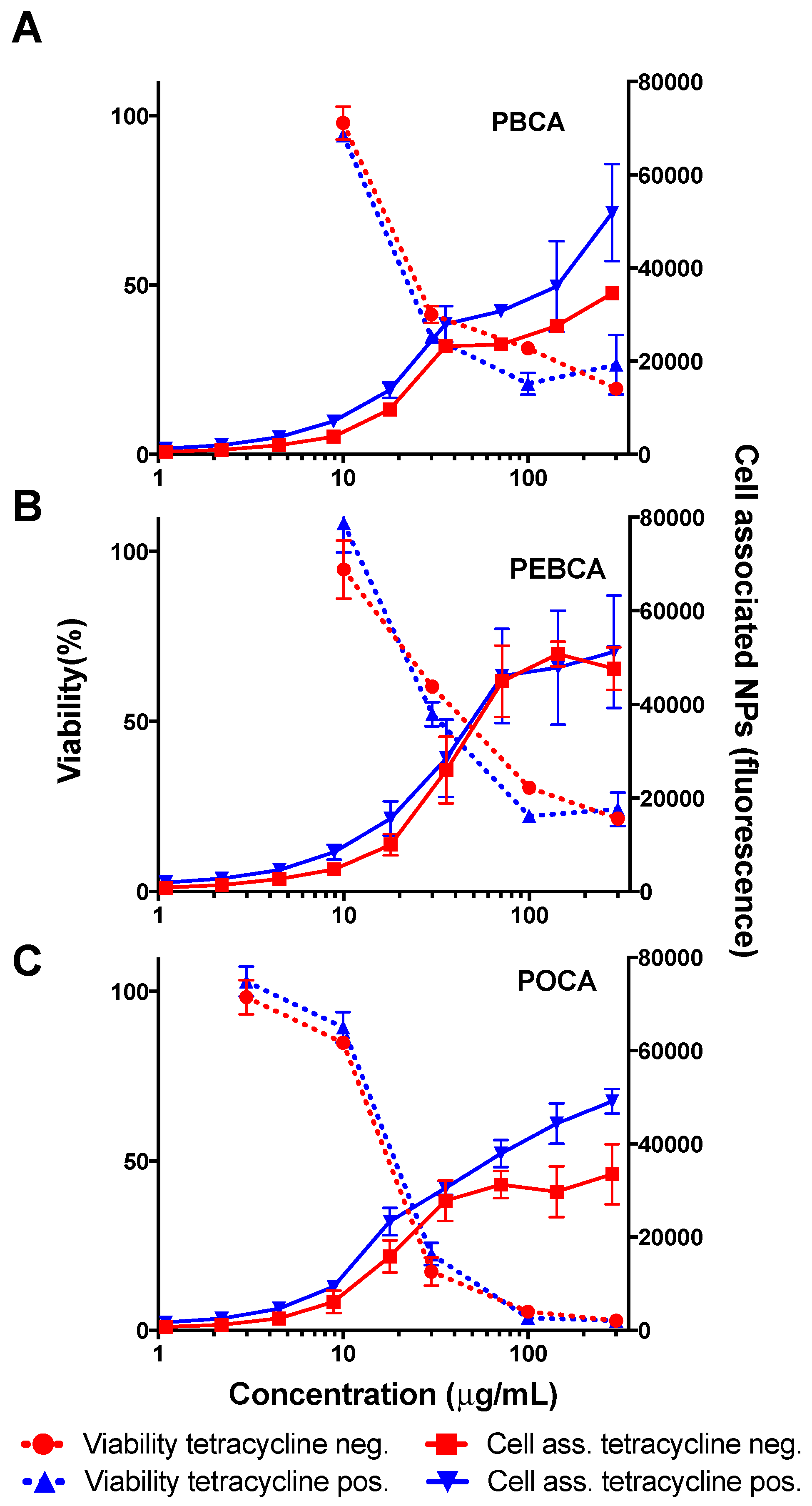
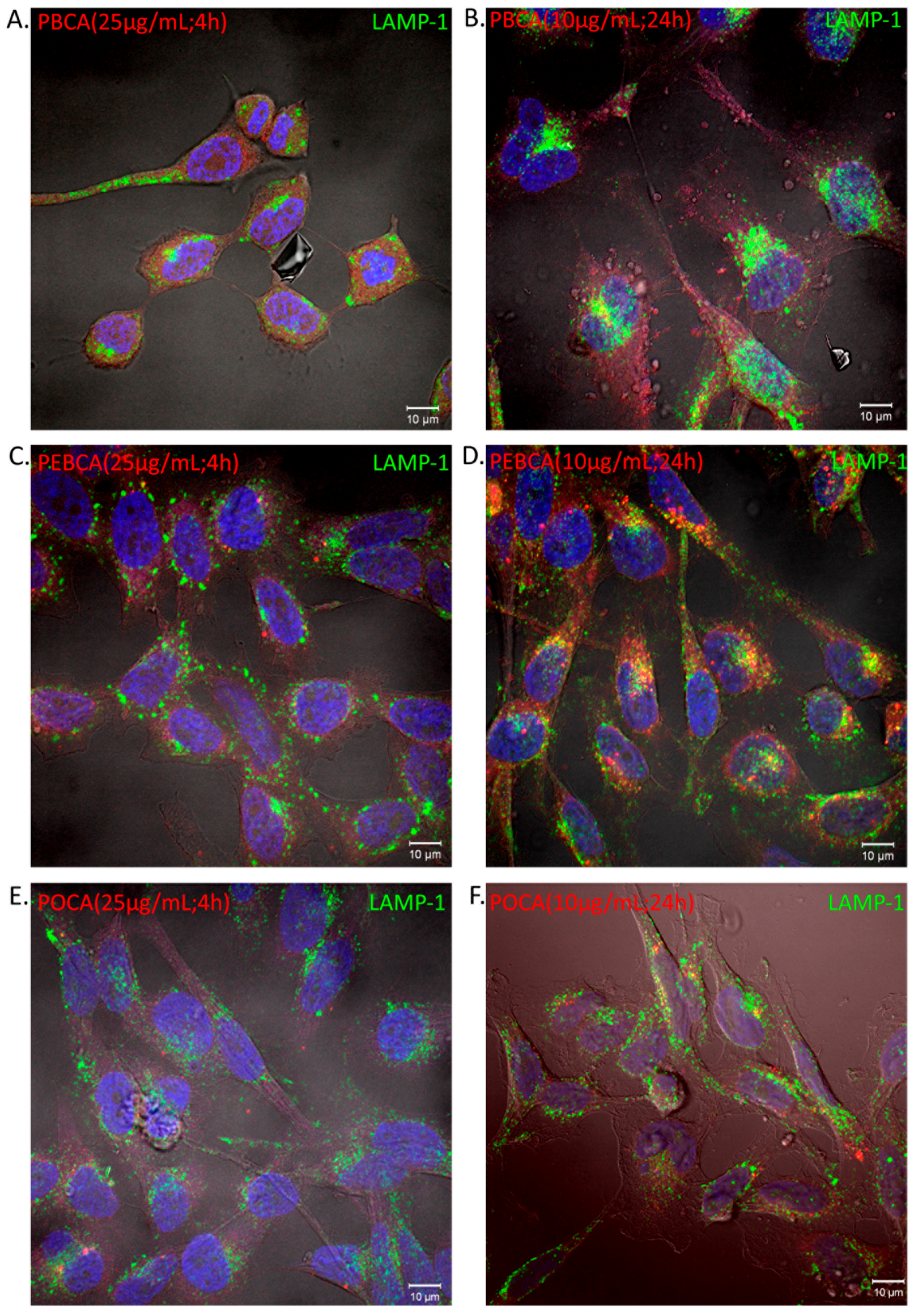
2.3. Is Toxicity Due to Cellular Association/Uptake of NPs?
2.4. Sterility and Immunology
3. Discussion
3.1. NP Toxicity
3.2. The Role of Cellular Uptake/Association on Toxicity
3.3. Evaluating In Vitro Toxicity
4. Methods
4.1. Nanoparticle Synthesis
4.2. NP Pre-Preparation for Toxicity Measurements
4.3. Cell Culture Conditions
4.4. High Throughput Screening Assays
4.5. Uptake Studies by Flow Cytometry
4.6. Cellular Uptake of PACA NPs by Confocal Fluorescence Microscopy
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Sulheim, E.; Baghirov, H.; von Haartman, E.; Boe, A.; Aslund, A.K.; Morch, Y.; Davies Cde, L. Cellular uptake and intracellular degradation of poly(alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Tekle, C.; van Deurs, B.; Sandvig, K.; Iversen, T.G. Cellular trafficking of quantum dot-ligand bioconjugates and their induction of changes in normal routing of unconjugated ligands. Nano Lett. 2008, 8, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.H.; McNeil, S.E. Preclinical Efficacy and Toxicity Testing of Engineered Nanomaterials. In Nanomedicine-Basic and Clinical Applications in Diagnostics and Therapy; Karger Publishers: Basel, Switzerland, 2011; Volume 2, pp. 71–79. [Google Scholar]
- Pradines, B.; Lievin-Le Moal, V.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Cell line-dependent cytotoxicity of poly(isobutylcyanoacrylate) nanoparticles coated with chitosan and thiolated chitosan: Insights from cultured human epithelial HeLa, Caco2/TC7 and HT-29/MTX cells. Int. J. Pharm. 2015, 491, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kolter, M.; Ott, M.; Hauer, C.; Reimold, I.; Fricker, G. Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo. J. Control. Release 2015, 197, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Lherm, C.; Muller, R.H.; Puisieux, F.; Couvreur, P. Alkylcyanoacrylate Drug Carriers. 2. Cytotoxicity of Cyanoacrylate Nanoparticles with Different Alkyl Chain-Length. Int. J. Pharm. 1992, 84, 13–22. [Google Scholar] [CrossRef]
- Aslund, A.K.; Sulheim, E.; Snipstad, S.; von Haartman, E.; Baghirov, H.; Starr, N.; Kvale Lovmo, M.; Lelu, S.; Scurr, D.; Davies, C.L.; et al. Quantification and Qualitative Effects of Different PEGylations on Poly(butyl cyanoacrylate) Nanoparticles. Mol. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- NCI-NCL. Available online: https://ncl.cancer.gov/resources/assay-cascade-protocols (accessed on 16 November 17).
- Damke, H.; Gossen, M.; Freundlieb, S.; Bujard, H.; Schmid, S.L. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995, 257, 209–220. [Google Scholar] [PubMed]
- Lira, M.C.B.; Santos-Magalhaes, N.S.; Nicolas, V.; Marsaud, V.; Silva, M.P.C.; Ponchel, G.; Vauthier, C. Cytotoxicity and cellular uptake of newly synthesized fucoidan-coated nanoparticles. Eur. J. Pharm. Biopharm. 2011, 79, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Gobbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part. Fibre Toxicol. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Crist, R.M.; Grossman, J.H.; Patri, A.K.; Stern, S.T.; Dobrovolskaia, M.A.; Adiseshaiah, P.P.; Clogston, J.D.; McNeil, S.E. Common pitfalls in nanotechnology: Lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr. Biol. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.M.; Limbach, L.K.; van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briede, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Kim, S.W.; Li, L.; Wang, S.Y.; Park, K.; Cheng, J.X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H.; Melikishvili, S.; Morch, Y.; Sulheim, E.; Aslund, A.K.; Hianik, T.; de Lange Davies, C. The effect of poly(ethylene glycol) coating and monomer type on poly(alkyl cyanoacrylate) nanoparticle interactions with lipid monolayers and cells. Colloids Surf. B Biointerfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Hak, S.; Baghirov, H.; Sulheim, E.; Morch, Y.; Lelu, S.; von Haartman, E.; Back, M.; Nilsson, K.P.; Klymchenko, A.S.; et al. Labeling nanoparticles: Dye leakage and altered cellular uptake. Cytom. A 2016. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Westrom, S.; Morch, Y.; Afadzi, M.; Aslund, A.; de Lange Davies, C. Contact-mediated intracellular delivery of hydrophobic drugs from polymeric nanoparticles. Cancer Nanotechnol. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kuempel, E.D.; Geraci, C.L.; Schulte, P.A. Risk Assessment and Risk Management of Nanomaterials in the Workplace: Translating Research to Practice. Ann. Occup. Hyg. 2012, 56, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Fontana, L.; Mauriello, M.C.; Iavicoli, I. Occupational Risk Assessment of Engineered Nanomaterials: Limits, Challenges and Opportunities. Curr. Nanosci. 2017, 13, 55–78. [Google Scholar] [CrossRef]
- Mørch, Y.; Hansen, R.; Berg, S.; Åslund, A.K.O.; Glomm, W.R.; Eggen, S.; Schmid, R.; Johnsen, H.; Kubowicz, S.; Snipstad, S.; et al. Nanoparticle-Stabilized Microbubbles for Multimodal Imaging and Drug Delivery. Contrast Media Mol. Imaging 2015. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Roger, E.; Anton, N.; Anton, H.; Shulov, I.; Vermot, J.; Mely, Y.; Vandamme, T.F. Highly lipophilic fluorescent dyes in nano-emulsions: Towards bright non-leaking nano-droplets. RSC Adv. 2012, 2, 11876–11886. [Google Scholar] [CrossRef] [Green Version]
- Åslund, A.; Sigurdson, C.J.; Klingstedt, T.; Grathwohl, S.; Bolmont, T.; Dickstein, D.L.; Glimsdal, E.; Prokop, S.; Lindgren, M.; Konradsson, P.; et al. Novel Pentameric Thiophene Derivatives for in Vitro and in Vivo Optical Imaging of a Plethora of Protein Aggregates in Cerebral Amyloidoses. ACS Chem. Biol. 2009, 4, 673–684. [Google Scholar] [CrossRef] [PubMed]
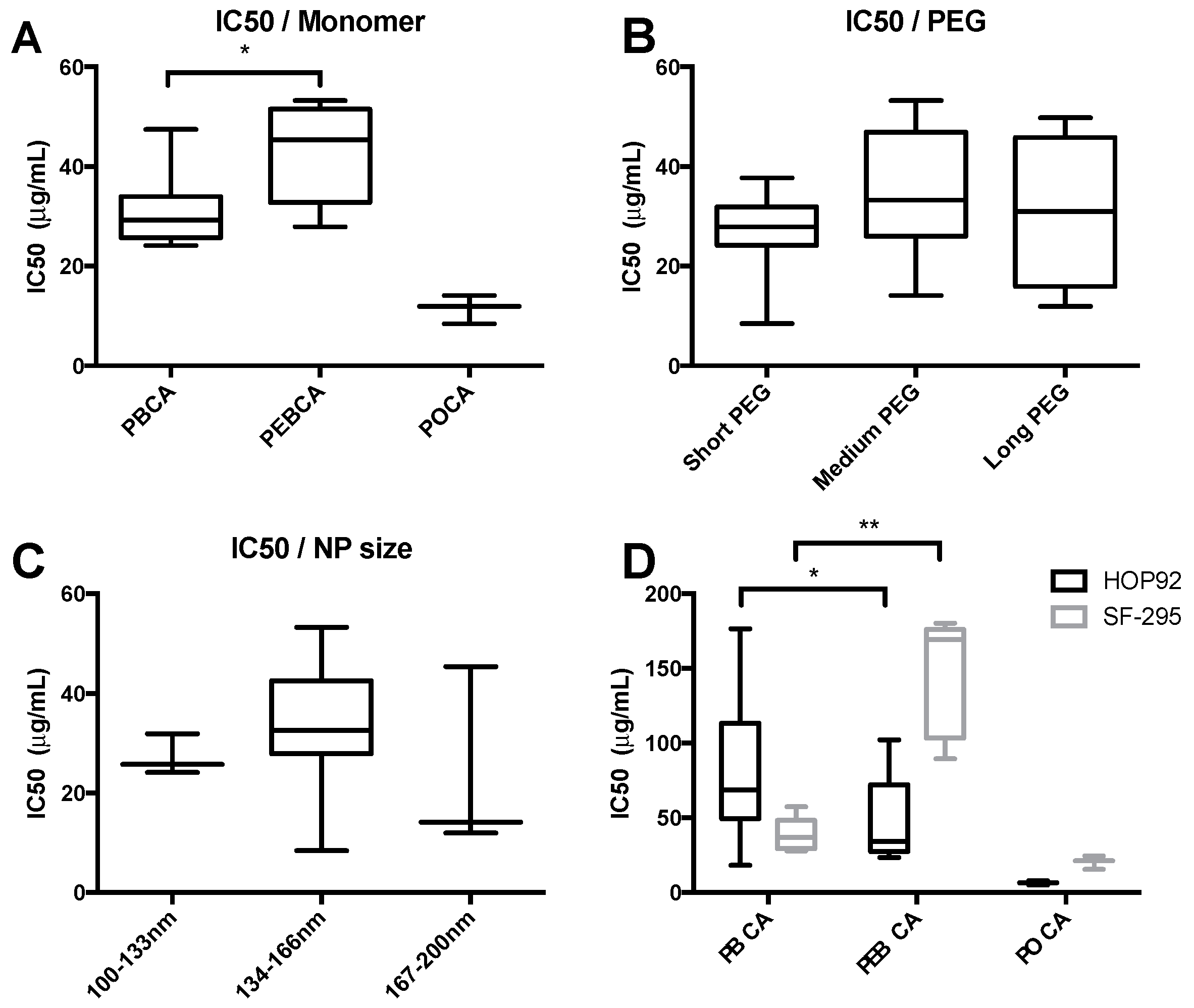
| Particle | PEG Surfactants | PEG Description | Size Groups | Zeta Potential | Polymer Chain Length (Mn and Monomer Units) |
|---|---|---|---|---|---|
| PBCA (N = 11) | Kolliphor/Brij (N = 4) | Short PEG | 100–133 nm (N = 3) | (-1) – (-7) mV | 50.5 ± 13.2 kD N: 327 ± 86 |
| Jeffamin/Brij (N = 5) | Medium PEG | ||||
| 134–166 nm (N = 8) | |||||
| Pluronic/Kolliphor (N = 2) | Long PEG | ||||
| PEBCA (N = 5) | Kolliphor/Brij (N = 2) | Short PEG | 134–166 nm(N = 4) | (-1) – (-7) mV | 48.0 ± 3.6 kD N: 265 ± 20 |
| Jeffamin/Brij (N = 2) | Medium PEG | ||||
| 167–200 nm (N = 1) | |||||
| Pluronic/Kolliphor (N = 1) | Long PEG | ||||
| POCA (N = 3) | Kolliphor/Brij (N = 1) | Short PEG | 134–166 nm (N = 1) | (-1) – (-7) mV | 53.0 ± 2.3 kD N: 254 ± 11 |
| Jeffamin/Brij (N = 1) | Medium PEG | ||||
| 167–200 nm (N = 2) | |||||
| Pluronic/Kolliphor (N = 1) | Long PEG |
| Cell Lines | Cell Origin | IC50 All NPs (μg/mL), N = 18 | IC50 PBCA NPs (μg/mL), N = 10 | IC50 PEBCA NPs (μg/mL), N = 5 | IC50 POCA NPs (μg/mL), N = 3 |
|---|---|---|---|---|---|
| IMR-90 | Lung fibroblast (normal) | 26 ± 19 | 32 ±2 4 | 22 ± 7 | 13 ± 3 |
| Hep G2 | Liver carcinoma | 26 ± 24 | 21 ± 3 | 70 ± 4 | 24 ± 1 |
| RBE4 | Rat brain endothelial cell | 44 ± 29 | 50 ± 5 | 74 ± 15 | 6 ± 1 |
| DU-145 | Prostate carcinoma | - | 30 ± 7 | >300 | 22 ± 8 |
| A498 | Kidney carcinoma | 23 ± 8 | 23 ± 5 | 30 ± 8 | 12 ± 3 |
| SW-620 | Colon adenocarcinoma | 37 ± 19 | 35 ± 7 | 56 ± 20 | 10 ± 3 |
| HOP-92 | Non-small lung carcinoma | 53 ± 45 | 77 ± 46 | 31 ± 7 | 7 ± 1 |
| MDA-MB-468 | Mammary gland adenocarcinoma | 19 ± 11 | 20 ± 9 | 26 ± 10 | 5 ± 1 |
| SF-295 | Glioblastoma multiform | 65 ± 58 | 38 ± 9 | 145 ± 40 | 20 ± 5 |
| UACC-62 | Mammary gland carcinoma | 15 ± 6 | 16 ± 2 | 19 ± 8 | 6 ± 1 |
| OVCAR-3 | Ovarian adenocarcinoma | 15 ± 7 | 15 ± 6 | 18 ± 7 | 8 ± 1 |
| COLO 205 | Colon adenocarcinoma | 23 ± 7 | 22 ± 6 | 31 ± 3 | 13 ± 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulheim, E.; Iversen, T.-G.; To Nakstad, V.; Klinkenberg, G.; Sletta, H.; Schmid, R.; Hatletveit, A.R.; Wågbø, A.M.; Sundan, A.; Skotland, T.; et al. Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles. Int. J. Mol. Sci. 2017, 18, 2454. https://doi.org/10.3390/ijms18112454
Sulheim E, Iversen T-G, To Nakstad V, Klinkenberg G, Sletta H, Schmid R, Hatletveit AR, Wågbø AM, Sundan A, Skotland T, et al. Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles. International Journal of Molecular Sciences. 2017; 18(11):2454. https://doi.org/10.3390/ijms18112454
Chicago/Turabian StyleSulheim, Einar, Tore-Geir Iversen, Vu To Nakstad, Geir Klinkenberg, Håvard Sletta, Ruth Schmid, Anne Rein Hatletveit, Ane Marit Wågbø, Anders Sundan, Tore Skotland, and et al. 2017. "Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles" International Journal of Molecular Sciences 18, no. 11: 2454. https://doi.org/10.3390/ijms18112454