A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine
Abstract
:1. Introduction
2. Results
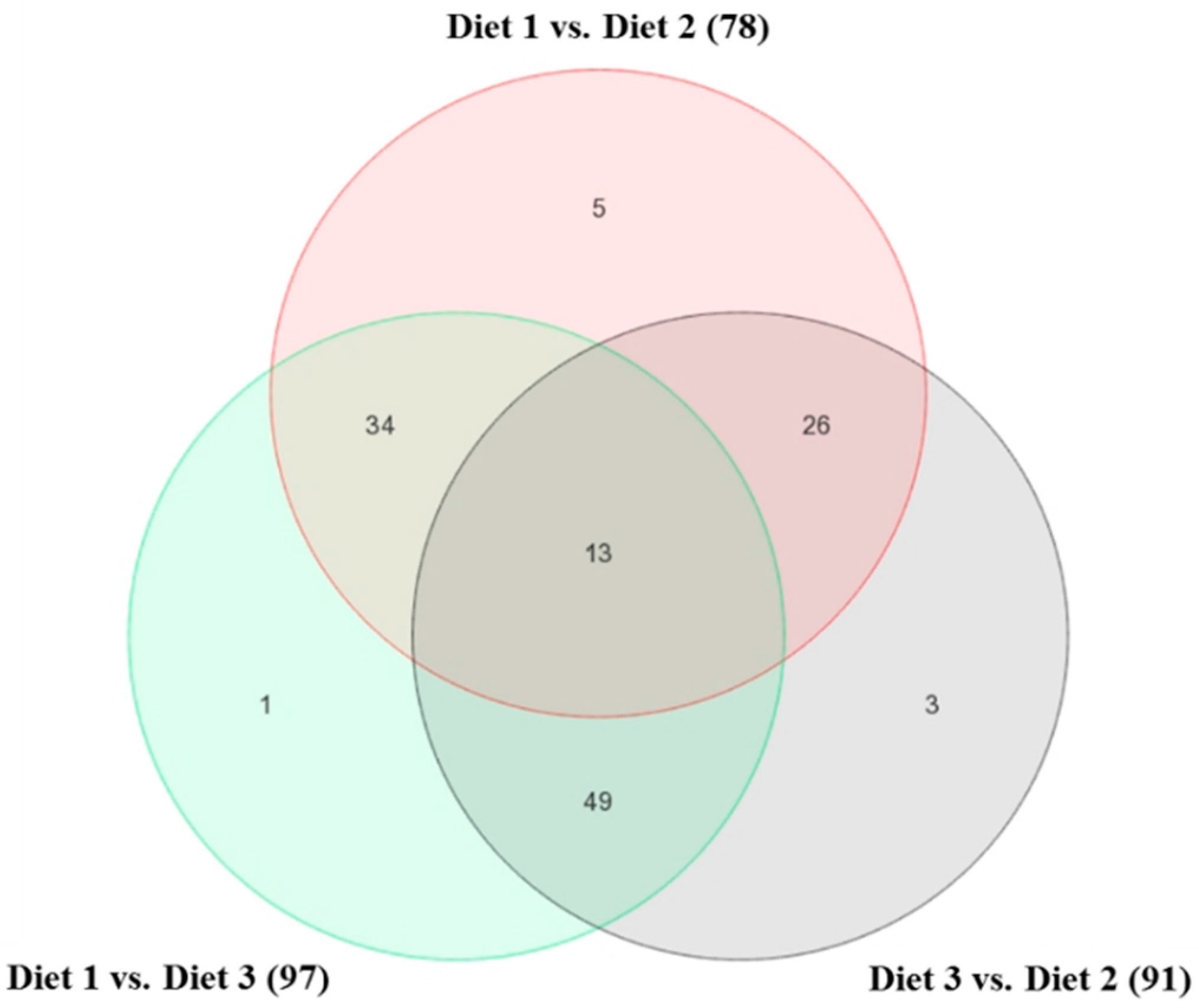
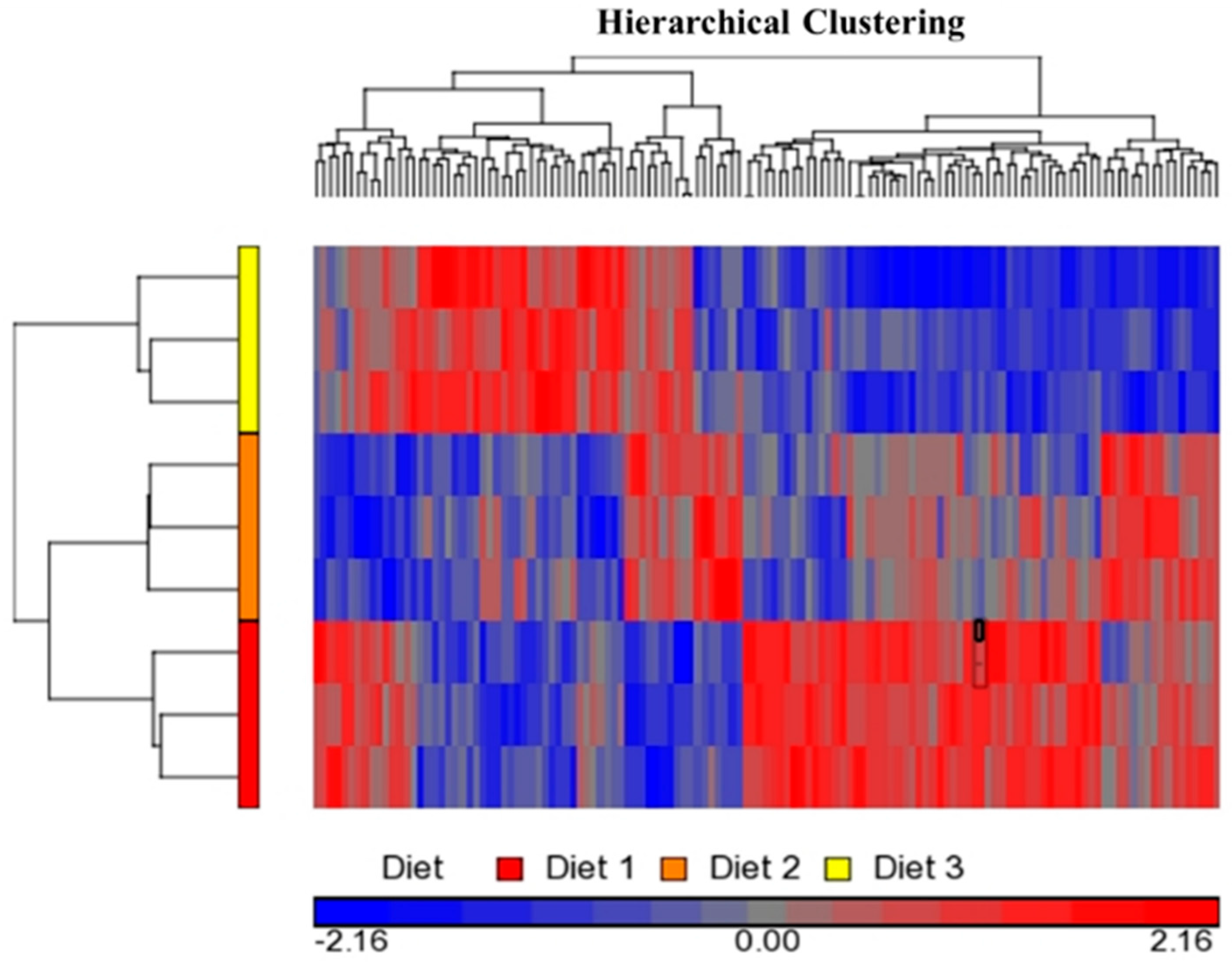
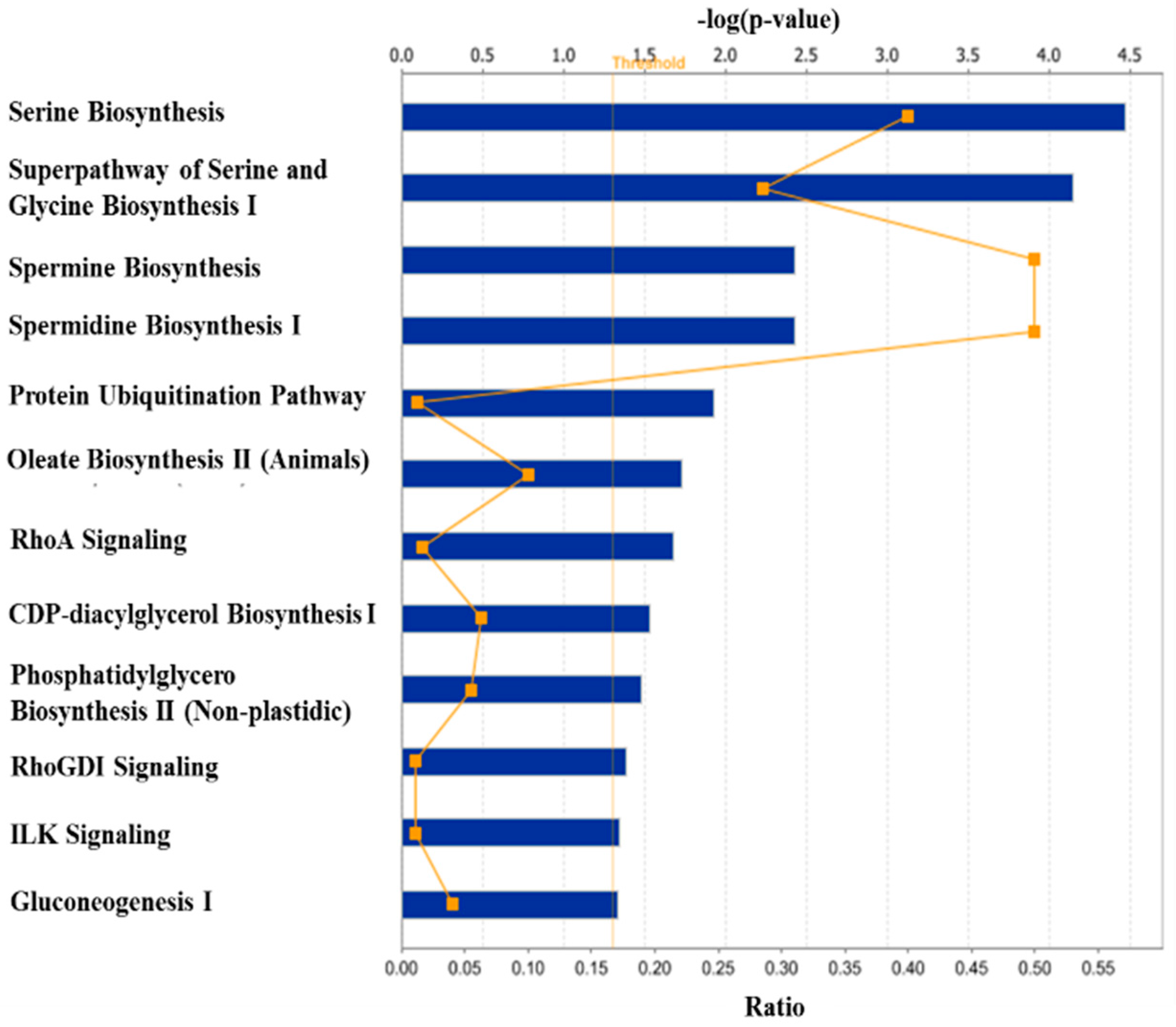
2.1. Bioinformatics Analyses of Microarray Data
2.2. Real-Time RT-PCR Analysis of Selected Genes
3. Discussion
3.1. Regulation of Protein Turnover
3.1.1. Protein Degradation
3.1.2. Protein Synthesis
3.2. Regulation of Lipid Metabolism
3.3. Other Biological Processes
4. Materials and Methods
4.1. Animal Trial and Sample Collection
4.2. Preparation of Total RNA
4.3. Microarray Analysis
4.4. Real-Time RT-PCR Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| ADG | Average daily gain |
| Akt | Protein Kinase B |
| ANOVA | Analysis of variance |
| ARS | Agricultural Research Service |
| CP | Crude protein |
| CT | Threshold cycle |
| DEG | Differentially expressed genes or gene transcripts |
| ER | Endoplasmic reticulum |
| GC | Guanine-cytosine |
| GEO | Gene Expression Omnibus |
| GCOS | GeneChip Operating Software |
| IPA | Ingenuity Pathways Analysis |
| MAPK | Mitogen-activated protein kinase |
| MIAMIE | Minimum information about a microarray experiment |
| NIFA | National Institute of Food and Agriculture |
| NRC | National Research Council |
| PGS | Partek Genomics Suite |
| PI3K | Phosphatidylinositol 3-kinase |
| PM | Plasma membrane |
| PTEN | Phosphatase and tensin homolog |
| RT | Reverse transcription |
| SAS | Statistical Analysis System |
| USDA | U.S. Department of Agriculture |
References
- National Research Council. Proteins and amino acids. In Nutrient Requirements of Swine, 11th revised ed.; The National Academies Press: Washington, DC, USA, 2012; pp. 15–44. [Google Scholar]
- Austin, J.L. Amino acids in swine nutrition. In Swine Nutrition, 2nd ed.; Lewis, A.J., Southern, L.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 131–150. [Google Scholar]
- Roy, N.; Lapierre, H.; Bernier, J.F. Whole-body protein metabolism and plasma profiles of amino acids and hormones in growing barrows fed diets adequate or deficient in lysine. Can. J. Anim. Sci. 2000, 80, 585–595. [Google Scholar] [CrossRef]
- Shelton, N.W.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Nelssen, J.L.; DeRouchey, J.M. Effects of increasing dietary standardized ileal digestible lysine for gilts grown in a commercial finishing environment. J. Anim. Sci. 2011, 89, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Witte, D.P.; Ellis, M.; McKeith, F.K.; Wilson, E.R. Effect of dietary lysine level and environmental temperature during the finishing phase on the intramuscular fat content of pork. J. Anim. Sci. 2000, 78, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Bidner, B.S.; Ellis, M.; Witte, D.P.; Carr, S.N.; McKeith, F.K. Influence of dietary lysine level, pre-slaughter fasting, and rendement napole genotype on fresh pork quality. Meat Sci. 2004, 68, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tous, N.; Lizardo, R.; Vila, B.; Gispert, M.; Font, I.F.M.; Esteve-Garcia, E. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 129–140. [Google Scholar] [CrossRef]
- Jefferson, L.S.; Kimball, S.R. Amino acid regulation of gene expression. J. Nutr. 2001, 131, 2460S–2466S. [Google Scholar] [PubMed]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [PubMed]
- Yao, K.; Yin, Y.L.; Chu, W.; Liu, Z.; Deng, D.; Li, T.; Huang, R.; Zhang, J.; Tan, B.; Wang, W.; Wu, G. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 2008, 138, 867–872. [Google Scholar] [PubMed]
- Bonetto, A.; Penna, F.; Minero, V.G.; Reffo, P.; Costamagna, D.; Bonelli, G.; Baccino, F.M.; Costelli, P. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-α. Amino Acids 2011, 40, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.O.; Hirabara, S.M.; de Souza, C.T.; Schenka, A.A.; Zecchin, H.G.; Vassallo, J.; Velloso, L.A.; Carneiro, E.; Carvalheira, J.B.; Curi, R.; et al. l-Glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia 2007, 50, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Crenshaw, M.A.; Regmi, N.; Armstrong, T.; Blanton, J.R.; Liao, S.F. Effect of dietary lysine fed to pigs at late finishing stage on market-value associated carcass characteristics. J. Anim. Vet. Adv. 2015, 14, 232–236. [Google Scholar]
- Brazma, A.; Hingamp, P.; Quackenbush, J.; Sherlock, G.; Spellman, P.; Stoeckert, C.; Aach, J.; Ansorge, W.; Ball, C.A.; Causton, H.C.; et al. Minimum information about a microarray experiment (MIAME) toward standards for microarray data. Nat. Genet. 2001, 29, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Fafournoux, P.; Bruhat, A.; Jousse, C. Amino acid regulation of gene expression. Biochem. J. 2000, 351, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Kyoya, T.; Nakashima, K.; Katsumata, M. Muscle protein metabolism during compensatory growth with changing dietary lysine levels from deficient to sufficient in growing rats. J. Nutr. Sci. Vitaminol. 2011, 57, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef]
- Fujimori, T.; Kamiya, Y.; Nagata, K.; Kato, K.; Hosokawa, N. Endoplasmic reticulum lectin XTP3-B inhibits endoplasmic reticulum-associated degradation of a misfolded α1-antitrypsin variant. FEBS J. 2013, 280, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Cassar-Malek, I.; Le Cunff, M.; Dubroeucq, H.; Renand, G.; Hocquette, J.F. New indicators of beef sensory quality revealed by expression of specific genes. J. Agric. Food Chem. 2007, 55, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- Chardin, P. Function and regulation of Rnd proteins. Nat. Rev. Mol. Cell Biol. 2006, 7, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, J.; Fan, T.; Li, S.; Ren, X. RhoE functions as a tumor suppressor in esophageal squamous cell carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumour Biol. 2012, 33, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Merzdorf, C.S.; Sive, H.L. The zic1 gene is an activator of Wnt signaling. Int. J. Dev. Biol. 2006, 50, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, S.; Xue, M.; Du, Q.; Cai, J.; Jin, H.; Si, J.; Wang, L. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI3K and MAPK signaling pathways in gastric cancer. BMC Cancer 2012, 12, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.B.; Fumagalli, S.; Thomas, G. Target of rapamycin (TOR): Balancing the opposing forces of protein synthesis and degradation. Curr. Opin. Genet. Dev. 1999, 9, 49–54. [Google Scholar] [CrossRef]
- Sato, T.; Ito, Y.; Nedachi, T.; Nagasawa, T. Lysine suppresses protein degradation through autophagic–lysosomal system in C2C12 myotubes. Mol. Cell Biochem. 2014, 391, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Synthesis of amino acids. In Amino Acids: Biochemistry and Nutrition, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 63–96. [Google Scholar]
- Regmi, N.; Wang, T.; Crenshaw, M.A.; Rude, B.J.; Wu, G.; Liao, S.F. Effects of dietary levels on plasma free amino acid profile in late-stage finishing pigs. SpringerPlus 2016, 5, 888. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Physiological functions of amino acids. In Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; pp. 339–390. [Google Scholar]
- Lee, N.K.L.; MacLean, H.E. Polyamines, androgens, and skeletal muscle hypertrophy. J. Cell Physiol. 2011, 226, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Von Deutsch, D.A.; Abukhalaf, I.K.; Wineski, L.E.; Silvestrov, N.A.; Bayorh, M.A.; Potter, D.E. Changes in muscle proteins and spermidine content in response to unloading and clenbuterol treatment. Can. J. Physiol. Pharmacol. 2003, 81, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Varona, L.; Oliver, M.A.; Noguera, J.L.; Sanchez, A.; Amills, M. Malic enzyme 1 genotype is associated with backfat thickness and meat quality traits in pigs. Anim. Genet. 2006, 37, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mourot, J.; Kouba, M. Development of intra- and intermuscular adipose tissue in growing large white and Meishan pigs. Reprod. Nutr. Dev. 1999, 39, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Bessa, R.J.; Hughes, R.A.; Jeronimo, E.; Moreira, O.C.; Prates, J.A.; Doran, O. Effect of pig breed and dietary protein level on selected fatty acids and stearoyl-coenzyme A desaturase protein expression in longissimus muscle and subcutaneous fat. J. Anim. Sci. 2013, 91, 4540–4546. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, N.; McGillivray, C.; Bai, Q.; Wood, J.D.; Evans, G.; Chang, K.-C. Restriction of dietary energy and protein induces molecular changes in young porcine skeletal muscles. J. Nutr. 2004, 134, 2191–2199. [Google Scholar] [PubMed]
- Vilà-Brau, A.; de Sousa-Coelho, A.L.; Gonçalves, J.F.; Haro, D.; Marrero, P.F. Fsp27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by FA oxidation rate. J. Lipid Res. 2013, 54, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Ronti, T.; Lupattelli, G.; Mannarino, E. The endocrine function of adipose tissue: An update. Clin. Endocrinol. 2006, 64, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Rabaglia, M.E.; Stoehr, J.P.; Nadler, S.T.; Schueler, K.L.; Zou, F.; Yandell, B.S.; Attie, A.D. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 2003, 52, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Regmi, N.; Wang, T.; Crenshaw, M.A.; Rude, B.J.; Liao, S.F. Limiting dietary lysine increased plasma concentration of total cholesterol in finishing pigs. J. Anim. Sci. 2015, 93, 66. [Google Scholar]
- Inuzuka, M.; Hayakawa, M.; Ingi, T. Serinc, an activity-regulated protein family, incorporates serine into membrane lipid synthesis. J. Biol. Chem. 2005, 280, 35776–35783. [Google Scholar] [CrossRef] [PubMed]
- Schauder, C.M.; Wu, X.; Saheki, Y.; Narayanaswamy, P.; Torta, F.; Wenk, M.R.; de Camilli, P.; Reinisch, K.M. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 2014, 510, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Hsieh, T.S.; Yang, T.T.; Rothberg, K.; Azizoglu, D.B.; Volk, E.; Liao, J.C.; Liou, J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013, 5, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R.; Villareal, D.T.; Agnusdei, D.; Nardi, P.; Avioli, L.V.; Gennari, C. Dietary l-lysine and calcium metabolism in humans. Nutrition 1992, 8, 400–405. [Google Scholar] [PubMed]
- Solberg, A.; Robertson, A.B.; Aronsen, J.M.; Rognmo, O.; Sjaastad, I.; Wisloff, U.; Klungland, A. Deletion of mouse Alkbh7 leads to obesity. J. Mol. Cell Biol. 2013, 5, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Sumegi, B.; Than, G.N.; Berente, Z.; Bohn, H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta 1999, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Romestaing, C.; Han, X.; Li, Y.; Hao, X.; Wu, Y.; Sun, C.; Liu, X.; Jefferson, L.S.; Xiong, J.; et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010, 12, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.D.; Ernst, L.K.; Nair, R.P.; Lowe, J.B. Molecular cloning, sequence, and expression of a human GDP-l-fucose:β-d-galactoside 2-α-l-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA 1990, 87, 6674–6678. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Gong, Y.; Qin, W.; Zhang, P.; Li, J.; Wei, L.; Zhou, X.; Li, H.; Qiu, X.; Zhong, F.; et al. Large-scale cDNA transfection screening for genes related to cancer development and progression. Proc. Natl. Acad. Sci. USA 2004, 101, 15724–15729. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Peng, X.; Sang, Y.; Qiu, M.; Luo, C.; He, Z.; Zhao, X.; Tong, A. Dichloroacetate induces protective autophagy in LoVo cells: Involvement of cathepsin D/thioredoxin-like protein 1 and Akt-mTOR-mediated signaling. Cell Death Dis. 2013, 4, e913. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ebihara, A.; Kajiho, H.; Kontani, K.; Nishina, H.; Katada, T. RASSF7 negatively regulates pro-apoptotic JNK signaling by inhibiting the activity of phosphorylated-MKK7. Cell Death Differ. 2011, 18, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 2002, 12, 14–21. [Google Scholar] [CrossRef]
- Recino, A.; Sherwood, V.; Flaxman, A.; Cooper, W.N.; Latif, F.; Ward, A.; Chalmers, A.D. Human RASSF7 regulates the microtubule cytoskeleton and is required for spindle formation, Aurora B activation and chromosomal congression during mitosis. Biochem. J. 2010, 430, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, J.; Andreasen, D.; Jensen, B.L.; Ainsworth, M.A.; Friis, U.G.; Johansen, T. NHE1, NHE2, and NHE3 contribute to regulation of intracellular pH in murine duodenal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, 197–206. [Google Scholar]
- Zou, C.; Li, J.; Bai, Y.; Gunning, W.T.; Wazer, D.E.; Band, V.; Gao, Q. Centrobin: A novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 2005, 171, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.R.; McLaughlin, J.N.; Skiba, N.P.; Hamm, H.E.; Willardson, B.M. Functional roles of the two domains of phosducin and phosducin-like protein. J. Biol. Chem. 2000, 275, 30399–30407. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.Q.; Yallowitz, A.R.; Sun, H.; Dressler, G.R.; Wellik, D.M. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol. Cell Biol. 2007, 27, 7661–7668. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Tashiro, H.; Katabuchi, H.; Suzuki, A.; Kurman, R.J.; Okamura, H. Neonatal estrogenic exposure suppresses PTEN-related endometrial carcinogenesis in recombinant mice. Lab. Investig. 2006, 86, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Tominaga, K.; Matzuk, M.M.; Pereira-Smith, O.M. MrgX is not essential for cell growth and development in the mouse. Mol. Cell Biol. 2005, 25, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Goto, M.; Sawa, C.; Ito, S.; Watanabe, H.; Sawada, J.; Handa, H. Functional interactions of transcription factor human GA-binding protein subunits. J. Biol. Chem. 1998, 273, 29302–29308. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Huang, Y.S. CPEB2–eEF2 interaction impedes HIF-1α RNA translation. EMBO J. 2012, 31, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Brown, K.R.; Stromberg, A.J.; Burris, W.R.; Boling, J.A.; Matthews, J.C. Dietary supplementation of selenium in inorganic and organic forms differentially and commonly alters blood and liver selenium concentrations and liver gene expression profiles of growing beef heifers. Biol. Trace Elem. Res. 2011, 140, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Boling, J.A.; Matthews, J.C. Gene expression profiling indicates an increased capacity for proline, serine, and ATP synthesis and mitochondrial mass by the liver of steers grazing high vs. low endophyte-infected tall fescue. J. Anim. Sci. 2015, 93, 5659–5671. [Google Scholar] [CrossRef] [PubMed]
- Partek, Inc. Partek Discovery Services: Turning Data into Discovery. Available online: http://www.partek.com/DiscoveryServices (accessed on 19 April 2017).
- Nygard, A.-B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Applied Biosystems. The comparative Ct method (ΔΔCt method). In Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR; Applied Biosystems: Foster City, CA, USA, 2008; pp. 52–59. [Google Scholar]
- Allison, D.B.; Cui, X.; Page, G.P.; Sabripour, M. Microarray data analysis: From disarray to consolidation and consensus. Nat. Rev. Genet. 2006, 7, 55–65. [Google Scholar] [CrossRef] [PubMed]

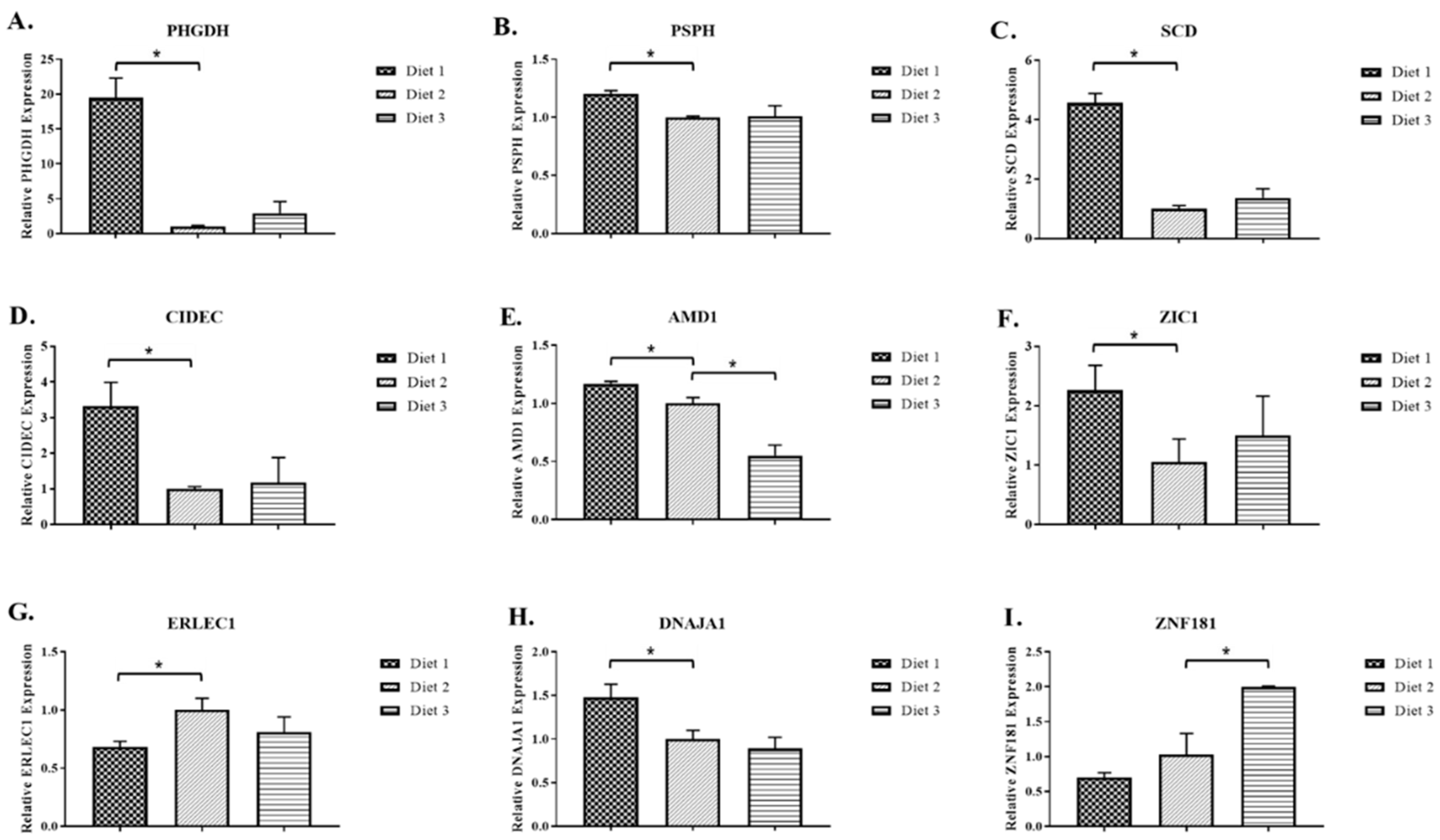
| Gene Symbol | Gene Description | GenBank 1 | p-Value 2 |
|---|---|---|---|
| Down-regulated genes | |||
| CPEB2 | Cytoplasmic polyadenylation element binding protein 2 | NM_001185049 | <0.001 |
| CST9L | Cystatin 9-like | XM_003134304 | 0.007 |
| ERLEC1 | Endoplasmic reticulum lectin 1 | XM_003125147 | 0.011 |
| ESYT1 | Extended synaptotagmin-like protein 1 | XM_003126262 | 0.009 |
| HOXA11B | Homeobox A11 | AF453292 | 0.001 |
| LCLAT1 | Lysocardiolipin acyltransferase 1 | NM_001142845 | 0.032 |
| MYL6 | Myosin, light chain 6 | NM_001163997 | 0.011 |
| RASSF7 | Ras association domain family member 7 | XM_003122393 | 0.004 |
| UBTD1 | Ubiquitin domain containing 1 | XM_003359315 | 0.003 |
| ZMAT5 | Zinc finger, matrin-type 5 | XM_001929010 | 0.035 |
| ZNF181 | Zinc finger protein 181 | NM_001244818 | 0.005 |
| Up-regulated genes | |||
| AMD1 | Adenosylmethionine decarboxylase 1 | XM_003121345 | 0.014 |
| ATP6V1G2 | Atpase, H+ transporting, lysosomal 13 kDa, V1 subunit G2 | NM_001145380 | 0.002 |
| C7H6orf136 | Chromosome 6 open reading frame 136 | NM_001243459 | 0.003 |
| CCDC25 | Coiled-coil domain containing 25 | NM_001243572 | 0.023 |
| CD164 | CD164 molecule | XM_001924626 | 0.022 |
| CHIT1 | Chitinase 1 | XM_003130296 | 0.005 |
| CHORDC1 | Cysteine and histidine-rich domain (CHORD) containing 1 | NM_001113446 | 0.010 |
| CHSY3 | Chondroitin sulfate synthase 3 | XM_003123906 | 0.049 |
| CIDEC | Cell death-inducing DFFA-like effector c | NM_001112689 | 0.024 |
| DNAJA1 | Dnaj (Hsp40) homolog, subfamily A, member 1 | NM_001244163 | 0.003 |
| eIF2S2 | Eukaryotic translation initiation factor 2 subunit 2 | XM_005672861 | 0.002 |
| FUT1 | Fucosyltransferase 1 | NM_214068 | 0.003 |
| GABPB1 | GA binding protein transcription factor, β subunit 1 | XM_005659610 | 0.002 |
| GPR182 | G protein-coupled receptor 182 | XM_003126290 | 0.000 |
| H1FOO | Oocyte-specific H1 histone | NM_001205063 | 0.016 |
| HOXA4 | Homeobox A4 | XM_003134841 | 0.037 |
| HSP90AB1 | Heat shock protein 90 kDa α (cytosolic), class B member 1 | NM_001244433 | 0.007 |
| LGALS13 | Lectin, galactoside-binding, soluble, 13 | NM_001142841 | 0.003 |
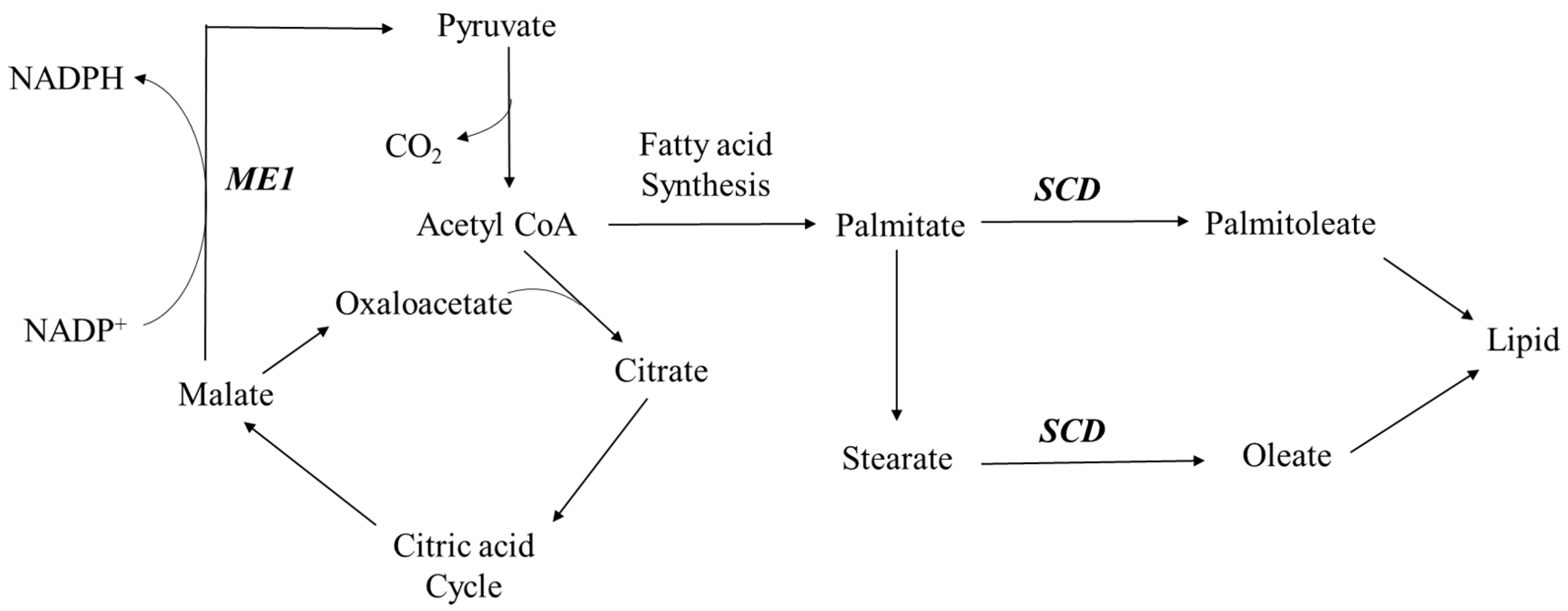
| ME1 | Malic enzyme 1, NADP+-dependent, cytosolic | XM_001924333 | 0.050 |
| MIR3187 | MicroRNA 3187 | NR_036154 | 0.002 |
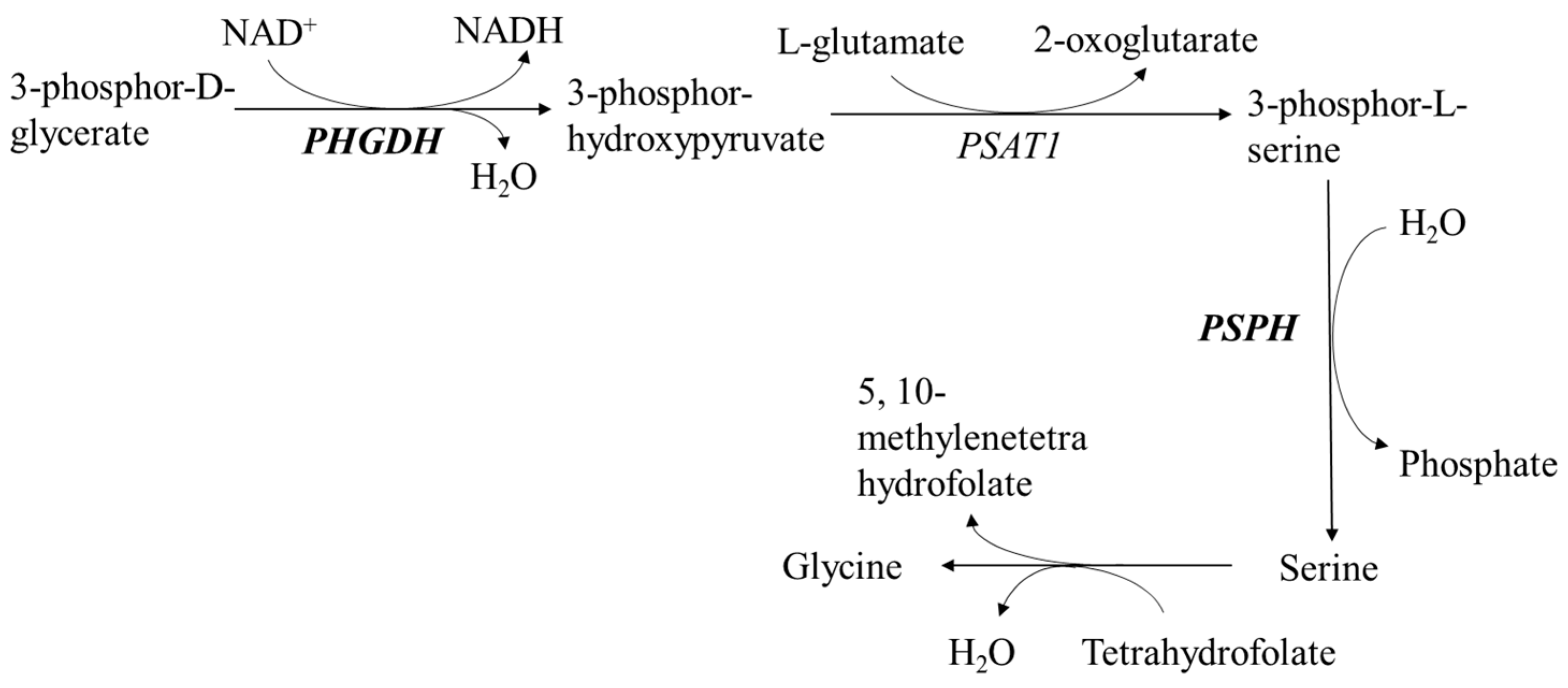
| PHGDH | Phosphoglycerate dehydrogenase | NM_001123162 | 0.000 |
| PSPH | Phosphoserine phosphatase | NM_001243221 | 0.009 |
| RND3 | Rho family GTPase 3 | NM_214296 | 0.003 |
| SCD | Stearoyl-CoA desaturase (δ-9-desaturase) | NM_213781 | 0.023 |
| SERP1 | Stress-associated endoplasmic reticulum protein 1 | NM_001243260 | 0.039 |
| TXNL1 | Thioredoxin-like 1 | NM_001244276 | 0.030 |
| UBE2B | Ubiquitin-conjugating enzyme E2B | NM_001257356 | 0.011 |
| ZIC1 | Zic family member 1 | XM_003358599 | 0.011 |
| Gene Symbol | Gene Description | GenBank 1 | p-Value 2 |
|---|---|---|---|
| Down-regulated genes | |||
| ALKBH7 | AlkB, alkylation repair homolog 7 | XM_003123112 | 0.001 |
| AMD1 | Adenosylmethionine decarboxylase 1 | XM_003121345 | 0.013 |
| CFAP20 | Cilia and flagella associated protein 20 | NM_001244786 | 0.001 |
| CFD | Complement factor D (adipsin) | XM_003122985 | 0.009 |
| CHORDC1 | Cysteine and histidine-rich domain (CHORD) containing 1 | NM_001113446 | 0.006 |
| CLCA2 | Chloride channel accessory 2 | XM_003125930 | 0.003 |
| CNTFR | Ciliary neurotrophic factor receptor | XM_003130672 | 0.027 |
| DNAJA1 | Dnaj (Hsp40) homolog, subfamily A, member 1 | NM_001244163 | 0.004 |
| ERLEC1 | Endoplasmic reticulum lectin 1 | XM_003125147 | 0.004 |
| HOXA4 | Homeobox A4 | XM_003134841 | 0.014 |
| HSP90AA1 | Heat shock protein 90 kDa α (cytosolic), class A member 1 | NM_213973 | 0.034 |
| HSPH1 | Heat shock 105 kDa/110 kDa protein 1 | NM_001097504 | 0.014 |
| LCLAT1 | Lysocardiolipin acyltransferase 1 | NM_001142845 | 0.001 |
| ME1 | Malic enzyme 1, NADP+-dependent, cytosolic | XM_001924333 | 0.027 |
| MFAP3 | Microfibrillar-associated protein 3 | XM_003134126 | 0.001 |
| MORF4L2 | Mortality factor 4 like 2 | XM_003135267 | 0.033 |
| SERP1 | Stress-associated endoplasmic reticulum protein 1 | NM_001243260 | 0.016 |
| SLC9A2 | Solute carrier family 9, subfamily A (NHE2, cation proton antiporter 2), member 2 | NM_001100189 | 0.010 |
| TMCO6 | Transmembrane and coiled-coil domains 6 | XM_003124040 | 0.003 |
| UBE2D2 | Ubiquitin-conjugating enzyme E2D 2 | NM_001078673 | 0.028 |
| UBTD1 | Ubiquitin domain containing 1 | XM_003359315 | 0.009 |
| ZMAT5 | Zinc finger, matrin-type 5 | XM_001929010 | 0.001 |
| Up-regulated genes | |||
| CHIT1 | Chitinase 1 (chitotriosidase) | XM_003130296 | 0.009 |
| CHSY3 | Chondroitin sulfate synthase 3 | XM_003123906 | 0.001 |
| CNTROB | Centrobin, centrosomal BRCA2 interacting protein | XM_003358269 | 0.007 |
| CXCR6 | Chemokine (C-X-C motif) receptor 6 | NM_001001623 | 0.000 |
| DBX1 | Developing brain homeobox 1 | XM_003122916 | 0.002 |
| DND1 | DND microrna-mediated repression inhibitor 1 | XM_003124043 | 0.009 |
| GPR182 | G protein-coupled receptor 182 | XM_003126290 | 0.008 |
| H1FOO | Oocyte-specific H1 histone | NM_001205063 | 0.003 |
| LGALS13 | Lectin, galactoside-binding, soluble, 13 | NM_001142841 | 0.005 |
| MYO5B | Myosin VB | XM_003121434 | 0.001 |
| PDCL | Phosducin-like | XM_001927696 | 0.003 |
| XKR4 | XK, Kell blood group complex subunit-related family, member 4 | XM_003355057 | 0.008 |
| ZNF181 | Zinc finger protein 181 | NM_001244818 | 0.037 |
| Item | Diet 1 | Diet 2 | Diet 3 |
|---|---|---|---|
| Ingredients (g/kg) | |||
| Corn | 908.44 | 904.94 | 901.44 |
| Soybean meal | 64.00 | 64.00 | 64.00 |
| Canola oil | 8.00 | 8.00 | 8.00 |
| l-Lysine-HCl (98.5%) | 0.00 | 3.50 | 7.00 |
| dl-Methionine (99.0%) | 0.40 | 0.40 | 0.40 |
| l-Threonine (98.5%) | 0.90 | 0.90 | 0.90 |
| l-Tryptophan (99.0%) | 0.35 | 0.35 | 0.35 |
| Limestone | 6.50 | 6.50 | 6.50 |
| Dicalcium phosphate | 9.00 | 9.00 | 9.00 |
| Salt | 2.00 | 2.00 | 2.00 |
| Mineral premix 2 | 0.33 | 0.33 | 0.33 |
| Vitamin premix 3 | 0.08 | 0.08 | 0.08 |
| Total | 1000.0 | 1000.0 | 1000.0 |
| Composition (g/kg) 4 | |||
| Metabolizable energy (kcal/kg) | 3319 | 3323 | 3326 |
| Crude protein | 104.5 | 107.5 | 110.5 |
| Total lysine | 4.33 | 7.08 | 9.82 |
| Total methionine | 2.37 | 2.36 | 2.36 |
| Total threonine | 5.02 | 5.01 | 5.00 |
| Total tryptophan | 1.40 | 1.40 | 1.39 |
| Total Ca | 4.58 | 4.58 | 4.58 |
| Total P | 4.32 | 4.31 | 4.30 |
| Gene Symbol 1 | GenBank 2 | Sequence (5′–3′) 3 | Amplicon Size (bp) |
|---|---|---|---|
| PHGDH | NM_001123162 | F: GCGGTTTGGTTTAGGTGTTTC | 113 |
| R: AAGGGTCCAGGCTATCACT | |||
| PSPH | NM_001243221 | F: CTGCAGGCTCCAGTTTAGTT | 97 |
| R: CTCGCAGAGTCTTTACCAACA | |||
| SCD | NM_213781 | F: CCCAAGGCAGACAAGAGAATAG | 91 |
| R: GTGTTGACGACTGAGGTTACAG | |||
| CIDEC | NM_001112689 | F: CCAACTCTCCCTCTCCCATAA | 106 |
| R: CATGTTCAGGCAACCAATGAAG | |||
| AMD1 | XM_003121345 | F: TCCACAAGTCAAGTCCTCTAATG | 108 |
| R: CCATGGAGAGGAACGAATCAA | |||
| ZIC1 | XM_003358599 | F: CGACCGACGCTTTGCTAATA | 97 |
| R: GTAGGACTTGTCGCACATCTT | |||
| ERLEC1 | XM_003125147 | F: GCTGGCTATCCTTTGTACTCTC | 109 |
| R: CAACACTGCTTGTGGACATTT | |||
| DNAJA1 | NM_001244163 | F: GGTGGTAAGAAAGGAGCAGTAG | 93 |
| R: CTGAACCATTCCAGGTCCTATT | |||
| ZNF181 | NM_001244818 | F: GCCTTCAGCCAAAGCAAATC | 85 |
| R: AGGCTTTCCCACATTCACTAC | |||
| HPRT1 4 | NM_001032376 | F: GCTATGCCCTTGACTACAATGA | 102 |
| R: TTGAACTCTCCTCTTAGGCTTTG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Feugang, J.M.; Crenshaw, M.A.; Regmi, N.; Blanton, J.R., Jr.; Liao, S.F. A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine. Int. J. Mol. Sci. 2017, 18, 885. https://doi.org/10.3390/ijms18040885
Wang T, Feugang JM, Crenshaw MA, Regmi N, Blanton JR Jr., Liao SF. A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine. International Journal of Molecular Sciences. 2017; 18(4):885. https://doi.org/10.3390/ijms18040885
Chicago/Turabian StyleWang, Taiji, Jean M. Feugang, Mark A. Crenshaw, Naresh Regmi, John R. Blanton, Jr., and Shengfa F. Liao. 2017. "A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine" International Journal of Molecular Sciences 18, no. 4: 885. https://doi.org/10.3390/ijms18040885
APA StyleWang, T., Feugang, J. M., Crenshaw, M. A., Regmi, N., Blanton, J. R., Jr., & Liao, S. F. (2017). A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine. International Journal of Molecular Sciences, 18(4), 885. https://doi.org/10.3390/ijms18040885