A Novel Brucine Gel Transdermal Delivery System Designed for Anti-Inflammatory and Analgesic Activities
Abstract
:1. Introduction
2. Results and Discussion
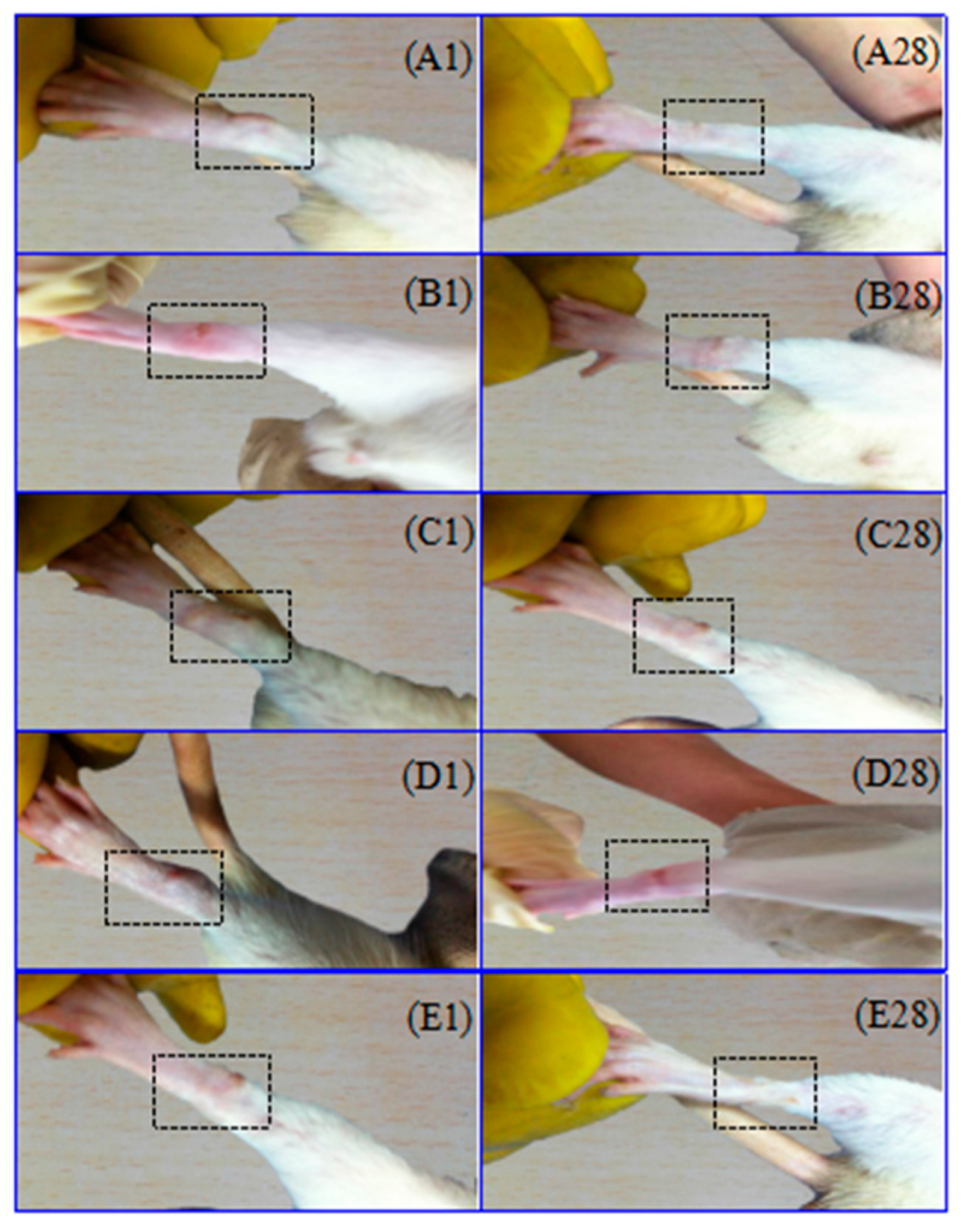
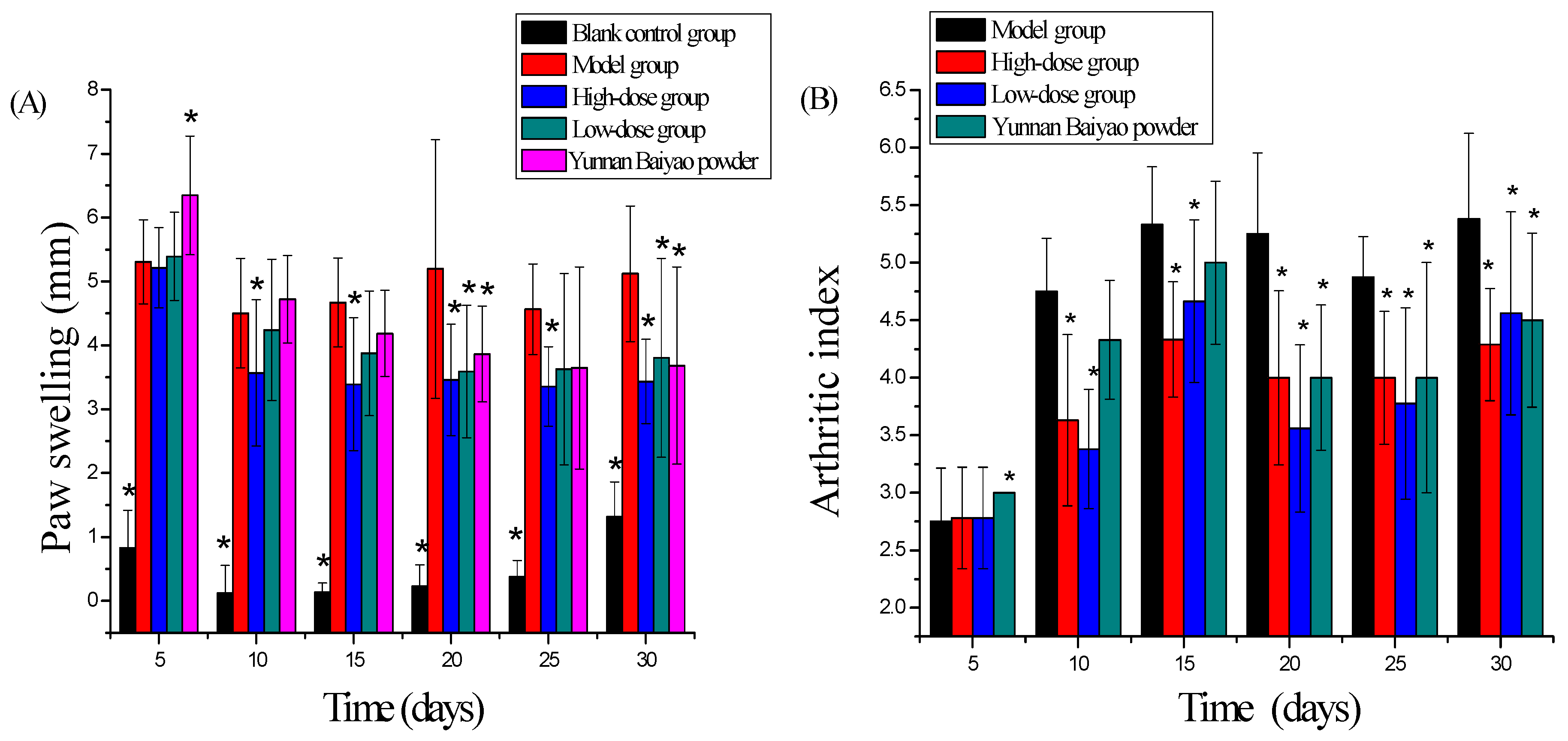
2.1. Adjuvant-Induced Arthritis
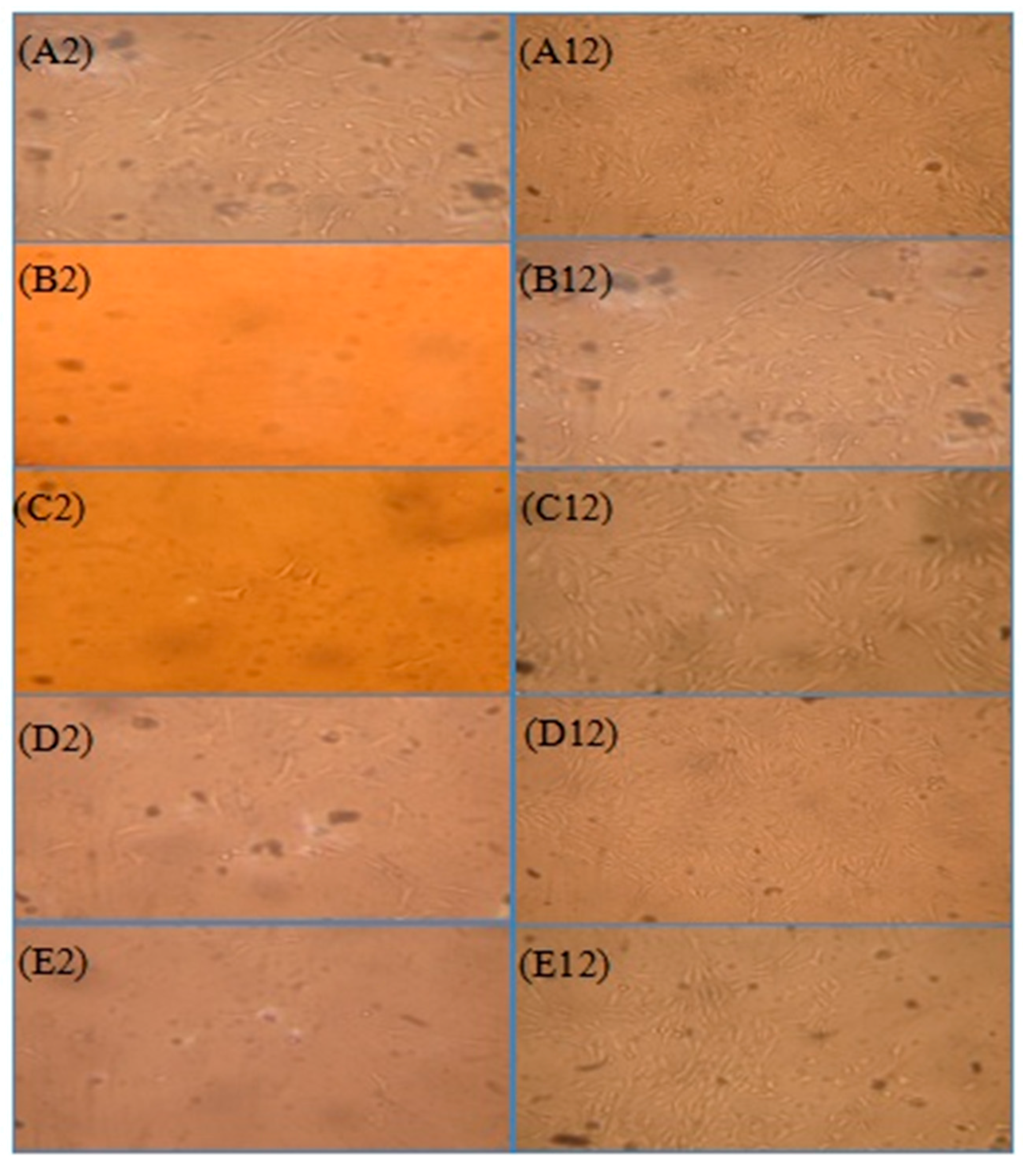
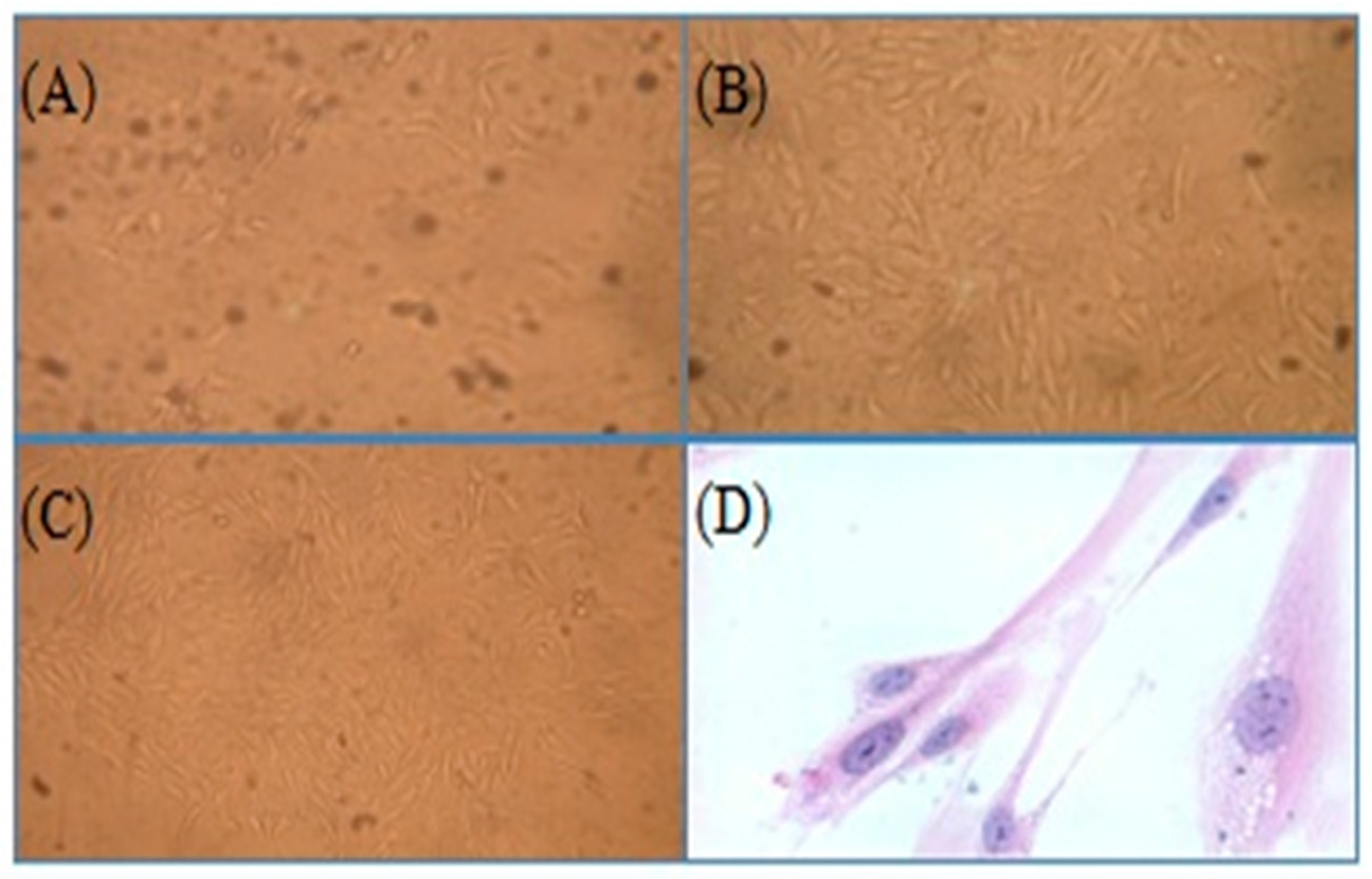
2.2. Synoviocytes
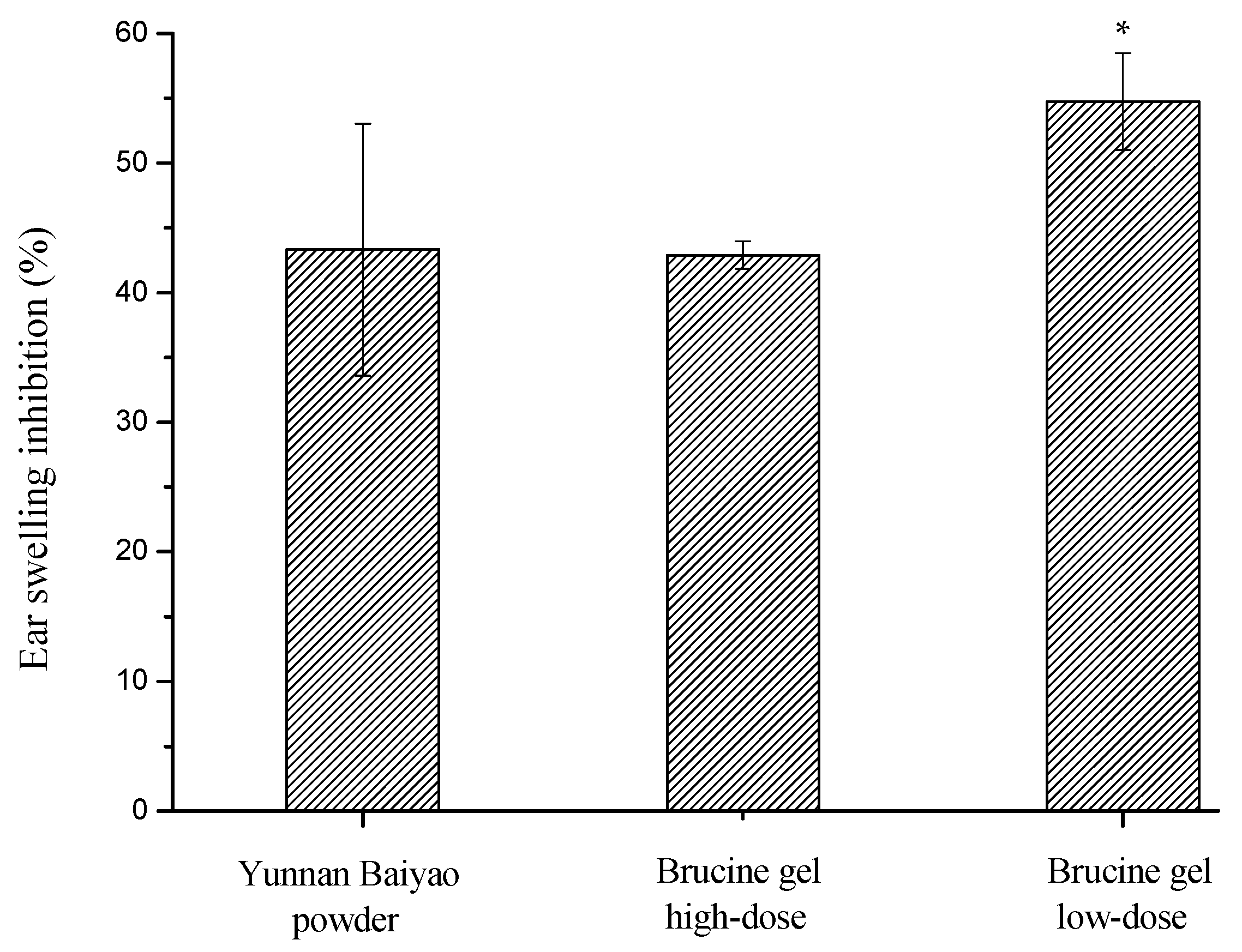
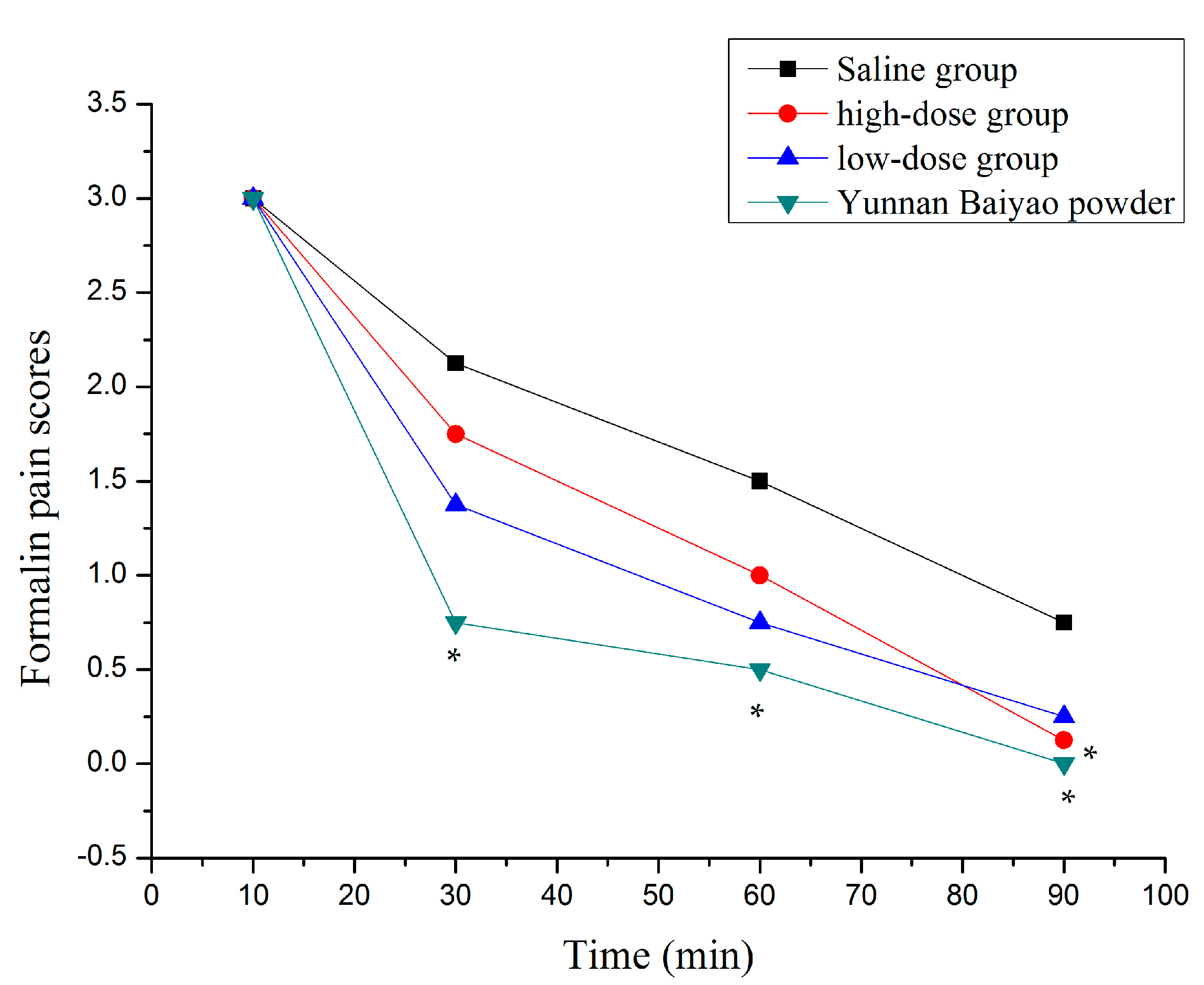
2.3. Inflammation and Soreness
2.4. Cytotoxicity Assay
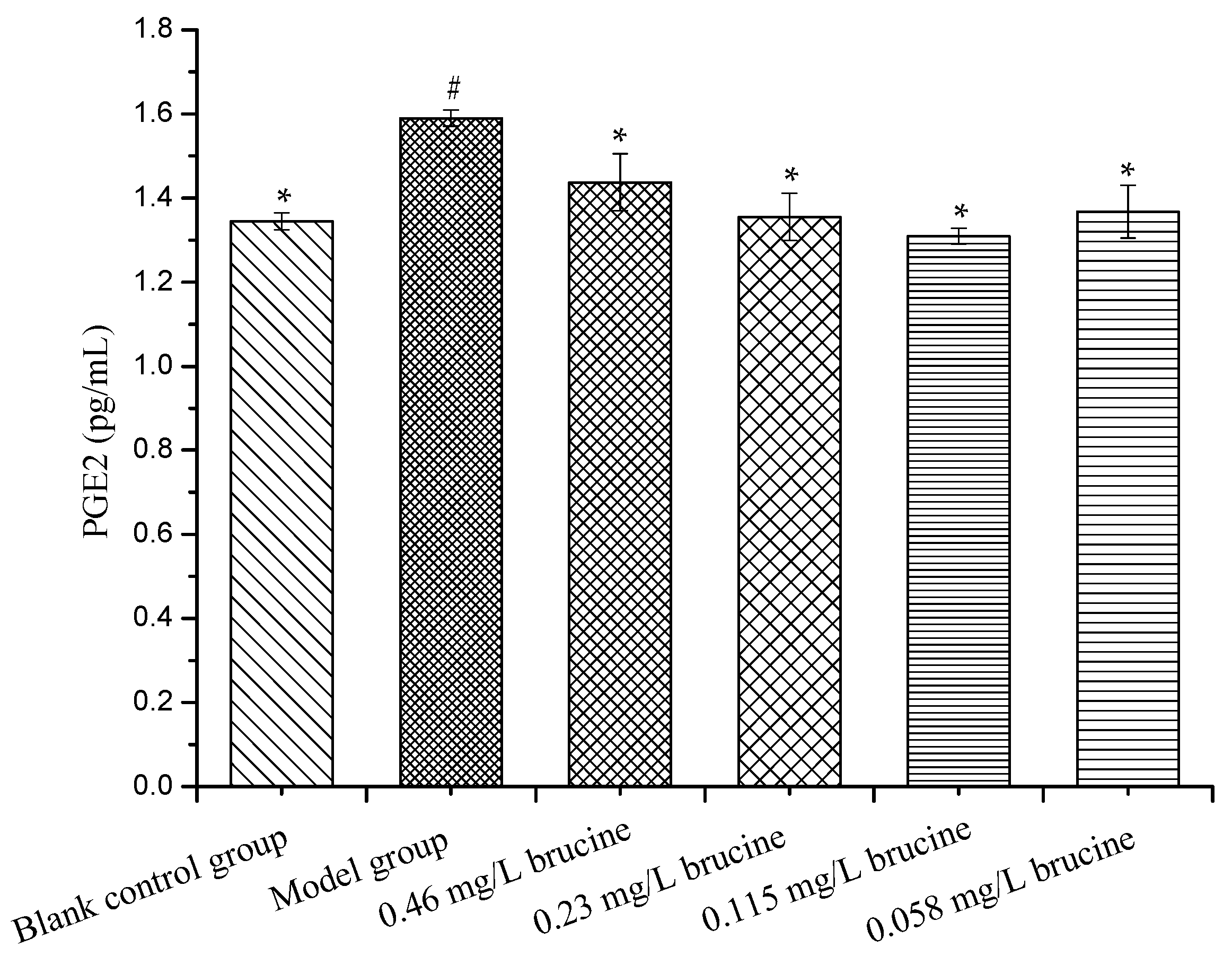
2.5. PGE2 Production Assay
3. Experimental Section
3.1. Drugs and Animals
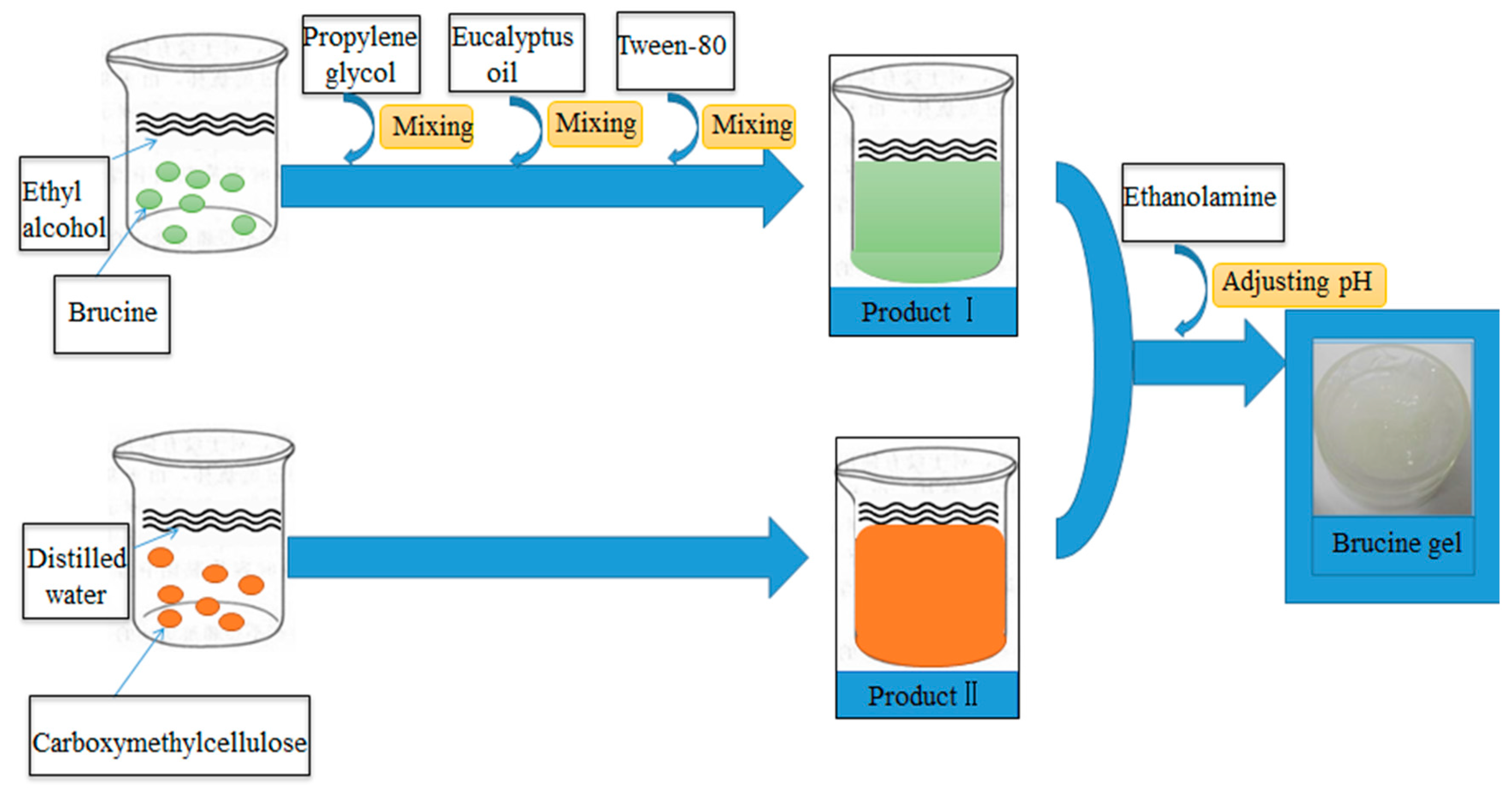
3.2. Brucine Gel Transdermal Delivery System
3.3. Rat Adjuvant Arthritis Model
3.4. Isolation and Culture of Synoviocytes
3.5. Mouse Ear Swelling Test
3.6. Formalin Test
3.7. Cytotoxicity Assay
3.8. PGE2 Production Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaurasia, S. Anti-inflammatory and antioxidant activity of Strychnos nux vomica Linn. Am. Eurasian J. Sustain. Agric. 2009, 3, 244–252. [Google Scholar]
- Li, Y.; Feng, H.; Li, Q.; Li, T. Decreasing toxicity and increasing efficacy on the compatibility of nux vomica powder: A experiment study. J. Tradit. Chin. Med. 2008, 10, 063. [Google Scholar]
- Mitra, S.; Kumar, V.; Ashok, B.K.; Acharya, R.N.; Ravishankar, B. A comparative anti-inflammatory activity of raw and processed Kupeelu (Strychnos nux-vomica Linn.) seeds on albino rats. Anc. Sci. Life 2011, 31, 73. [Google Scholar] [PubMed]
- Kushwaha, R.K.; Berval, R.; Sharma, A. The therapeutic and toxicological effect of kupilu (Strychnos nux-vomica L.)—A Review. Ayushdhara 2014, 1, 1–4. [Google Scholar]
- Cohen, M.R. Herbal and complementary and alternative medicine therapies for liver disease: A focus on Chinese traditional medicine in hepatitis C virus. Clin. Liver Dis. 2001, 5, 461–478. [Google Scholar] [CrossRef]
- Cai, B.; Nagasawa, T.; Kadota, S.; Hattori, M.; Namba, T.; Kuraishi, Y. Processing of nuxvomica. VII. Antinociceptive effects of crude alkaloids from the processed and unprocessed seeds of Strychnosnux-vomica in mice. Biol. Pharm. Bull. 1996, 19, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Qi, S.; Gao, W.; Chen, X.; Hu, Z. Separation and determination of strychnine and brucine in Strychnos nux-vomica L. and its preparation by nonaqueous capillary electrophoresis. J. Pharm. Biomed. 2006, 41, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, H.L.; Hu, R.R.; Hu, W.; Chen, Z.P.; Cai, H.; Liu, X.; Lu, T.; Fang, Y.; Cai, B.C. Pharmacokinetics of brucine after intravenous and oral administration to rats. Fitoterapia 2011, 82, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, A.; Yadav, A.K.; Singh, S.; Gautam, H.; Singh, H.N.; Sharma, A.; Yadav, C. Theoretical aspects of transdermal drug delivery system. Bull. Pharm. Res. 2013, 3, 78–89. [Google Scholar]
- Chen, J.; Wang, X.; Qu, Y.G.; Chen, Z.P.; Cai, H.; Liu, X.; Xu, F.; Lu, T.; Cai, B.C. Analgesic and anti-inflammatory activity and pharmacokinetics of alkaloids from seeds of Strychnosnux-vomica after transdermal administration: Effect of changes in alkaloid composition. Ethnopharmacology 2012, 139, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, Y.; Liu, C.S.; Wang, Q.Y.; Shen, X.; Yang, B.C. Preparation of liposomal brucine and its pharmaceutical/pharmacodynamic characterization. Acta Pharmacol. Sin. 2007, 28, 1851. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, O.A.; Abdel-Daim, M.M. Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnosnux-vomica. Cytotechnology 2015, 67, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chaphalkar, S.R. Cytotoxic and anti-inflammatory activity of aqueous extract of Strychnosnux-vomica. J. Biol. Nat. 2015, 4, 217–223. [Google Scholar]
- Kuruvilla, A.P.; Shah, R.; Hochwald, G.M.; Liggitt, H.D.; Palladino, M.A.; Thorbecke, G.J. Protective effect of transforming growth factor β1 on experimental autoimmune diseases in mice. Proc. Natl. Acad. Sci. USA 1991, 88, 2918–2921. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.J.; Abbott, F.V. The formalin test: A dose-response analysis at three developmental stages. Pain 1998, 76, 337–347. [Google Scholar] [CrossRef]
- Leelaprakash, G.; Dass, S.M. Invitro anti-inflammatory activity of methanol extract of enicostemmaaxillare. Int. J. Drug Dev. Res. 2011, 3, 189–196. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. Inflammatory response on the pancreatic acinar cell injury. Scand. J. Surg. 2005, 94, 97–102. [Google Scholar] [PubMed]
- Simon, J.; Anzil, A.P. Immunohistological evidence of perivascular localization of basic protein in early development of experimental allergic encephalomyelitis. Actaneuropathologica 1974, 27, 33–42. [Google Scholar] [CrossRef]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Khatri, N. Analgesic and anti-inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and carrageenan induced paw edema in mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, D. Pain mechanisms in patients with chronic pain. Clin. Drug Investig. 2012, 32, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Chew, E.Y.; Ferris, F.L.; Klein, M.L. Prevalence of anti-retinal autoantibodies in different stages of age-related macular depassage. BMC Ophthalmol. 2014, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Suke, S.G.; Negi, H.; Mediratta, P.K.; Banerjee, B.D.; Sharma, K.K. Anti-arthritic and anti-inflammatory activity of combined pioglitazone and prednisolone on adjuvant-induced arthritis. Eur. J. Pharmacol. 2013, 718, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S. Comparative Evaluation of Nyctanthes arbor tristis and Alstonia scholaris Leaves Extracts in Freunds Complete Adjuvant Induced Arthritis in Rats. Int. J. Pharm. Biol. Arch. 2013, 4, 903–908. [Google Scholar]
- Cai, X.; Zhou, H.; Wong, Y.F.; Xie, Y.; Liu, Z.Q.; Jiang, Z.H.; Bian, Z.X.; Xu, H.X.; Liu, L. Suppression of the onset and progression of collagen-induced arthritis in rats by QFGJS, a preparation from an anti-arthritic Chinese herbal formula. J. Ethnopharmacol. 2007, 110, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, Y.; Gao, D.; Zhang, H. Antibacterial and anti-inflammatory activities of extract and fractions from Pyrrosiapetiolosa (Christ et Bar.) Ching. J. Ethnopharmacol. 2014, 155, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: Characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Takada, Y.; Ihara, H.; Urano, T.; Takada, A. Changes in blood and plasma serotonergic measurements in rats-effect of nicotine and/or exposure to different stresses. Thromb. Res. 1995, 80, 307–316. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, S.F.; Sun, N.J. Direct determination of brucine by square wave voltammetry on 4-amino-2-mercaptopyrimidine self-assembled monolayer gold electrode. Bioelectrochemistry 2004, 65, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, T.S.; Yin, F.Z.; Cai, B.C. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of strychnosnux-vomica. J. Ethnopharmacol. 2003, 88, 205–214. [Google Scholar] [CrossRef]
- Gu, L.; Wang, X.; Liu, Z.; Ju, P.; Zhang, L.; Zhang, Y.; Ma, B.; Bi, K.; Chen, X. A study of Semen Strychni-induced renal injury and herb-herb interaction of Radix Glycyrrhizae extract and/or Rhizoma Ligustici extract on the comparative toxicokinetics of strychnine and brucine in rats. Food Chem. Toxicol. 2014, 68, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, W.; Zhang, L.; Xu, H.M. Effects and mechanisms of total glucosides of paeony on synoviocytes activities in rat collagen-induced arthritis. J. Ethnopharmacol. 2009, 121, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, S.; Crowyn, G. Structure determination of fexofenadine-α-cyclodextrin complex by quantitative 2D ROESY analysis and molecular mechanics studies. Magn. Reson. Chem. 2012, 50, 299–304. [Google Scholar] [CrossRef] [PubMed]


| Groups | BW (g) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Blank control group | 200.52 ± 12.461 | 221.06 ± 13.936 * | 248.48 ± 20.071 * | 262.04 ± 23.671 | 283.14 ± 21.453 |
| Model group | 196.53 ± 19.643 | 193.12 ± 20.343 # | 212.85 ± 22.778 # | 224.27 ± 19.413 # | 246.92 ± 12.95 # |
| High-dose group | 195.15 ± 13.943 | 211.52 ± 15.736 *# | 238.71 ± 27.822 * | 241.44 ± 30.686 | 250.90 ± 35.31 # |
| Low-dose group | 202.11 ± 20.067 | 208.52 ± 14.293 * | 227.48 ± 19.924 | 242.25 ± 22.407 | 255.21 ± 25.150 |
| Yunnan Baiyao powder group | 198.67 ± 16.918 | 210.08 ± 20.560 * | 228.98 ± 24.936 | 233.12 ± 26.119 | 234.08 ± 32.79 # |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Liang, Q.; Feng, P.; Li, C.; Yang, C.; Liang, H.; Tang, H.; Shuai, C. A Novel Brucine Gel Transdermal Delivery System Designed for Anti-Inflammatory and Analgesic Activities. Int. J. Mol. Sci. 2017, 18, 757. https://doi.org/10.3390/ijms18040757
Wu P, Liang Q, Feng P, Li C, Yang C, Liang H, Tang H, Shuai C. A Novel Brucine Gel Transdermal Delivery System Designed for Anti-Inflammatory and Analgesic Activities. International Journal of Molecular Sciences. 2017; 18(4):757. https://doi.org/10.3390/ijms18040757
Chicago/Turabian StyleWu, Ping, Qin Liang, Pei Feng, Chunyan Li, Chunguang Yang, Hongsuo Liang, Huaibo Tang, and Cijun Shuai. 2017. "A Novel Brucine Gel Transdermal Delivery System Designed for Anti-Inflammatory and Analgesic Activities" International Journal of Molecular Sciences 18, no. 4: 757. https://doi.org/10.3390/ijms18040757






