Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability
Abstract
:1. Introduction
2. Results
2.1. RA Counters Proliferation but Has No Negative Impact on the Survival of Human Skin-Derived MCs
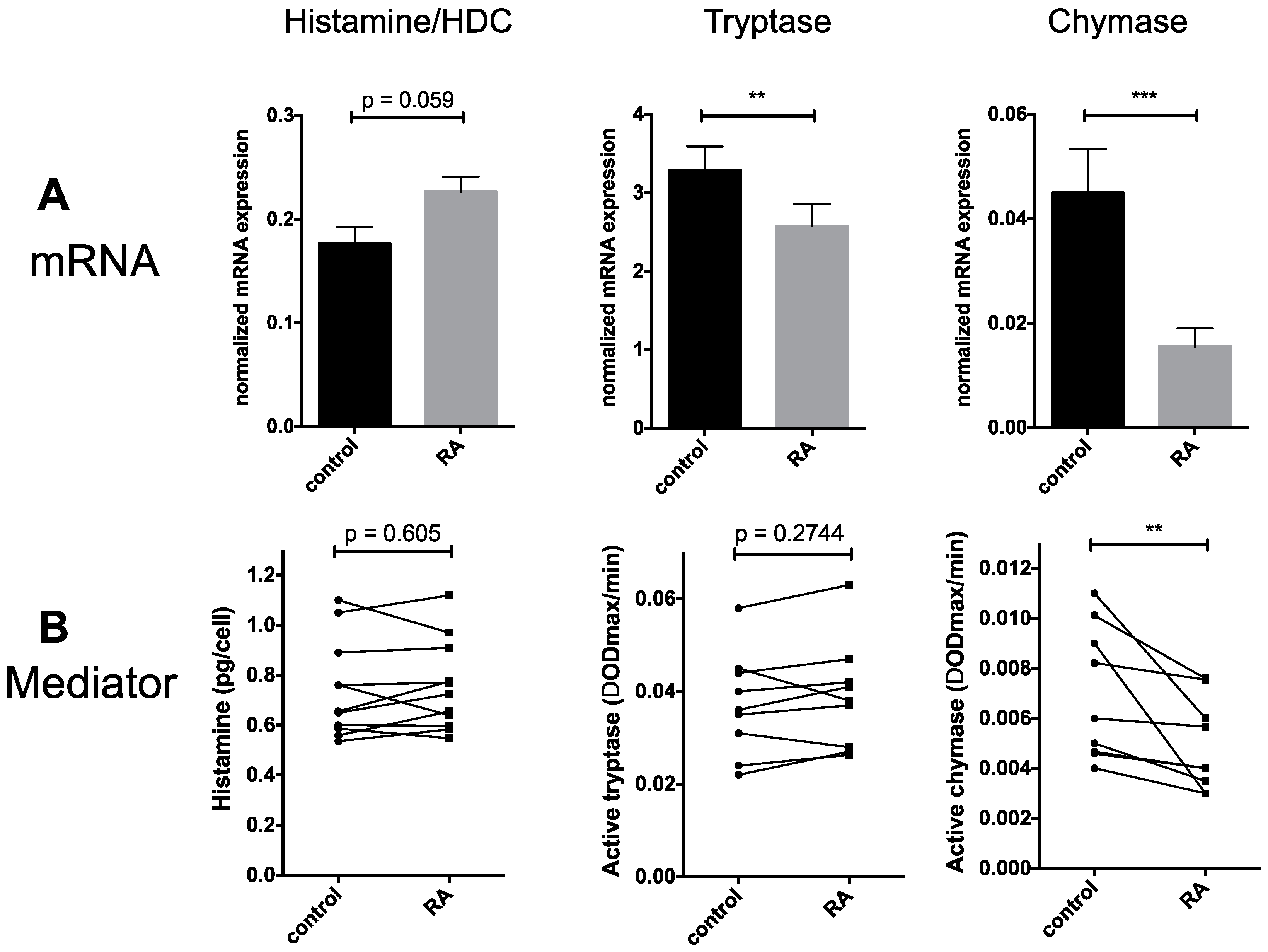
2.2. Granule Constituents
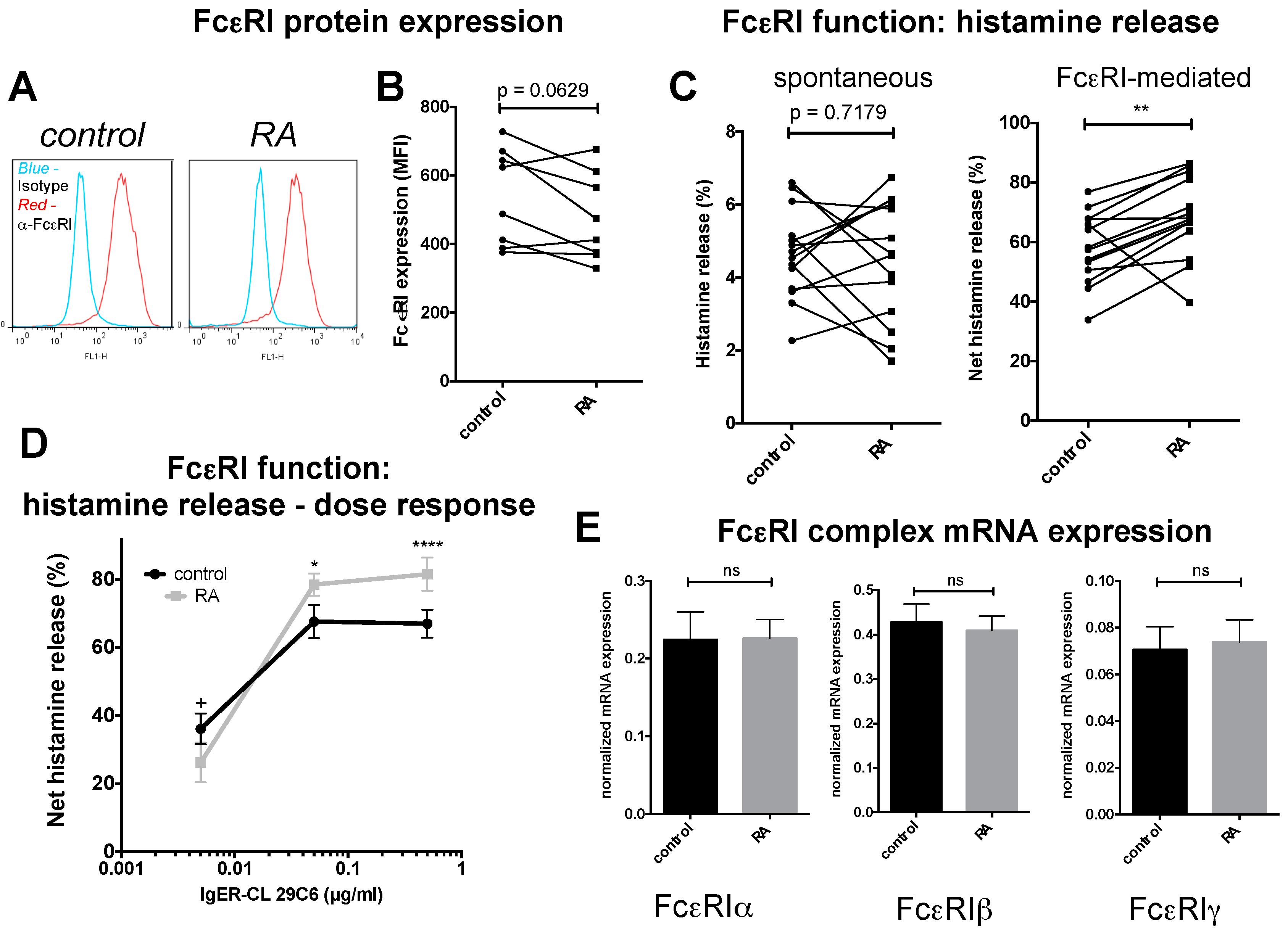
2.3. FcεRI Expression and Allergic MC Stimulation
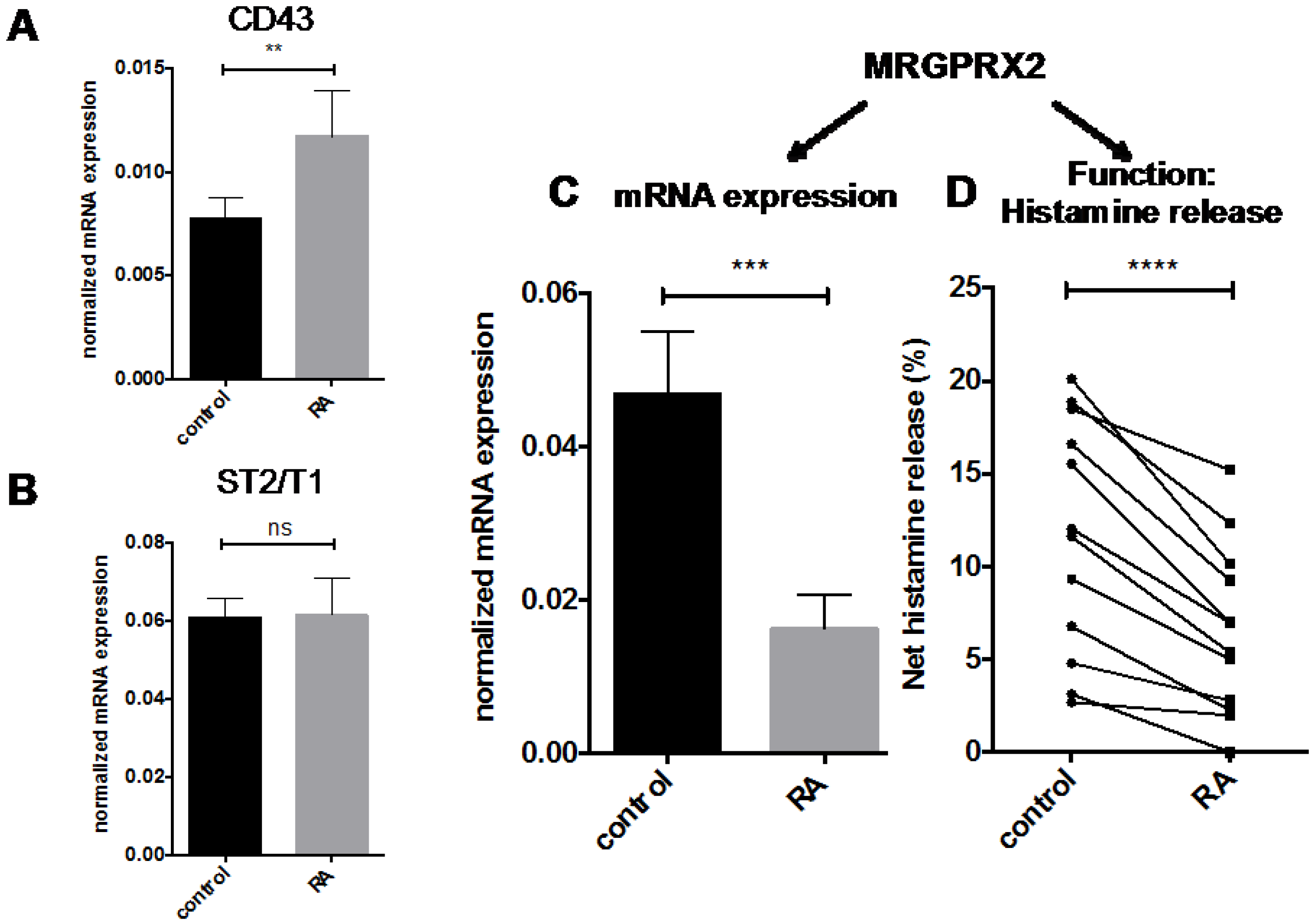
2.4. Other MC Selective Traits
3. Discussion
4. Experimental Section
4.1. Isolation and Culture of Human Skin MCs
4.2. MC Treatment
4.3. Toluidine-Blue Staining and Microscopy
4.4. Annexin-FITC/PI Staining
4.5. BrdU Incorporation
4.6. Reverse Transcription-Quantitative PCR (RT-qPCR)
4.7. FcεRI Surface Expression
4.8. Histamine Quantitation
4.9. Histamine Release Assay
4.10. MC Protease Activity
4.11. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Motakis, E.; Zuberbier, T. Mast cell transcriptome elucidation: What are the implications for allergic disease in the clinic and where do we go next? Expert Rev. Clin. Immunol. 2014, 10, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Oboki, K.; Ito, A. Development of mast cells. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2008, 38, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Papoiu, A.D. What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep. 2008, 8, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Nilsson, G.; Suttle, M.M.; Naukkarinen, A. Is there a role for mast cells in psoriasis? Arch. Dermatol. Res. 2008, 300, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Kaplan, A.P. Progress and challenges in the understanding of chronic urticaria. Allergy Asthma Clin. Immunol. 2007, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bretterklieber, A.; Beham-Schmid, C.; Sturm, G.J.; Berghold, A.; Brezinschek, R.; Aberer, W.; Aberer, E. Anaphylaxis with clonal mast cells in normal looking skin—A new entity? Allergy 2015, 70, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Trivedi, N.N.; Zuberbier, T. Phenotypic variability in human skin mast cells. Exp. Dermatol. 2016, 25, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Immunological Genome Project Consortium. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Voorhees, J.J. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996, 10, 1002–1013. [Google Scholar] [PubMed]
- Mihaly, J.; Gamlieli, A.; Worm, M.; Ruhl, R. Decreased retinoid concentration and retinoid signalling pathways in human atopic dermatitis. Exp. Dermatol. 2011, 20, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Fisher, G.J.; Kang, S.; Voorhees, J.J. Molecular mechanisms of intrinsic skin aging and retinoid-induced repair and reversal. J. Investig. Dermatol. Symp. Proc. 1998, 3, 57–60. [Google Scholar] [PubMed]
- Appa, Y. Retinoid therapy: Compatible skin care. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 111–119. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, Y.G.; Euvrard, S.; Bouwes Bavinck, J.N. Systemic and topical retinoids in the management of skin cancer in organ transplant recipients. Dermatol. Surg. 2004, 30, 656–661. [Google Scholar] [PubMed]
- Zhang, C.; Duvic, M. Treatment of cutaneous T-cell lymphoma with retinoids. Dermatol. Ther. 2006, 19, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Li, K. A review of acitretin for the treatment of psoriasis. Expert Opin. Drug Saf. 2009, 8, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Digiovanna, J.J.; Mauro, T.; Milstone, L.M.; Schmuth, M.; Toro, J.R. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol. Ther. 2013, 26, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Timm, K.; Kuhl, A.A.; Heine, G.; Worm, M. Impact of systemic alitretinoin treatment on skin barrier gene and protein expression in patients with chronic hand eczema. Br. J. Dermatol. 2016, 175, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Starke, A.; Kirchhof, L.; Zuberbier, T.; Henz, B.M. Comparative cytokine profile of human skin mast cells from two compartments—Strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J. Leukoc. Biol. 2004, 75, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. IL-4 and human skin mast cells revisited: Reinforcement of a pro-allergic phenotype upon prolonged exposure. Arch. Dermatol. Res. 2016, 308, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.L.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; Itoh, M.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babina, M.; Guhl, S.; Motakis, E.; Artuc, M.; Hazzan, T.; Worm, M.; Forrest, A.R.; Zuberbier, T. Retinoic acid potentiates inflammatory cytokines in human mast cells: Identification of mast cells as prominent constituents of the skin retinoid network. Mol. Cell. Endocrinol. 2015, 406, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.; Daub, C.O.; Vitting-Seerup, K.; Andersson, R.; Lilje, B.; Drablos, F.; Lennartsson, A.; Ronnerblad, M.; Hrydziuszko, O.; Vitezic, M.; et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 2015, 347, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Guhl, S.; Artuc, M.; Neou, A.; Babina, M.; Zuberbier, T. Long-term cultured human skin mast cells are suitable for pharmacological studies of anti-allergic drugs due to high responsiveness to fcepsilonri cross-linking. Biosci. Biotechnol. Biochem. 2011, 75, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Guhl, S.; Neou, A.; Artuc, M.; Zuberbier, T.; Babina, M. Skin mast cells develop non-synchronized changes in typical lineage characteristics upon culture. Exp. Dermatol. 2014, 23, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G.; Abrink, M.; Ringvall, M.; Wernersson, S. Mast cell proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [PubMed]
- Potaczek, D.P.; Kabesch, M. Current concepts of IgE regulation and impact of genetic determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Zoltowska, A.; Ketelaar, M.E.; Nilsson, G. IL-33 and thymic stromal lymphopoietin in mast cell functions. Eur. J. Pharmacol. 2016, 778, 68–76. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Weber, S.; Henz, B.M. Cd43 (leukosialin, sialophorin) expression is differentially regulated by retinoic acids. Eur. J. Immunol. 1997, 27, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Purton, L.E. Roles of retinoids and retinoic acid receptors in the regulation of hematopoietic stem cell self-renewal and differentiation. PPAR Res. 2007, 2007, 87934. [Google Scholar] [CrossRef] [PubMed]
- Rochette-Egly, C.; Germain, P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl. Recept. Signal. 2009, 7, e005. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Weber, S.; Henz, B.M. Retinoic acids and dexamethasone alter cell-surface density of β2-integrins and ICAM-1 on human leukemic (HMC-1) mast cells. Arch. Dermatol. Res. 1997, 289, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Mammeri, K.; Henz, B.M. Retinoic acid up-regulates myeloid ICAM-3 expression and function in a cell-specific fashion—Evidence for retinoid signaling pathways in the mast cell lineage. J. Leukoc. Biol. 2001, 69, 361–372. [Google Scholar] [PubMed]
- Kinoshita, T.; Koike, K.; Mwamtemi, H.H.; Ito, S.; Ishida, S.; Nakazawa, Y.; Kurokawa, Y.; Sakashita, K.; Higuchi, T.; Takeuchi, K.; et al. Retinoic acid is a negative regulator for the differentiation of cord blood-derived human mast cell progenitors. Blood 2000, 95, 2821–2828. [Google Scholar] [PubMed]
- Hjertson, M.; Kivinen, P.K.; Dimberg, L.; Nilsson, K.; Harvima, I.T.; Nilsson, G. Retinoic acid inhibits in vitro development of mast cells but has no marked effect on mature human skin tryptase- and chymase-positive mast cells. J. Investig. Dermatol. 2003, 120, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Iavarone, A.; Freedman, L.P. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J. Biol. Chem. 1996, 271, 31723–31728. [Google Scholar] [CrossRef]
- Lavelle, D.; Chen, Y.H.; Hankewych, M.; Desimone, J. Inhibition of myeloma cell growth by all-trans retinoic acid is associated with upregulation of p21WAF1 and dephosphorylation of the retinoblastoma protein. Leuk. Lymphoma 1999, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Kyriakou, D.S.; Seretakis, D.; Boucher, W.; Letourneau, R.; Kempuraj, D.; Theoharides, T.C. Inhibitory effect of retinoic acid on proliferation, maturation and tryptase level in human leukemic mast cells (HMC-1). Int. J. Immunopathol. Pharmacol. 2003, 16, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Blom, T.; Kusche-Gullberg, M.; Kjellen, L.; Butterfield, J.H.; Sundstrom, C.; Nilsson, K.; Hellman, L. Phenotypic characterization of the human mast-cell line HMC-1. Scand. J. Immunol. 1994, 39, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Douer, D.; Ramezani, L.; Parker, J.; Levine, A.M. All-trans-retinoic acid effects the growth, differentiation and apoptosis of normal human myeloid progenitors derived from purified CD34+ bone marrow cells. Leukemia 2000, 14, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. The yin and yang of IL-33 in human skin mast cell biology—Reinforcement of proliferation and histamine synthesis combined with profound decline in immunological and non-immunological histamine releasability. Nutrients 2017. in preparation. [Google Scholar]
- Ohtsu, H.; Tanaka, S.; Terui, T.; Hori, Y.; Makabe-Kobayashi, Y.; Pejler, G.; Tchougounova, E.; Hellman, L.; Gertsenstein, M.; Hirasawa, N.; et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001, 502, 53–56. [Google Scholar] [CrossRef]
- Blank, U.; Madera-Salcedo, I.K.; Danelli, L.; Claver, J.; Tiwari, N.; Sánchez-Miranda, E.; Vázquez-Victorio, G.; Ramírez-Valadez, K.A.; Macias-Silva, M.; González-Espinosa, C. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front. Immunol. 2014, 5, 453. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic acid as a modulator of T cell immunity. Nutrients 2016, 8, E349. [Google Scholar] [CrossRef] [PubMed]
- Guhl, S.; Babina, M.; Neou, A.; Zuberbier, T.; Artuc, M. Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells—Drastically reduced levels of tryptase and chymase in mast cell lines. Exp. Dermatol. 2010, 19, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Regulation of FoxP3 regulatory T cells and Th17 cells by retinoids. Clin. Dev. Immunol. 2008, 2008, 416910. [Google Scholar] [CrossRef] [PubMed]
- Ertesvåg, A.; Naderi, S.; Blomhoff, H.K. Regulation of B cell proliferation and differentiation by retinoic acid. Semin. Immunol. 2009, 21, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Artuc, M.; Steckelings, U.M.; Grutzkau, A.; Smorodchenko, A.; Henz, B.M. A long-term coculture model for the study of mast cell-keratinocyte interactions. J. Investig. Dermatol. 2002, 119, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guhl, S.; Babina, M.; Zuberbier, T.; Artuc, M. Integration of the human dermal mast cell into the organotypic co-culture skin model. Methods Mol. Biol. 2014, 1192, 69–85. [Google Scholar] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Skin mast cell phenotypes between two highly divergent cohorts—More pronounced variability within than between groups. Exp. Dermatol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Schulke, Y.; Kirchhof, L.; Guhl, S.; Franke, R.; Bohm, S.; Zuberbier, T.; Henz, B.M.; Gombart, A.F. The transcription factor profile of human mast cells in comparison with monocytes and granulocytes. Cell. Mol. Life Sci. 2005, 62, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Karkola, K.; Harvima, R.J.; Naukkarinen, A.; Neittaanmaki, H.; Horsmanheimo, M.; Fraki, J.E. Biochemical and histochemical evaluation of tryptase in various human tissues. Arch. Dermatol. Res. 1989, 281, 231–237. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babina, M.; Artuc, M.; Guhl, S.; Zuberbier, T. Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability. Int. J. Mol. Sci. 2017, 18, 525. https://doi.org/10.3390/ijms18030525
Babina M, Artuc M, Guhl S, Zuberbier T. Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability. International Journal of Molecular Sciences. 2017; 18(3):525. https://doi.org/10.3390/ijms18030525
Chicago/Turabian StyleBabina, Magda, Metin Artuc, Sven Guhl, and Torsten Zuberbier. 2017. "Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability" International Journal of Molecular Sciences 18, no. 3: 525. https://doi.org/10.3390/ijms18030525




