Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease
Abstract
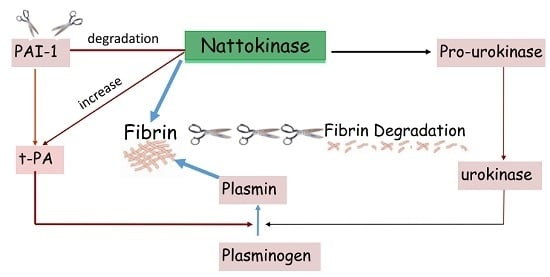
:1. Introduction
2. Benefits of Nattokinase
3. Nattokinase Safety Assessment
4. Production and Purification of Nattokinase
5. Analysis of the Nattokinase Gene and Protein
6. Recombinant Nattokinase Production via Genetic Engineering
7. Plants as Potential Factories for Nattokinase Production
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NK | nattokinase |
| PMF | plant molecular farming |
| BeYDV | bean yellow dwarf virus |
| TSP | total soluble protein |
References
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Yatagai, C.; Maruyama, M.; Kawahara, T.; Sumi, H. Nattokinase promoted tissue plasminogen activator release from human cells. Pathophysiol. Haemost. Thromb. 2008, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Hong, K.; Ito, Y.; Misawa, S.; Takeuchi, N.; Kariya, K.; Nishimuro, S. Transport of nattokinase across the rat intestinal tract. Biol. Pharm. Bull. 1995, 18, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ohnishi, K.; Takaoka, S.; Ogasawara, K.; Fukuyama, R.; Nakamuta, H. Antihypertensive effects of continuous oral administration of nattokinase and its fragments in spontaneously hypertensive rats. Biol. Pharm. Bull. 2011, 34, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, F.; Negahdaripour, M.; Berenjian, A.; Behfar, A.; Mohammadi, F.; Zamani, M.; Irajie, C.; Ghasemi, Y. Nattokinase: Production and application. Appl. Microbiol. Biotechnol. 2014, 98, 9199–9206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, S.; Liu, M.; Gao, C.; Yang, J.; Zhang, X.; Ding, B. Ultrasmall and anionic starch nanospheres: Formation and vitro thrombolytic behavior study. Carbohydr. Polym. 2013, 96, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sumi, H.; Hamada, H.; Nakanishi, K.; Hiratani, H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990, 84, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: Results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr. Blood Press. Control. 2016, 9, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, Y.; Nirengi, S.; Homma, T.; Esaki, K.; Ohta, M.; Clark, J.F.; Hamaoka, T. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci. Rep. 2015, 5, 11601. [Google Scholar] [CrossRef] [PubMed]
- Lampe, B.J.; English, J.C. Toxicological assessment of nattokinase derived from Bacillus subtilis var. natto. Food Chem. Toxicol. 2016, 88, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kondo, K.; Matsumoto, Y.; Zhao, B.-Q.; Otsuguro, K.; Maeda, T.; Tsukamoto, Y.; Urano, T.; Umemura, K. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci. 2003, 73, 1289–1298. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Kim, T.-S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.-S.; Park, S.K.; Lee, S.-P.; Choi, E.-K.; et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim. Res. 2013, 29, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, M.; Yang, X.; Chen, Q.; Chen, H.; Lin, D.H. Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Acta Haematol. 2014, 132, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.-H.; Shen, M.-C.; Lin, J.-S.; Wen, Y.-K.; Hwang, K.-L.; Cham, T.-M.; Yang, N.-C. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr. Res. 2009, 29, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ero, M.P.; Ng, C.M.; Mihailovski, T.; Harvey, N.R.; Lewis, B.H. A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern. Ther. Health Med. 2013, 19, 16–19. [Google Scholar] [PubMed]
- Chang, Y.-Y.; Liu, J.-S.; Lai, S.-L.; Wu, H.-S.; Lan, M.-Y. Cerebellar hemorrhage provoked by combined use of nattokinase and aspirin in a patient with cerebral microbleeds. Intern. Med. 2008, 47, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Fadl, N.N.; Ahmed, H.H.; Booles, H.F.; Sayed, A.H. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum. Exp. Toxicol. 2013, 32, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Dogné, J.M.; Hanson, J.; de Leval, X.; Pratico, D.; Pace-Asciak, C.R.; Drion, P.; Pirotte, B.; Ruan, K.H. From the design to the clinical application of thromboxane modulators. Curr. Pharm. Des. 2006, 12, 903–923. [Google Scholar] [CrossRef] [PubMed]
- Ibe, S.; Kumada, K.; Yoshida, K.; Otobe, K. Natto (fermented soybean) extract extends the adult lifespan of Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2013, 77, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2006, 43, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, Q.; Zhang, R.-H.; Zhang, Y.-Z. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douche, a traditional Chinese soybean food. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 45–52. [Google Scholar] [CrossRef]
- Kim, W.; Choi, K.; Kim, Y.; Park, H.; Choi, J.; Lee, Y.; Oh, H.; Kwon, I.; Lee, S. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl. Environ. Microbiol. 1996, 62, 2482–2488. [Google Scholar] [PubMed]
- Fujita, M.; Nomura, K.; Hong, K.; Ito, Y.; Asada, A.; Nishimuro, S. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem. Biophys. Res. Commun. 1993, 197, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Mizutani, O.; Yamagata, Y.; Ichishima, E.; Nakajima, T. Alkaline-resistance model of subtilisin ALP I, a novel alkaline subtilisin. J. Biochem. 2001, 129, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.A.; Thuan, D.H.T.; Tam, T.T.M.; Huong, N.T. Determination the optimum fermentation in obtaining nattokinase by Bacillus subtilis natto. Int. J. Innov. Appl. Stud. 2015, 13, 663–668. [Google Scholar]
- Tuan, N.A.; Huong, N.T. Optimization of the fermentation medium to receive the highest biomass yield by Bacillus subtilis natto and the initial test of nattokinase yield. IOSR J. Eng. 2014, 4, 35–40. [Google Scholar]
- Ku, T.W.; Tsai, R.L.; Pan, T.M. A simple and cost-saving approach to optimize the production of subtilisin NAT by submerged cultivation of Bacillus subtilis natto. J. Agric. Food Chem. 2009, 57, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Chen, H.-J.; Liang, T.-W.; Lin, Y.-D. A novel nattokinase produced by Pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochem. 2009, 44, 70–76. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, Y.Y.; Liang, T.W. Purification and biochemical characterization of a nattokinase by conversion of shrimp shell with Bacillus subtilis TKU007. N. Biotechnol. 2011, 28, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Berenjian, A.; Mahanama, R.; Kavanagh, J.; Dehghani, F.; Ghasemi, Y. Nattokinase production: Medium components and feeding strategy studies. Chem. Ind. Chem. Eng. 2014, 20, 541–547. [Google Scholar] [CrossRef]
- Deepak, V.; Kalishwaralal, K.; Ramkumarpandian, S.; Babu, S.V.; Senthilkumar, S.R.; Sangiliyandi, G. Optimization of media composition for nattokinase production by Bacillus subtilis using response surface methodology. Bioresour. Technol. 2008, 99, 8170–8174. [Google Scholar] [CrossRef] [PubMed]
- Rasagnya, P.S.; Vangalapati, M. Studies on optimization of process parameters for nattokinase production by Bacillus subtilis NCIM 2724 and purification by liquid-liquid extraction. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 4516–4521. [Google Scholar]
- Garg, R.; Thorat, B.N. Nattokinase purification by three phase partitioning and impact of t-butanol on freeze drying. Sep. Purif. Technol. 2014, 131, 19–26. [Google Scholar] [CrossRef]
- Ito, K. Grain and legume allergy. Chem. Immunol. Allergy 2015, 101, 145–151. [Google Scholar] [PubMed]
- Nakamura, T.; Yamagata, Y.; Ichishima, E. Nucleotide sequence of the subtilisin NAT gene, aprN, of Bacillus subtilis (natto). Biosci. Biotechnol. Biochem. 1992, 56, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, X.; Zhang, Y. Microbial fibrinolytic enzymes: An overview of source, production, properties, and thrombolytic activity in vivo. Appl. Microbiol. Biotechnol. 2005, 69, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-L.; Zuo, Z.-Y.; Liu, Z.-G.; Tsai, K.-C.; Liu, A.-F.; Zou, G.-L. Construction of a 3D model of nattokinase, a novel fibrinolytic enzyme from Bacillus natto: A novel nucleophilic catalytic mechanism for nattokinase. J. Mol. Graph. Model. 2005, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-L.; Ye, M.-Q.; Zuo, Z.-Y.; Liu, Z.-G.; Tai, K.-C.; Zou, G.-L. Probing the importance of hydrogen bonds in the active site of the subtilisin nattokinase by site-directed mutagenesis and molecular dynamics simulation. Biochem. J. 2006, 395, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Feng, C.; Zhong, J.; Huan, L. Roles of s3 site residues of nattokinase on its activity and substrate specificity. J. Biochem. 2007, 142, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.M.; Nayak, S.; Lele, S.S. Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: Media optimization, purification and characterization. J. Biosci. Bioeng. 2012, 113, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.D.; Vaithilingam, M.; Shanker, R.; Kumar, S.; Thiyur, S.; Babu, V.; Selvakumar, J.N.; Prakash, S. Exploring the in vitro thrombolytic activity of nattokinase from a New Strain Pseudomonas aeruginosa CMSS. Jundishapur J. Microbiol. 2015, 8, e23567. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Guo, P.-C.; Jiang, W.-L.; Fan, X.-M.; Luo, X.-Y.; Li, H.-H. Expression of nattokinase in Escherichia coli and renaturation of its inclusion body. J. Biotechnol. 2016, 231, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Jia, S.; Sun, Y.; Chen, M.; Chen, X.; Zhong, J.; Huan, L. Secretory expression of nattokinase from Bacillus subtilis YF38 in Escherichia coli. Mol. Biotechnol. 2007, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-J.; Chen, H.-C.; Chao, Y.-P.; Tzen, J.-T. Efficient system of artificial oil bodies for functional expression and purification of recombinant nattokinase in Escherichia coli. J. Agric. Food Chem. 2005, 53, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-T.; Chiang, C.-J.; Chao, Y.-P. Strategy to approach stable production of recombinant nattokinase in Bacillus subtilis. Biotechnol. Prog. 2007, 23, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Kim, K.-M.; Kim, M.-K.; Lee, I.-Y.; Kim, B.-S. Production of nattokinase by high cell density fed-batch culture of Bacillus subtilis. Bioprocess Biosyst. Eng. 2011, 34, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.T.; Chiang, C.J.; Chao, Y.P. Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol. Prog. 2007, 23, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Zhong, J.; Huan, L. Secretory expression of a heterologous nattokinase in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2007, 75, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Feng, C.; Zhong, J.; Huan, L.-D. Enhanced production of recombinant nattokinase in Bacillus subtilis by promoter optimization. World J. Microbiol. Biotechnol. 2011, 27, 99–106. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quyen, T.D.; Le, H.T. Cloning and enhancing production of a detergent- and organic-solvent-resistant nattokinase from Bacillus subtilis VTCC-DVN-12-01 by using an eight-protease-gene-deficient Bacillus subtilis WB800. Microb. Cell Fact. 2013, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Xiong, S.; Zhang, J.; Cai, L.; Yang, Y. Expression and purification of recombinant nattokinase in Spodoptera frugiperda cells. Biotechnol. Lett. 2007, 29, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, Z.; Pan, L.; Yang, R.; Liang, S. Expression of nattokinase gene in yeast Pichia pastoris. J. South China Univ. Technol. 2003, 31, 1–4. [Google Scholar]
- Shahid, N.; Daniell, H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol. J. 2016, 14, 2079–2099. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and Challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef] [PubMed]
- Hiwasa-Tanase, K.; Ezura, H. Molecular Breeding to Create Optimized Crops: From Genetic Manipulation to Potential Applications in Plant Factories. Front Plant Sci. 2016, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472. [Google Scholar] [CrossRef]
- Arntzen, C. Plant-made pharmaceuticals: From “Edible Vaccines” to Ebola therapeutics. Plant Biotechnol. 2015, 13, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Loos, A.; Steinkellner, H. Plant glyco-biotechnology on the way to synthetic biology. Front Plant Sci. 2014, 5, 523. [Google Scholar] [CrossRef] [PubMed]
- Tschofen, M.; Knopp, D.; Hood, E.; Stöger, E. Plant molecular farming: Much more than medicines. Annu. Rev. Anal. Chem. 2016, 9, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Bosch, D.; Castilho, A.; Loos, A.; Schots, A.; Steinkellner, H. N-glycosylation of plant-produced recombinant proteins. Curr. Pharm. Des. 2013, 19, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Streatfield, S.J.; Rybicki, E.P. Advances in molecular farming: Key technologies, scaled up production and lead targets. Plant Biotechnol. J. 2015, 13, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Spök, A.; Karner, S. Plant Molecular Farming: Opportunities and Challenges; JRC Technical Report EUR 23383 EN; Office for Official Publications of the European Communities: Kopstal, Luxembourg, 2008. [Google Scholar]
- Drake, P.M.; Szeto, T.H.; Paul, M.J.; Teh, A.Y.; Ma, J.K. Recombinant biologic products versus nutraceuticals from plants—A regulatory choice? Br. J. Clin. Pharmacol. 2017, 83, 82–87. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, D.; Rybicki, E.P.; Fujiyama, K.; Franconi, R.; Benvenuto, E. Editorial: Plant Molecular Farming: Fast, scalable, cheap, sustainable. Front. Plant Sci. 2016, 7, 1148. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. When plant virology met Agrobacterium: The rise of the deconstructed clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Catrice, E.V.; Sainsbury, F. Assembly and purification of polyomavirus-like particles from plants. Mol. Biotechnol. 2015, 57, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Phoolcharoen, W.; Lai, H.; Piensook, K.; Cardineau, G.; Zeitlin, L.; Whaley, K.J.; Arntzen, C.J.; Mason, H.S.; Chen, Q. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol. Bioeng. 2010, 106, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Rosenthal, S.H.; Mason, H.S. 5′ and 3′ Untranslated regions strongly enhance performance of geminiviral replicons in Nicotiana benthamiana leaves. Front. Plant Sci. 2016, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tull, L. Expression of blood clot-dissolving proteins in transgenic plant. FASEB J. 2014, 28 (Suppl. LB852), 1. [Google Scholar]
- Duprez, L.; Wirawan, E.; Vanden Berghe, T.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ning, T.; Xie, T.; Qiu, Q.; Zhang, L.; Sun, Y.; Jiang, D.; Fu, K.; Yin, F.; Zhang, W.; et al. Large-scale production of functional human serum albumin from transgenic rice seeds. Proc. Natl. Acad. Sci. USA 2011, 108, 19078–19083. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, S.P.; Requesens, D.V.; Chang, Y.K.; Hood, K.R.; Flory, A.; Howard, J.A.; Hood, E.E. Heterologous expression of cellobiohydrolase II (Cel6A) in maize endosperm. Transgenic Res. 2013, 22, 477–488. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, G.; Scheffer, S.; Jacobs, A.; Zambre, M.; Zobell, O.; Goossens, A.; Depicker, A.; Angenon, G. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat. Biotechnol. 2002, 20, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Leps, M.; Conrad, U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant Biotechnol. J. 2006, 4, 243–249. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, N.B.; Murad, A.M.; Vianna, G.R.; Coelho, C.; Rech, E.L. Expression and characterization of recombinant molecules in transgenic soybean. Curr. Pharm. Des. 2013, 19, 5553–5563. [Google Scholar] [CrossRef] [PubMed]
- Bost, K.; Piller, K. Protein expression systems: Why soybean seeds? In Soybean—Molecular Aspects of Breeding; Sudaric, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 1–18. [Google Scholar]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Y.; Yao, J.; Sparks, S.; Wang, K.Y. Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 523. https://doi.org/10.3390/ijms18030523
Weng Y, Yao J, Sparks S, Wang KY. Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. International Journal of Molecular Sciences. 2017; 18(3):523. https://doi.org/10.3390/ijms18030523
Chicago/Turabian StyleWeng, Yunqi, Jian Yao, Sawyer Sparks, and Kevin Yueju Wang. 2017. "Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease" International Journal of Molecular Sciences 18, no. 3: 523. https://doi.org/10.3390/ijms18030523
APA StyleWeng, Y., Yao, J., Sparks, S., & Wang, K. Y. (2017). Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. International Journal of Molecular Sciences, 18(3), 523. https://doi.org/10.3390/ijms18030523