Bioactivity of NANOZR Induced by Alkali Treatment
Abstract
:1. Introduction
2. Results
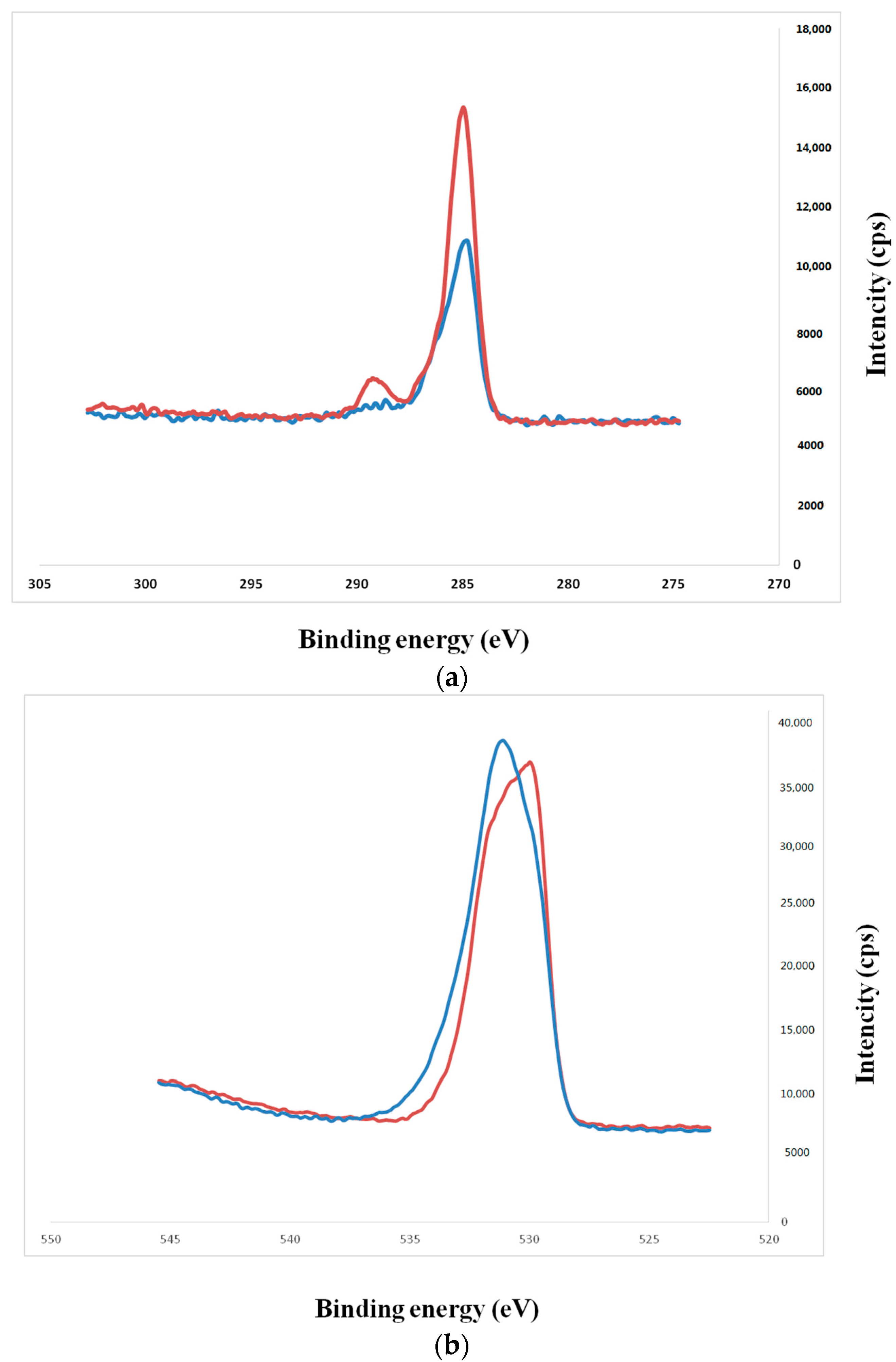
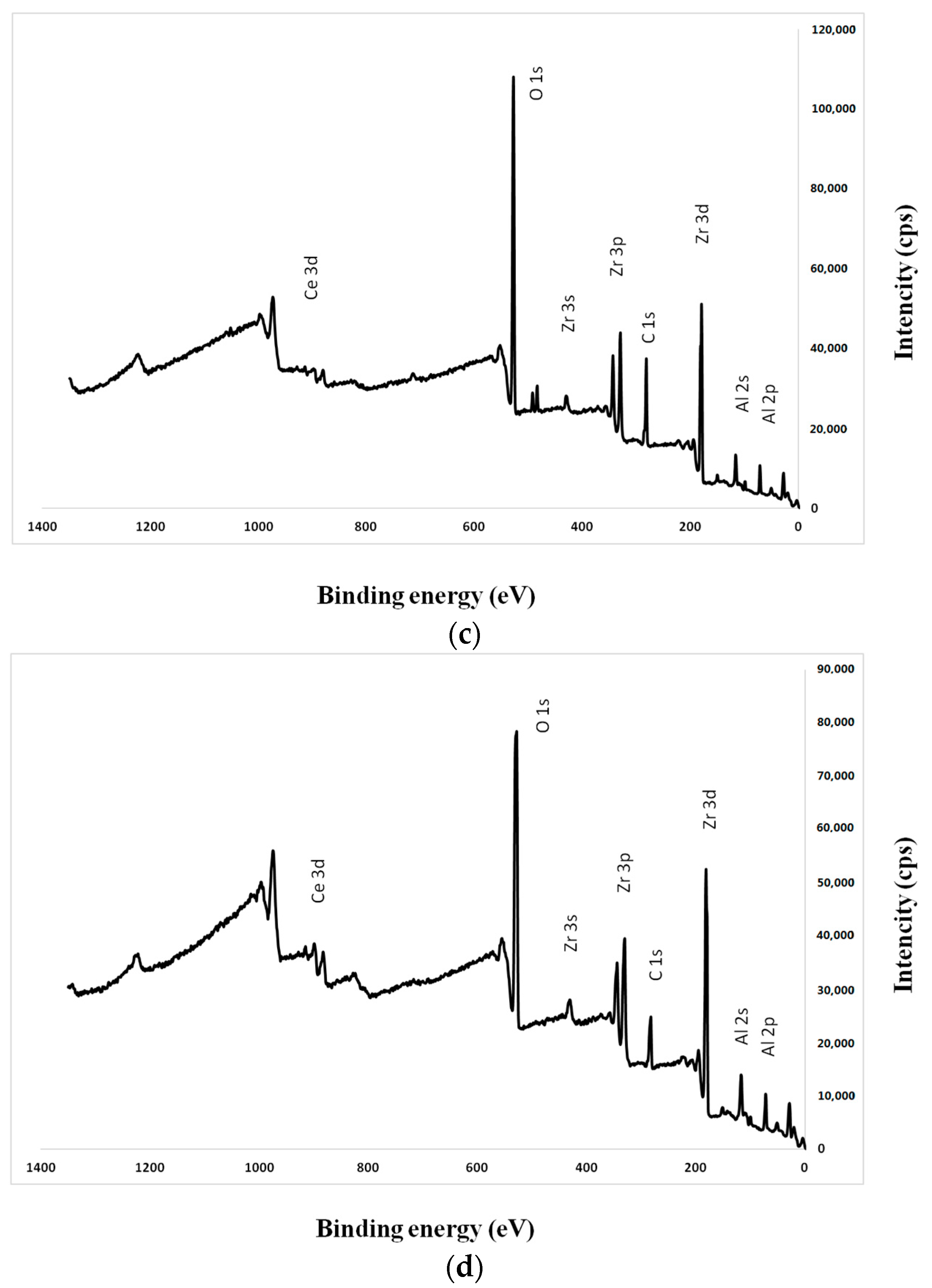
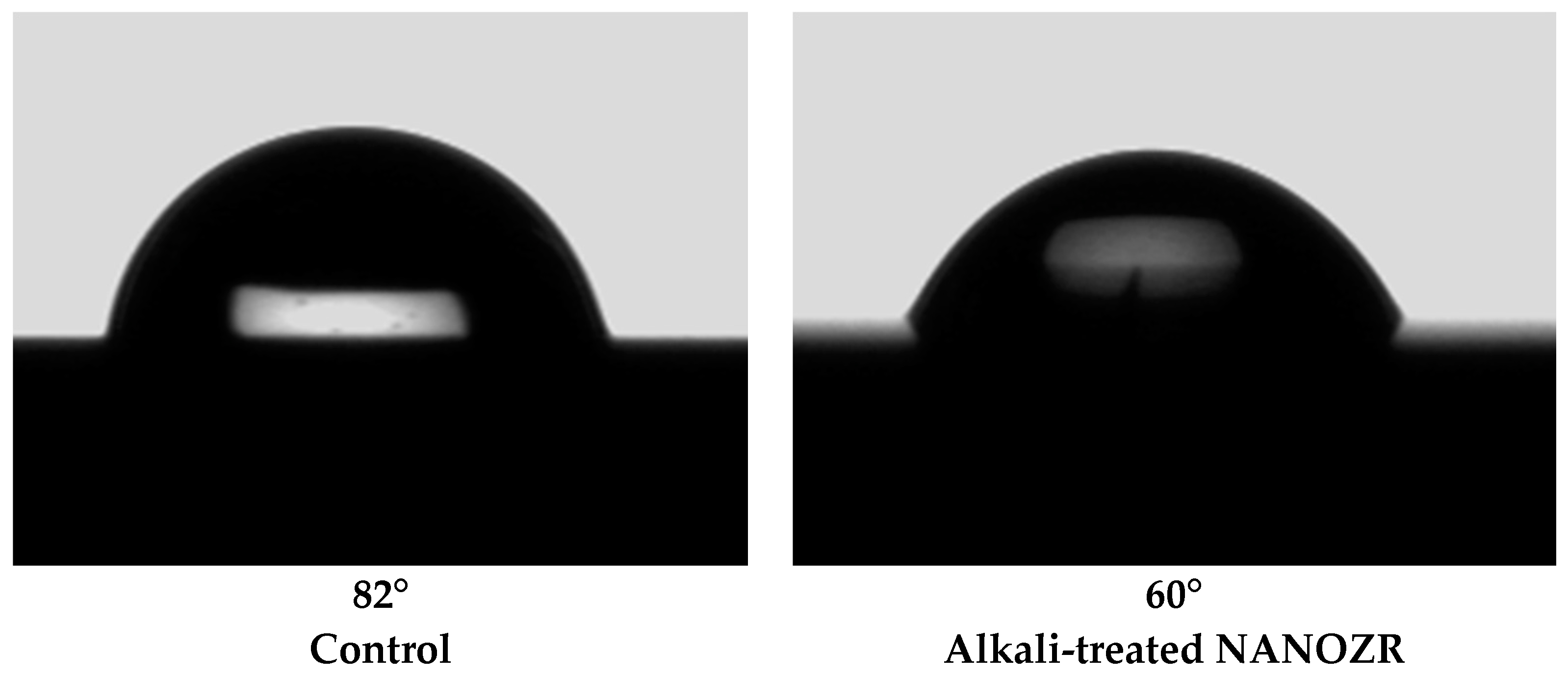
2.1. Sample Preparation
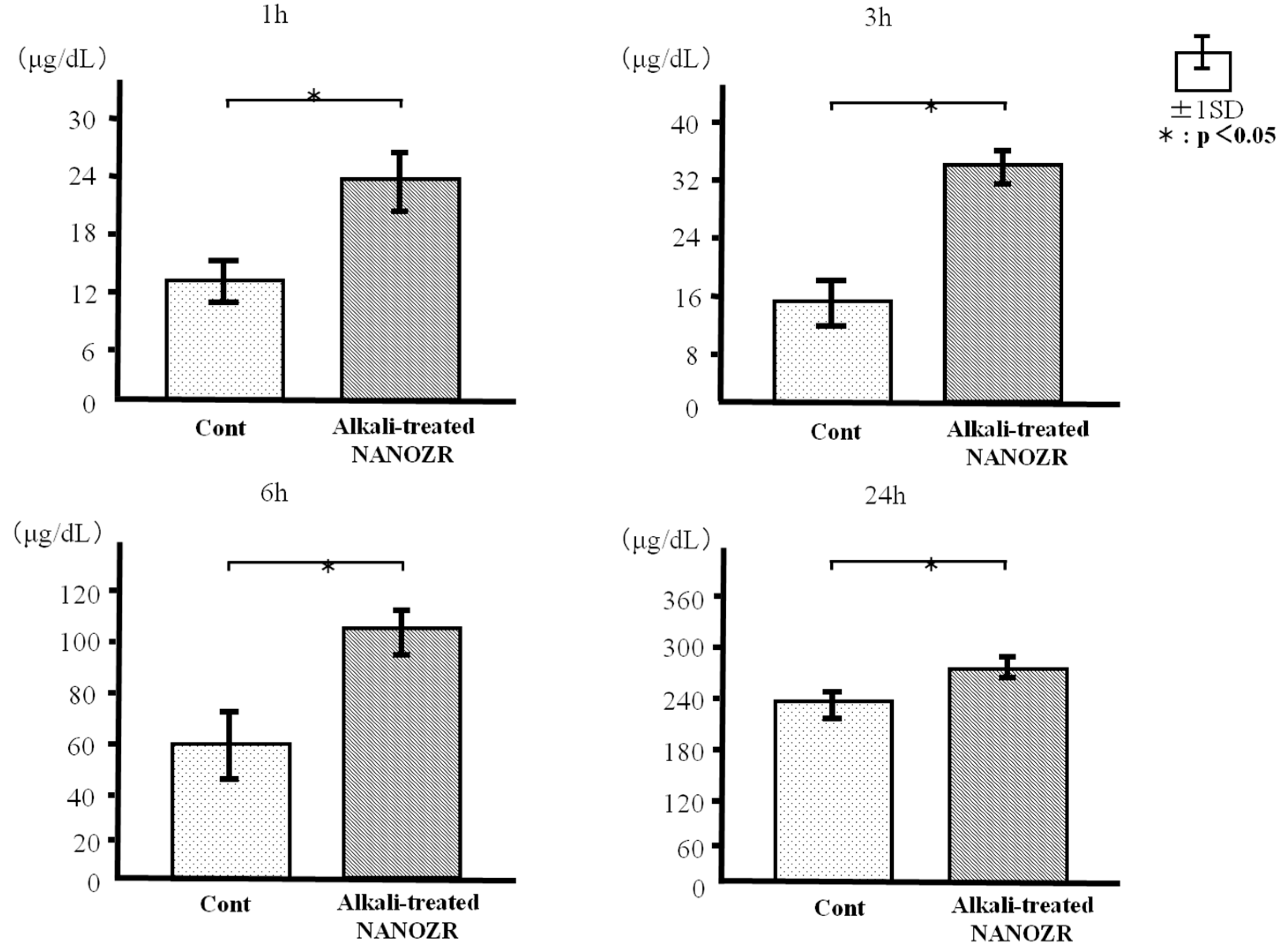
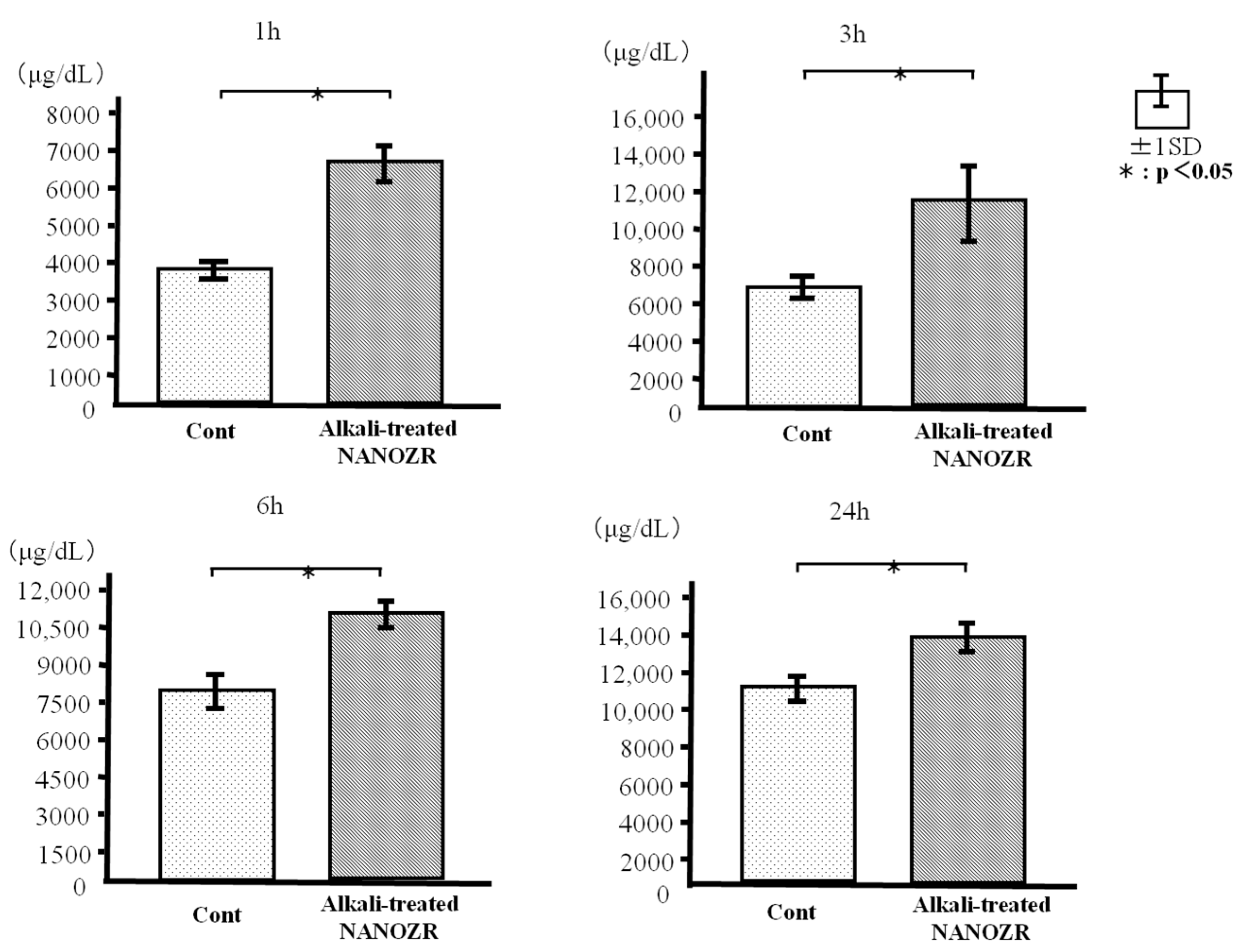
2.2. Protein Adsorption
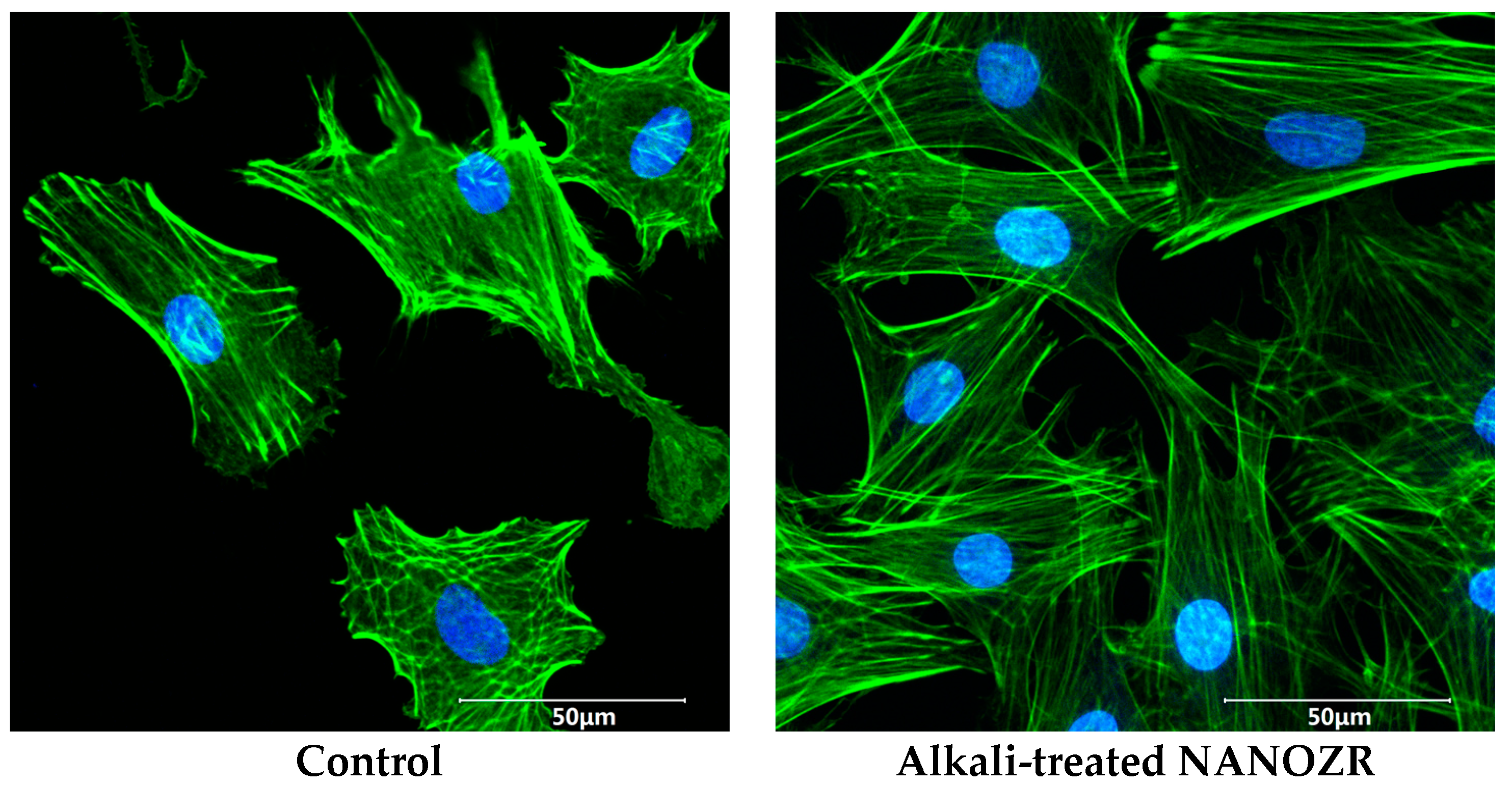
2.3. Cell Adhesion and Morphology
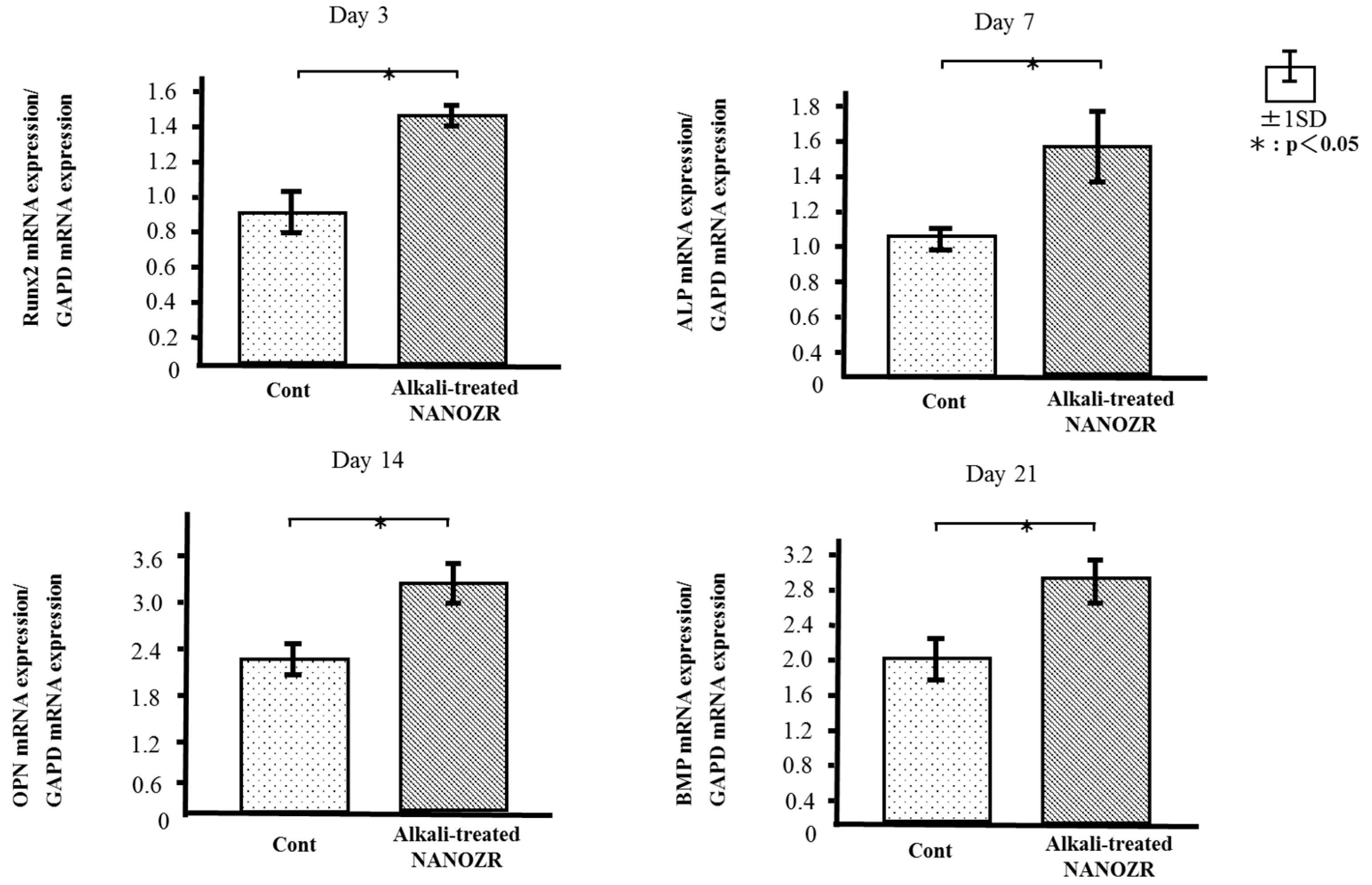
2.4. Quantitative Real-Time (qRT-)PCR Analysis of Osteogenesis-Related Gene Expression
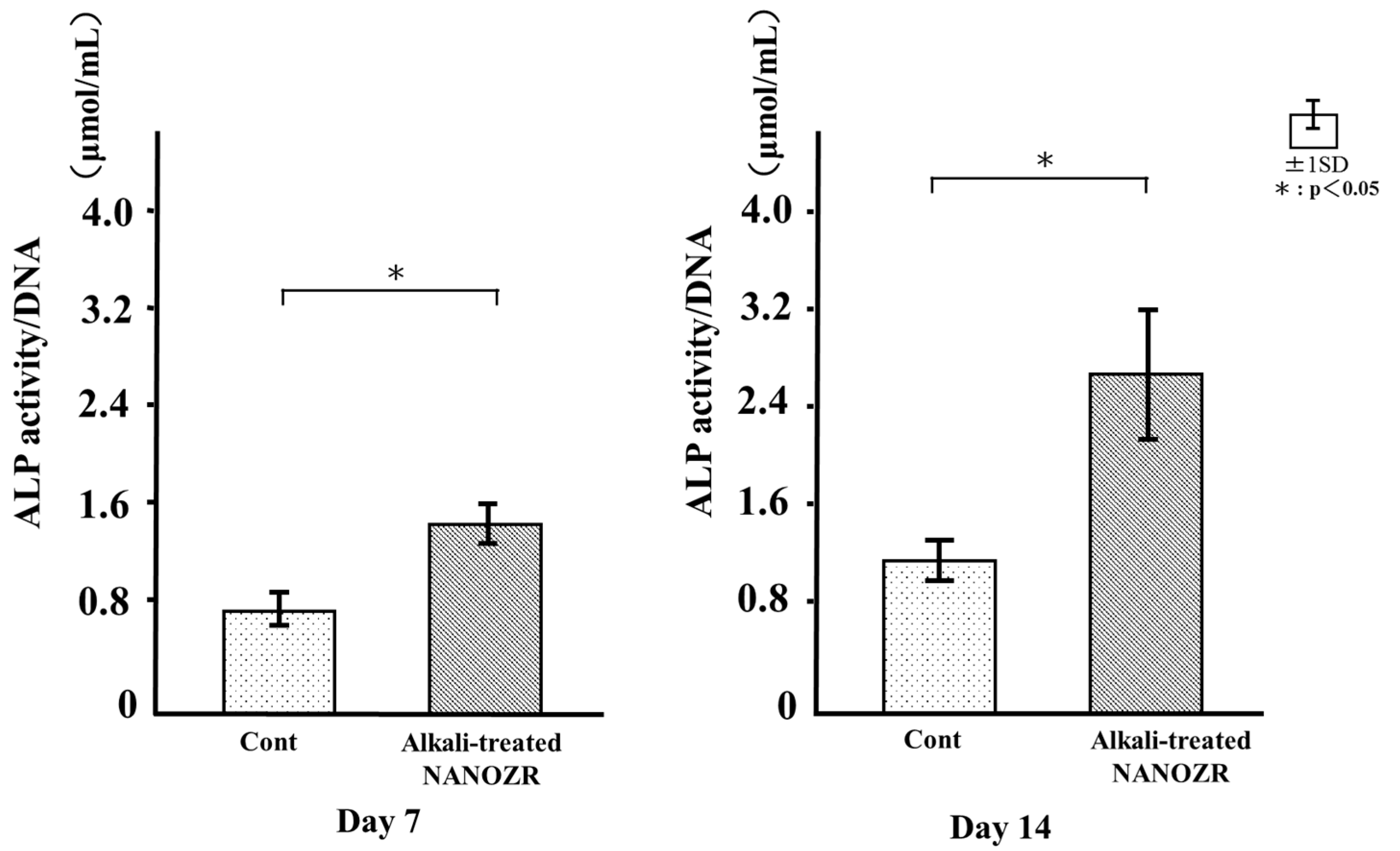
2.5. ALP Activity
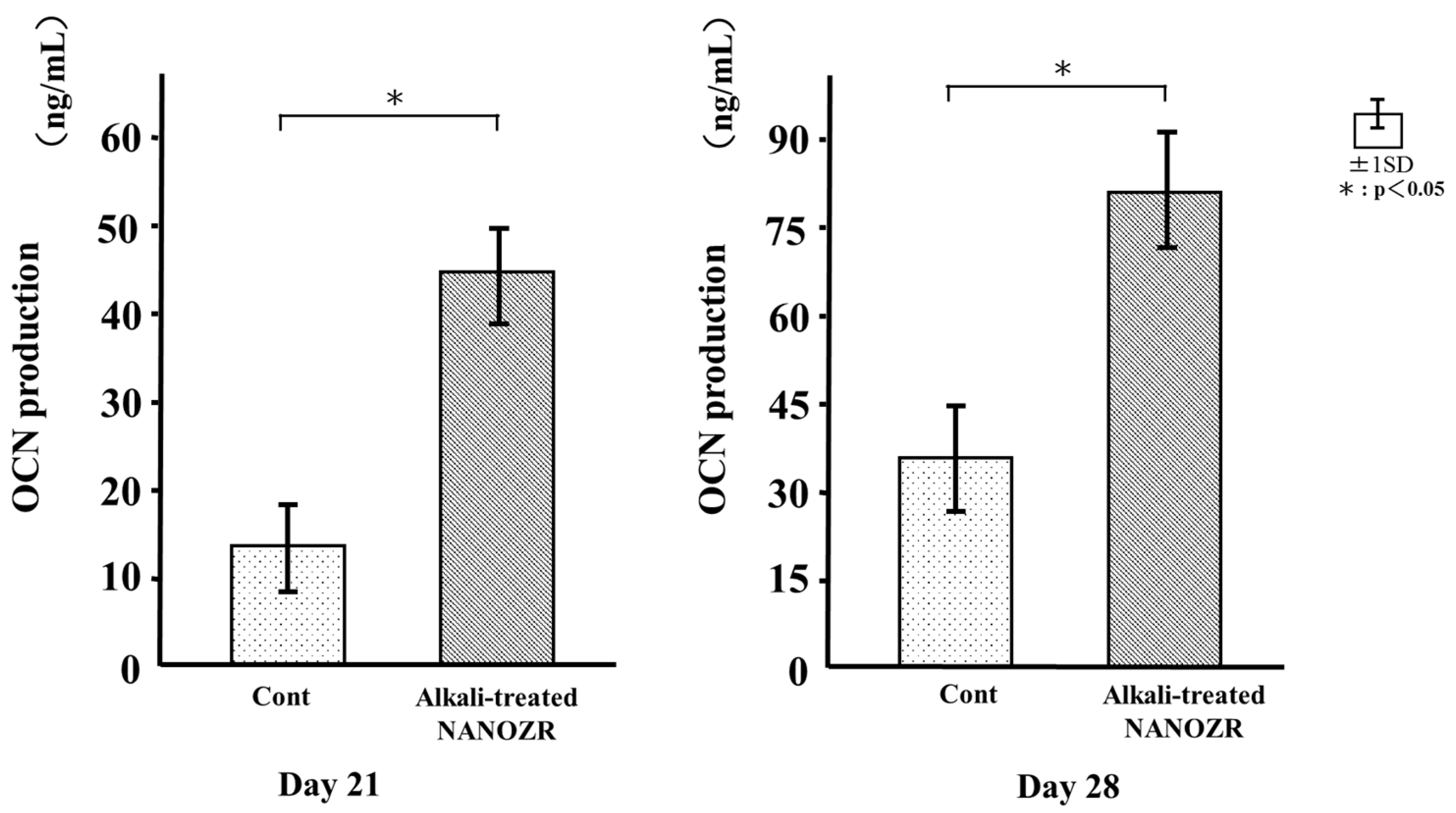
2.6. Osteocalcin (OCN) Production
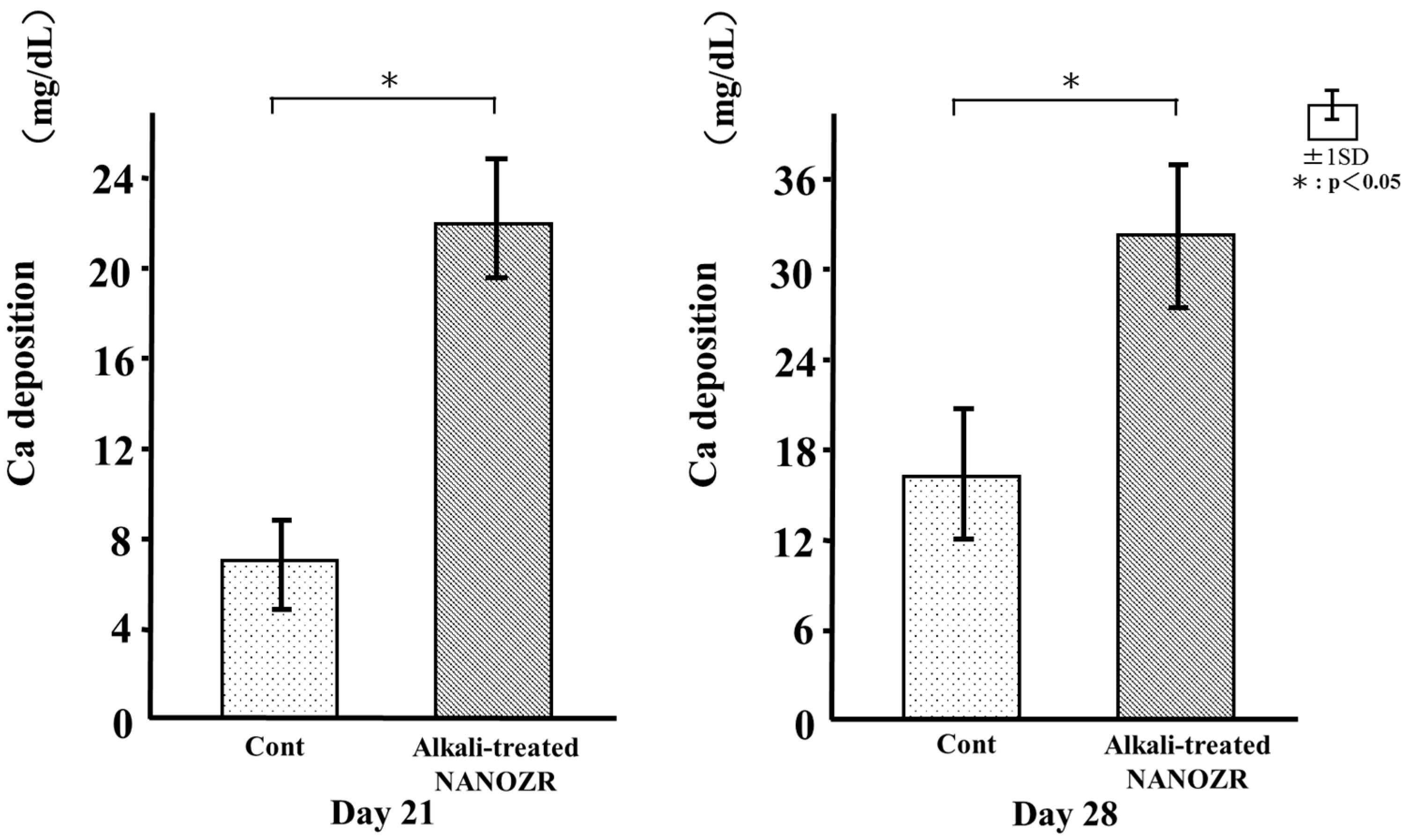
2.7. Mineralization
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Surface Characterization
4.3. Protein Adsorption
4.4. Cell Culture
4.5. Cell Adhesion
4.6. Cell Morphology
4.7. qRT-PCR Analysis
4.8. ALP Activity
4.9. OCN ELISA Analysis
4.10. Mineralization
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3Y-TZP | yttria-stabilized tetragonal zirconia polycrystals |
| Al2O3 | alumina polycrystals |
| ALP | alkaline phosphatase |
| BMP | bone morphogenetic protein |
| BSA | bovine serum albumin |
| Ce-TZP | ceria-stabilized tetragonal zirconia polycrystals |
| DAPI | 4′,6-diamidino-2-phenylindole |
| NANOZR | ceria-stabilized tetragonal ZrO2 polycrystalline ceramic-based nanostructured zirconia/alumina composite |
| OCN | osteocalcin |
| OPN | osteopontin |
| PBS | phosphate-buffered saline |
| RBM | rat bone marrow |
| Runx2 | runt-related transcription factor 2 |
| SEM | scanning electron microscopy |
| SPM | scanning probe microscopy |
| TiO2 | titania |
| TNS | titanium nanosheets |
| XPS | X-ray photoelectron spectroscopy |
References
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. A 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implants 2003, 18, 200–210. [Google Scholar] [PubMed]
- Guo, J.; Padilla, R.J.; Ambrose, W.; de Kok, I.J.; Cooper, L.F. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz Filho, H.O.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.; Mendonca, D.; Aragao, F.J.L.; Cooper, L.F. Advancing dental implant surface technology-From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Wadamoto, M.; Akagawa, Y.; Sato, Y.; Kubo, T. The three-dimensional bone interface of an osseointegrated implant. I: A morphometric evaluation in initial healing. J. Prosthet. Dent. 1996, 76, 170–175. [Google Scholar] [CrossRef]
- Meirelles, L.; Currie, F.; Jacobsson, M.; Albrektsson, T.; Wennerberg, A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int. J. Oral Maxillofac. Implants 2008, 23, 641–647. [Google Scholar] [PubMed]
- Vandrovcov, M.; Bakov, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar]
- Piconi, C.; Maccauro, G. Zircnoia as a ceramic biomaterials. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Yildirim, M.; Edelhoff, D.; Henisch, O.; Spiekermann, H. Ceramic abutments—A new era in achieving optimal esthetics in implant dentistry. Int. J. Periodontics Restor. Dent. 2000, 20, 81–91. [Google Scholar]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Sailer, I.; Hammele, C.H.; Attin, T.; Schmidlin, P. In vitro color changes of soft tissue caused by restorative materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251–257. [Google Scholar]
- Bianco, P.D.; Ducheyne, P.; Cuckler, J.M. Local accumulation of titanium released from a titanium implant in the absence of wear. J. Biomed. Mater. Res. 1996, 31, 227–234. [Google Scholar] [CrossRef]
- Weingart, D.; Steinemann, S.; Schilli, W.; Strub, J.R.; Hellerich, U.; Assenmacher, J.; Simpson, J. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int. J. Oral Maxillofac. Surg. 1994, 23, 450–452. [Google Scholar] [CrossRef]
- Scott, H.G. Phase relationships in the zirconia-yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Oka, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Ce-TZP/Al2O3 nanocomposite as a bearing material in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Nawa, M.; Nakamoto, S.; Sekino, T.; Niihara, K. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceram. Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. The effect of TiO2 addition on strengthening and toughening in intragranular type of 12Ce-TZP/Al2O3 nanocomposites. J. Eur. Ceram. Soc. 1998, 18, 209–219. [Google Scholar] [CrossRef]
- Guazzato, M.; Proos, K.; Quach, L.; Swain, M.V. Strength, reliability and mode of fracture of bilayered porcelain/zirconia (Y-TZP) dental ceramics. Biomaterials 2004, 25, 5045–5052. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.C.; Han, J.S.; Bächle, M.; Jang, J.H.; Shin, S.W.; Kim, D.J. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent. Mater. 2007, 23, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Kübler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4, 29. [Google Scholar]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. A 1992, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Periodontics Res. 2012, 56, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surfaces with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale features of a titanium surface: Effects of deposition time in NaOH solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Chian, K.S. Effects of CO2 laser irradiation on the surface properties of magnesia-partially stabilised zirconia (MgO-PSZ) bioceramic and the subsequent improvements in human osteoblast cell adhesion. J. Biomater. Appl. 2004, 19, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Bächle, M.; Butz, F.; Hübner, U.; Bakalinis, E.; Kohal, R.J. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin. Oral Implants Res. 2007, 18, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Yang, D.; Wu, X.; Wang, H.; Miao, X.; Li, W. In vitro and in vivo biocompatibility of graded hydroxyapatite–zirconia composite bioceramic. J. Mater. Sci. Mater. Med. 2008, 19, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, M.; Komasa, S.; Fujio, M.; Terada, C.; Kusumoto, T.; Nishizaki, H.; Tanaka, M.; Okazaki, J. Biocompatibility of surface modified implant materials. In Proceedings of the 30th Japan Association of Oral Rehabilitation, Kyoto, Japan, 20 November 2016. [Google Scholar]
- Sul, Y.-T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Baier, R.E.; Meyer, A.E.; Natiella, J.R.; Natiella, R.R.; Carter, J.M. Surface properties determine bioadhesive outcomes: Methods and results. J. Biomed. Mater. Res. 1984, 18, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Bächle, M.; Kohal, R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implants Res. 2004, 15, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Setzer, B.; Bächle, M.; Metzger, M.C.; Kohal, R.J. The gene-expression and phenotypic response of hFOB 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 2009, 30, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Endo, K.; Araki, Y.; Ohno, H.; Matsuda, K. ESCA analysis of tarnish films on dental alloys removed from the oral cavities (Part 1) Ag-In alloys. J. Dent. Mater. 1988, 7, 184–191. [Google Scholar]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR Induced by Alkali Treatment. Int. J. Mol. Sci. 2017, 18, 780. https://doi.org/10.3390/ijms18040780
Nishizaki M, Komasa S, Taguchi Y, Nishizaki H, Okazaki J. Bioactivity of NANOZR Induced by Alkali Treatment. International Journal of Molecular Sciences. 2017; 18(4):780. https://doi.org/10.3390/ijms18040780
Chicago/Turabian StyleNishizaki, Mariko, Satoshi Komasa, Yoichiro Taguchi, Hiroshi Nishizaki, and Joji Okazaki. 2017. "Bioactivity of NANOZR Induced by Alkali Treatment" International Journal of Molecular Sciences 18, no. 4: 780. https://doi.org/10.3390/ijms18040780





