Immunohistochemical Characterization of Connexin43 Expression in a Mouse Model of Diabetic Retinopathy and in Human Donor Retinas
Abstract
:1. Introduction
2. Results
2.1. High Glucose Exacerbates Pro-Inflammatory Cytokine-Mediated Increase in Connexin43 Expression
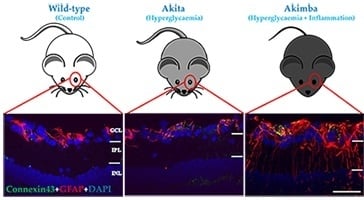
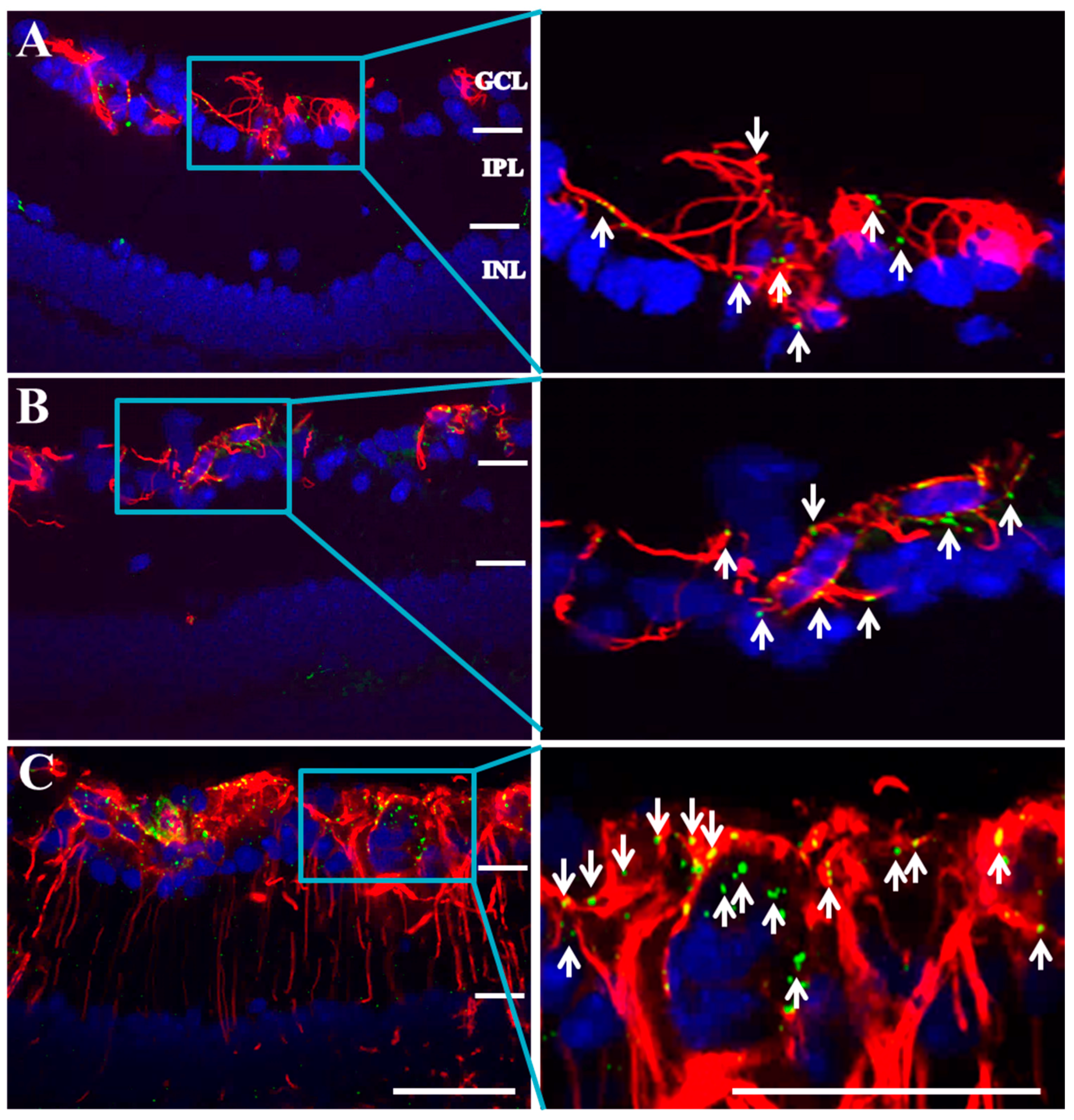
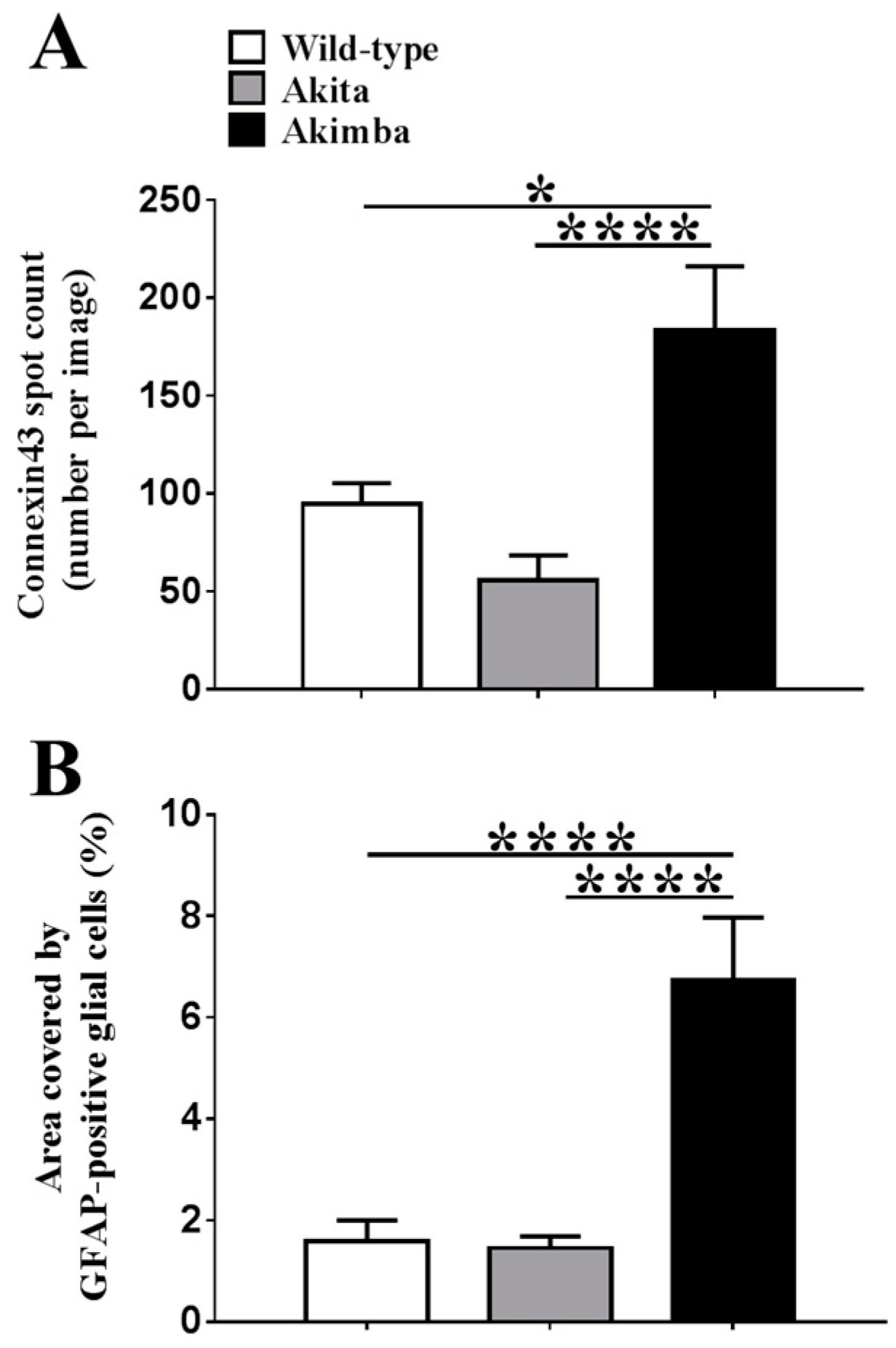
2.2. Connexin43 and Glial Fibrillary Acidic Protein (GFAP) Expression Increase in Akimba Compared to Wild-Type and Akita Retinas
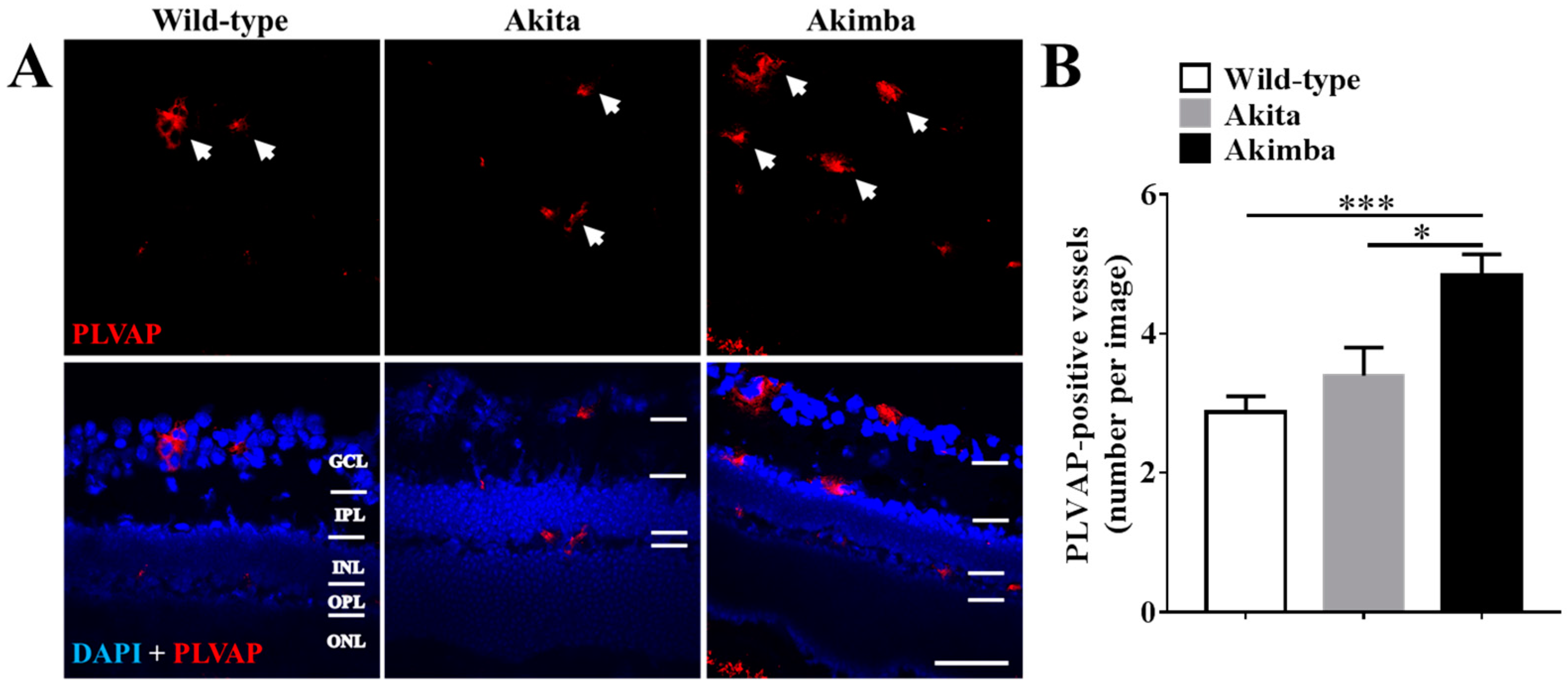
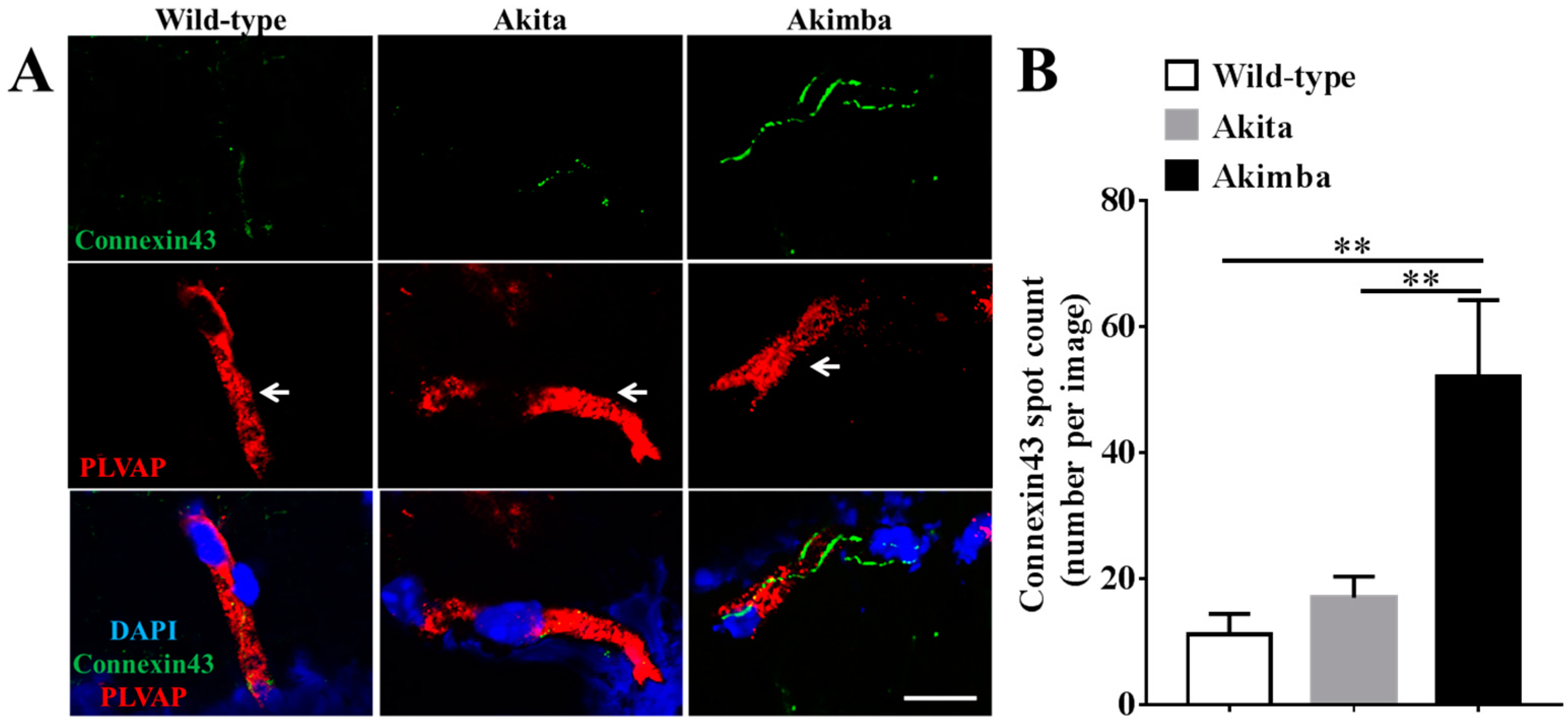
2.3. Connexin43 Expression Increases in Blood Vessels of Akimba Compared to Wild-Type and Akita Retinas
2.4. Connexin43 and GFAP Expression Increases in Human Donor DR Retinas Compared to Age-Matched Controls
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies
4.2. Animal Tissues
4.3. Human Donor Tissues
4.4. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Lieth, E.; Barber, A.J.; Gardner, T.W. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin. Ophthalmol. 1999, 14, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.K.; Merges, C.; McLeod, D.S.; Lutty, G.A. Vascular endothelial growth factor and vascular permeability changes in human diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2729–2741. [Google Scholar]
- Jiang, X.; Yang, L.; Luo, Y. Animal Models of Diabetic Retinopathy. Curr. Eye Res. 2015, 40, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Animal Models of Diabetic Retinopathy. In Retinal and Choroidal Angiogenesis; Springer: New York, NY, USA, 2008; pp. 81–102. [Google Scholar]
- Rakoczy, E.P.; Ali Rahman, I.S.; Binz, N.; Li, C.R.; Vagaja, N.N.; de Pinho, M.; Lai, C.M. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am. J. Pathol. 2010, 177, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Kruk, J.; Klaassen, I.; Vogels, I.M.; Magno, A.L.; Lai, C.M.; Van Noorden, C.J.; Schlingemann, R.O.; Rakoczy, E.P. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp. Eye Res. 2014, 122, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Zhang, J.; Acosta, M.L.; Rupenthal, I.D.; Green, C.R. Connexin43 in retinal injury and disease. Prog. Retin. Eye Res. 2016, 51, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.M.; Johnson, C.S.; de Souza, C.F.; Chee, K.S.; Good, W.R.; Green, C.R.; Danesh-Meyer, H.V. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4028–4034. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Gorrie, C.A.; Velamoor, S.; Green, C.R.; Nicholson, L.F. Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci. Res. 2013, 75, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tonkin, R.S.; Nguyen, T.; O’Carroll, S.J.; Nicholson, L.F.; Green, C.R.; Moalem-Taylor, G.; Gorrie, C.A. Systemic Administration of Connexin43 Mimetic Peptide Improves Functional Recovery after Traumatic Spinal Cord Injury in Adult Rats. J. Neurotrauma 2017, 34, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Green, C.R.; Bennet, L.; Nicholson, L.F.; Danesh-Meyer, H.; O’Carroll, S.J.; Gunn, A.J. A key role for connexin hemichannels in spreading ischemic brain injury. Curr. Drug Targets 2013, 14, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Kerr, N.M.; Zhang, J.; Eady, E.K.; O’Carroll, S.J.; Nicholson, L.F.; Johnson, C.S.; Green, C.R. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain 2012, 135, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Green, C.R.; Danesh-Meyer, H.V.; Rupenthal, I.D. Neuroprotection in the treatment of glaucoma—A focus on connexin43 gap junction channel blockers. Eur. J. Pharm. Biopharm. 2015, 95, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Green, C.R.; Teague, R.; Perrett, J.; Danesh-Meyer, H.V.; Toth, I.; Rupenthal, I.D. Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv. Transl. Res. 2015, 5, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Theriault, E.; Frankenstein, U.; Hertzberg, E.; Nagy, J. Connexin 43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J. Comp. Neurol. 1997, 382, 199–214. [Google Scholar] [CrossRef]
- Cronin, M.; Anderson, P.N.; Cook, J.E.; Green, C.R.; Becker, D.L. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol. Cell. Neurosci. 2008, 39, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bobbie, M.W.; Roy, S.; Trudeau, K.; Munger, S.J.; Simon, A.M.; Roy, S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3758–3763. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Muto, T.; Barrette, K.; Challyandra, L.; Roy, S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Mol. Vis. 2014, 20, 732–741. [Google Scholar] [PubMed]
- Tien, T.; Barrette, K.F.; Chronopoulos, A.; Roy, S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6518–6525. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Haimovici, R.; Kao, R.; Li, A.F.; Roy, S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 2002, 51, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Green, C.R.; Nicholson, L.F.; O’Carroll, S.J.; Fraser, M.; Bennet, L.; Gunn, A.J. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann. Neurol. 2012, 71, 121–132. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Wang, N.; Bol, M.; Decrock, E.; Ponsaerts, R.; Bultynck, G.; Dupont, G.; Leybaert, L. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J. Biol. Chem. 2012, 287, 12250–12266. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Alkadhi, M.; Nicholson, L.F.; Green, C.R. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun. Adhes. 2008, 15, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; O’Carroll, S.J.; Danesh-Meyer, H.V.; van der Heyde, H.C.; Becker, D.L.; Nicholson, L.F.; Green, C.R. Connexin-based therapeutic approaches to inflammation in the central nervous system. In Connexin Cell Communication Channels: Roles in the Immune System and Immunopathology; CRC Press: Boca Raton, FL, USA, 2013; pp. 273–305. [Google Scholar]
- Zhang, J.; O’Carroll, S.J.; Henare, K.; Ching, L.M.; Ormonde, S.; Nicholson, L.F.; Danesh-Meyer, H.V.; Green, C.R. Connexin hemichannel induced vascular leak suggests a new paradigm for cancer therapy. FEBS Lett. 2014, 588, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Nor, M.N.M.; Danesh-Meyer, H.V.; Vessey, K.A.; Fletcher, E.L.; O’Carroll, S.J.; Acosta, M.L.; Green, C.R. Connexin43 Mimetic Peptide Improves Retinal Function and Reduces Inflammation in a Light-Damaged Albino Rat ModelConnexin43 Mimetic Peptide Preserves Retinal Function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3961–3973. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Chen, X.; McMenamin, P.G.; Rakoczy, E.P. Absence of clinical correlates of diabetic retinopathy in the Ins2Akita retina. Clin. Exp. Ophthalmol. 2013, 41, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.R.; Renteria, R.C.; Duong, T.Q. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Magno, A.L.; Ramos, D.; Catita, J.; McMenamin, P.G.; Chen, F.K.; Rakoczy, E.P.; Ruberte, J. Angiography reveals novel features of the retinal vasculature in healthy and diabetic mice. Exp. Eye Res. 2015, 138, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.; Hirabara, S.; Rebelato, E.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.; Carpinelli, A.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Calado, S.M.; Alves, L.S.; Simao, S.; Silva, G.A. GLUT1 activity contributes to the impairment of PEDF secretion by the RPE. Mol. Vis. 2016, 22, 761–770. [Google Scholar] [PubMed]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef] [PubMed]
- Li, A.F.; Sato, T.; Haimovici, R.; Okamoto, T.; Roy, S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5376–5382. [Google Scholar] [CrossRef]
- Tien, T.; Muto, T.; Zhang, J.; Sohn, E.H.; Mullins, R.F.; Roy, S. Association of reduced Connexin 43 expression with retinal vascular lesions in human diabetic retinopathy. Exp. Eye Res. 2016, 146, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Girao, H.; Pereira, P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J. Biol. Chem. 2004, 279, 27219–27224. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Naranjo, A.; Cormie, P.; Serrano, A.E.; Wang, C.H.M.; Thrasivoulou, C.; Sutcliffe, J.E.S.; Gilmartin, D.J.; Tsui, J.; Serena, T.E.; Phillips, A.R.J.; et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol. Int. 2012, 36, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Basilio, D.; Saez, J.C.; Orellana, J.A.; Raine, C.S.; Bukauskas, F.; Bennett, M.V.; Berman, J.W. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J. Neuroimmun. Pharmacol. 2012, 7, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Davidson, J.O.; Gunn, K.C.; Phillips, A.R.; Green, C.R.; Gunn, A.J. Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv. Protein Chem. Struct. Biol. 2016, 104, 1–37. [Google Scholar] [PubMed]
- Coutinho, P.; Qiu, C.; Frank, S.; Tamber, K.; Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 2003, 27, 525–541. [Google Scholar] [CrossRef]
- Kerr, N.M.; Johnson, C.S.; Zhang, J.; Eady, E.K.; Green, C.R.; Danesh-Meyer, H.V. High pressure-induced retinal ischaemia reperfusion causes upregulation of gap junction protein connexin43 prior to retinal ganglion cell loss. Exp. Neurol. 2012, 234, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Nivison-Smith, L.; O’Brien, B.J.; Truong, M.; Guo, C.X.; Kalloniatis, M.; Acosta, M.L. Vinpocetine modulates metabolic activity and function during retinal ischemia. Am. J. Physiol. Cell Physiol. 2015, 308, 737–749. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugisho, O.O.; Green, C.R.; Zhang, J.; Binz, N.; Acosta, M.L.; Rakoczy, E.; Rupenthal, I.D. Immunohistochemical Characterization of Connexin43 Expression in a Mouse Model of Diabetic Retinopathy and in Human Donor Retinas. Int. J. Mol. Sci. 2017, 18, 2567. https://doi.org/10.3390/ijms18122567
Mugisho OO, Green CR, Zhang J, Binz N, Acosta ML, Rakoczy E, Rupenthal ID. Immunohistochemical Characterization of Connexin43 Expression in a Mouse Model of Diabetic Retinopathy and in Human Donor Retinas. International Journal of Molecular Sciences. 2017; 18(12):2567. https://doi.org/10.3390/ijms18122567
Chicago/Turabian StyleMugisho, Odunayo O., Colin R. Green, Jie Zhang, Nicolette Binz, Monica L. Acosta, Elizabeth Rakoczy, and Ilva D. Rupenthal. 2017. "Immunohistochemical Characterization of Connexin43 Expression in a Mouse Model of Diabetic Retinopathy and in Human Donor Retinas" International Journal of Molecular Sciences 18, no. 12: 2567. https://doi.org/10.3390/ijms18122567
APA StyleMugisho, O. O., Green, C. R., Zhang, J., Binz, N., Acosta, M. L., Rakoczy, E., & Rupenthal, I. D. (2017). Immunohistochemical Characterization of Connexin43 Expression in a Mouse Model of Diabetic Retinopathy and in Human Donor Retinas. International Journal of Molecular Sciences, 18(12), 2567. https://doi.org/10.3390/ijms18122567