Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins
Abstract
:1. Introduction
2. Results
2.1. Fatty Acid Composition
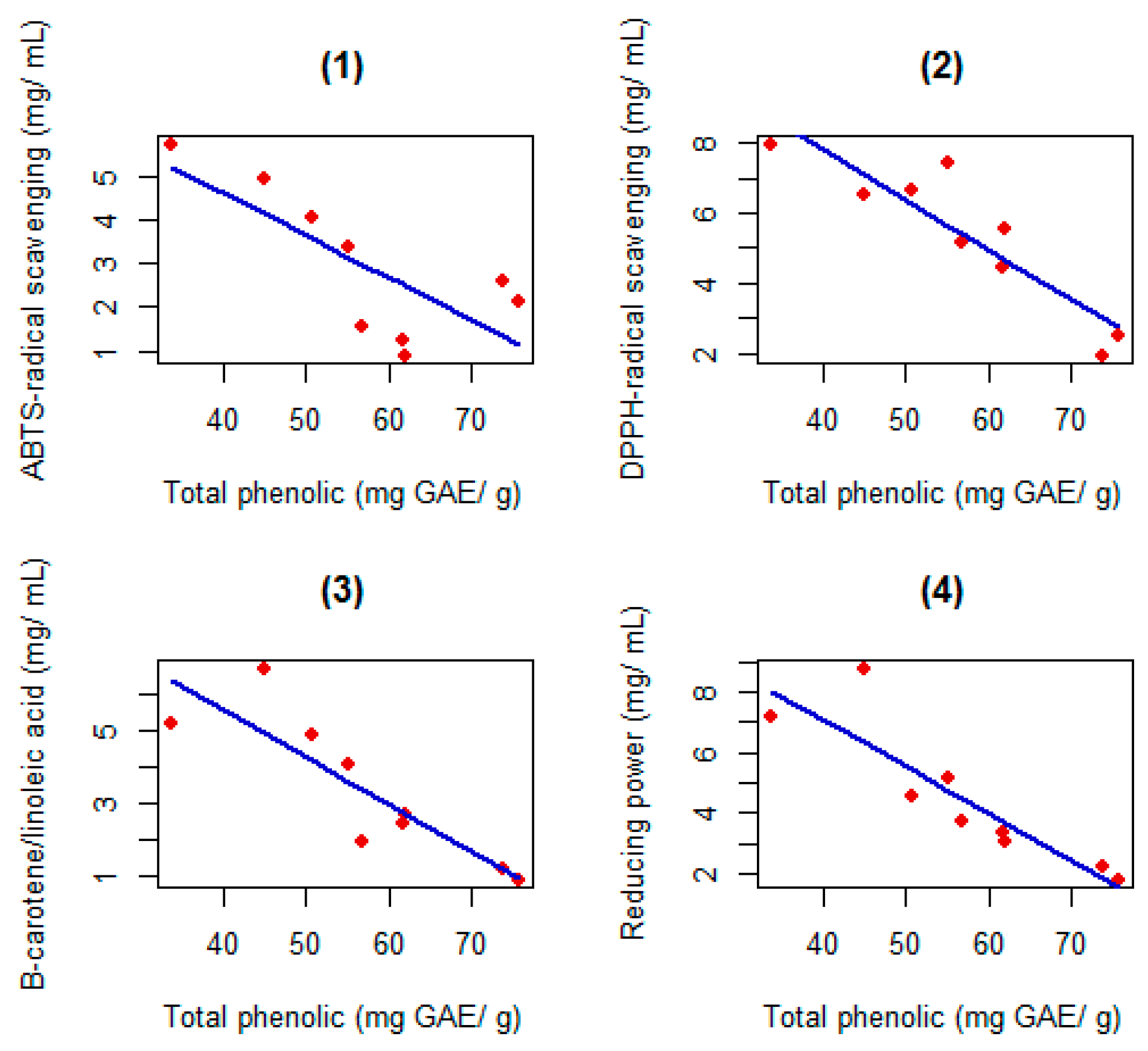
2.2. Antioxidant Activities
2.3. Total Phenolic and Flavonoid
2.4. Enzyme Inhibitory Activities
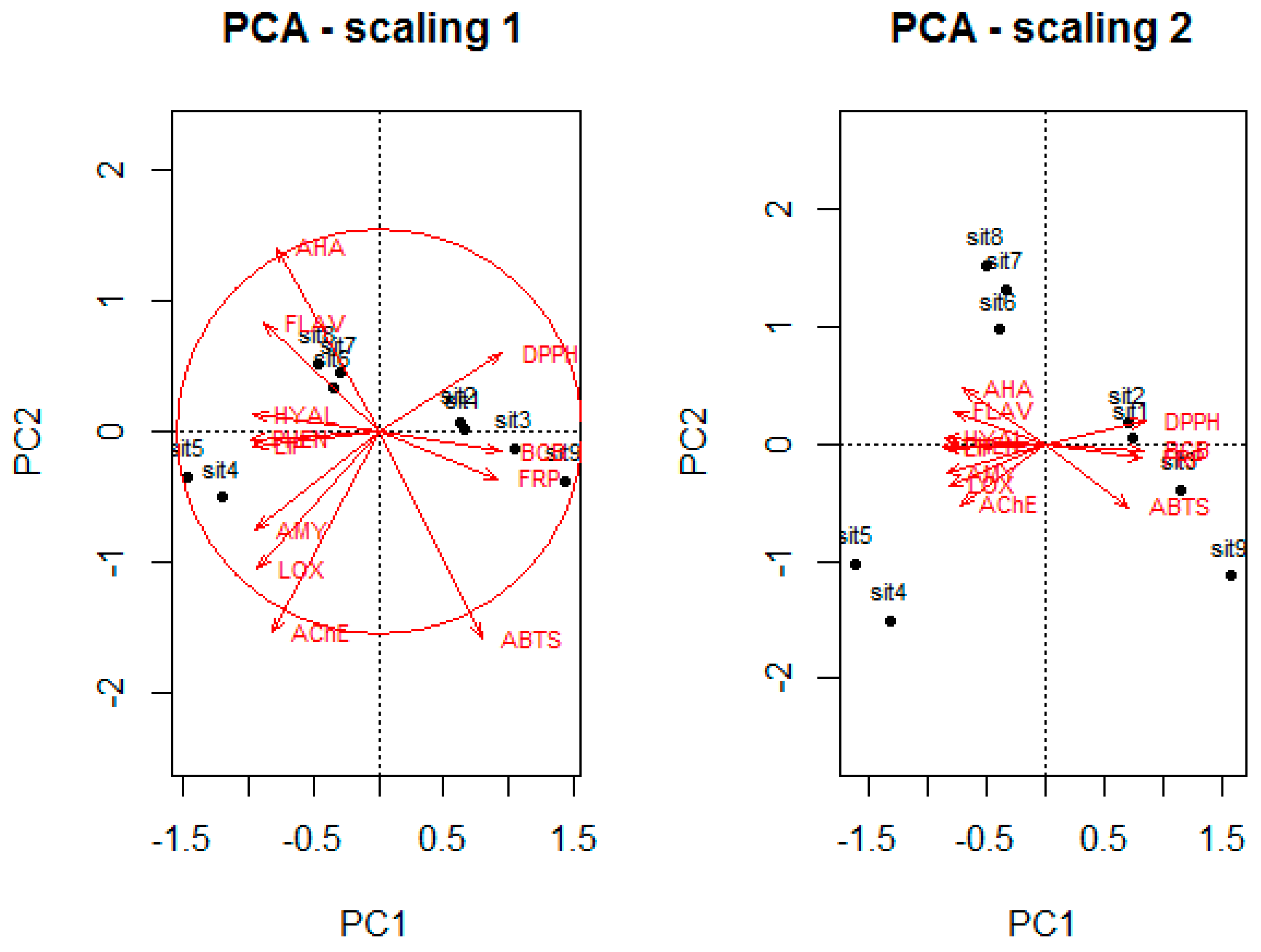
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Determination of Fatty Acids
4.3. Preparation of Pollen Methanolic Extracts (PME)
4.4. Total Phenols and Flavonoids, and Antioxidant Activity
4.5. Enzymatic Activities
4.5.1. Lipase
4.5.2. α-Amylase
4.5.3. Acetylcholinesterase (AchE)
4.5.4. Tyrosinase
4.5.5. Hyaluronidase
4.5.6. Lipoxygenase
4.5.7. Antihemolytic Assay
4.6. Statistical Analyzes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campos, M.G.R.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of bee-pollen? J. ApiProd. ApiMed. Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Rodrigues, S.; Pereira, A.P.; Féas, X. Portuguese bee pollen: Palynological study, nutritional and microbiological evaluation. Int. J. Food Sci. Technol. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arabian J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Reis, L.C.B.; de Souza, C.O.; da Silva, J.B.A.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active biocomposites of cassava starch: The effect of yerba mate extract andmango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Karampour, N.S.; Hemmati, A.A.; Malmir, A. The anxiolytic effect of bee pollen hydroalcoholic extract in mice. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7. [Google Scholar] [CrossRef]
- Yildiz, O.; Karahalil, F.; Can, Z.; Sahin, H.; Kolayli, S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and própolis. J. Enzyme Inhib. Med. Chem. 2014, 29, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic contente, antioxidante properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of anti-mutagenic, anti-histopathologic and antioxidante capacities of Egyptian bee pollen and propolis extracts. Cytotechnology 2014, 66, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Rajagopalan, R. Diabetes and insulin resistance associated disorders: Disease and the therapy. Curr. Sci. 2002, 83, 1533–1538. [Google Scholar]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine-ferulic acid hybrids as multipotent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Féas, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Schneider, H.; Ludwing, M.; Schnitker, J.; Brahler, E.; Weidner, W. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis–chronic pelvic pain syndrome: A multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur. Urol. 2009, 56, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Mareczek, A.; Wyzgolik, G.; Klepacz-Baniak, J.; Czekonska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Melo, A.A.M.; Estevinho, M.L.M.F.; Sattler, J.A.G.; Souza, B.R.; Freitas, A.S.; Barth, O.M.; Almeida-Muradian, L.B. Effect of processing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. Food Sci. Technol. 2016, 65, 808–815. [Google Scholar]
- Nurdianah, H.F.; Ahmad Firdaus, A.H.; Eshaifol Azam, O.; Wan Adnan, W.O. Antioxidant activity of bee pollen ethanolic extracts from Malaysian stingless bee measured using DPPH-HPLC assay. Int. Food Res. J. 2016, 23, 403–405. [Google Scholar]
- Estevinho, L.M.; Chambó, E.D.; Pereira, A.P.R.; Carvalho, C.A.L.; Toledo, V.A.A. Characterization of Lavandula spp. honey using multivariate techniques. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.D.S.; Maciel, L.F.; Miranda, M.S.; Druzian, J.I. Compostos bioativos e potencial antioxidante do pólen apícola produzido por abelhas africanizadas (Apis mellifera L.). Rev. Inst. Adolfo Lutz 2010, 69, 233–242. [Google Scholar]
- Rebiai, A.; Lanez, T. Chemical composition and antioxidante activity of Apis mellifera bee pollen from northwest Algeria. J. Fundam. Appl. Sci. 2012, 4, 155–163. [Google Scholar] [CrossRef]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Féas, X.; Vásquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef] [PubMed]
- Carpes, S.T.; Prado, A.; Moreno, I.A.M.; Mourão, G.B.; Alencar, S.M.; Masson, M.L. Avaliação do potencial antioxidante do pólen apícola produzido na Região Sul do Brasil. Quim. Nova 2008, 31, 1660–1664. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; Campos, M.G.; Ávila-Reyes, J.A.; Naranjo-Jiménez, N.; Herrera-Corral, J.; González-Valdez, L.S. Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Interciencia 2004, 29, 574–578. [Google Scholar]
- King, A.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolismo and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczyńska, J.; Borawska, M.H. Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J. Apic. Res. 2013, 57, 147–157. [Google Scholar] [CrossRef]
- Sagona, S.; Pozzo, L.; Peiretti, P.G.; Biondi, C.; Giusti, M.; Gabriele, M.; Felicioli, A. Palynological origin, chemical composition, lipid peroxidation and fatty acid profile of organic Tuscanian bee-pollen. J. Apic. Res. 2017, 56, 136–143. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N.; Zaheri, L. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2013, 3, 138. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices, of raw roe of blue fin tuna (Thunnus thynnus L.) and their salted product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method. A revised description. Svensk Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutricional content of fresh. Bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists International. Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995; Volume 2, p. 474. [Google Scholar]
- Bárbara, M.S.; Machado, C.S.; Sodré, G.D.S.; Dias, L.G.; Estevinho, L.M.; de Carvalho, C.A.L. Microbiological Assessment, Nutritional Characterization and Phenolic Compounds of Bee Pollen from Mellipona mandacaia Smith, 1983. Molecules 2015, 20, 12525–12544. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of própolis sample from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.R.; Kumazawa, S.; Hamasaka, T.; Bang, K.S.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J. Agric. Food Chem. 2004, 52, 7286–7292. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of própolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Roh, C.; Jung, U. Screening of Crude Plant Extracts with Anti-Obesity Activity. Int. J. Mol. Sci. 2012, 13, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kawabata, J. α-Glucosidase inhibition of 6-hydroxyflavones. Part 3: Synthesis and evaluation of 2, 3, 4-trihydroxybenzoylcontaining flavonoid analogs and 6-aminoflavones as α-glucosidase inhibitors. Bioorgan. Med. Chem. 2005, 13, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.; Gao, B.; Xu, P.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008, 106, 1195–1201. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.; Celep, F.; Kahraman, A.; Dogan, M.; Yilmaz, G.; Sener, B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase and antioxidant activities. Food Chem. 2010, 120, 34–43. [Google Scholar] [CrossRef]
- Orhan, I.E.; Khan, M.T. Flavonoid derivatives as potent tyrosinase inhibitors—A survey of recent findings between 2008–2013. Curr. Top. Med. Chem. 2014, 14, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Tanaka, T.; Kouno, I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by β-glucosidase in the presence of amino acids. Biol. Pharm. Bull. 2003, 26, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.N.; Hsiao, G.; Kuo, Y.H. Protection of oxidative hemolysis by demethyldiisoeugenol in normal and β-thalassemic red blood cells. Free Radic. Biol. Med. 1997, 22, 215–222. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, 2014. Available online: http://www.R-project.org/ (accessed on 20 December 2016).

| Fatty Acid 2 | Pollen Extracts (Mean ± SD) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
| C4:0 | 0.035 ± 0.005 d | 0.021 ± 0.002 ab | 0.026 ± 0.002 bc | 0.040 ± 0.002 d | 0.037 ± 0.002 cd | 0.020 ± 0.002 ab | 0.074 ± 0.006 e | 0.019 ± 0.001 ab | 0.011 ± 0.001 a |
| C6:0 | 0.036 ± 0.004 b | 0.040 ± 0.000 b | 0.039 ± 0.002 b | 0.043 ± 0.002 b | 0.038 ± 0.003 b | 0.037 ± 0.004 b | 0.070 ± 0.000 c | 0.025 ± 0.001 a | 0.043 ± 0.003 b |
| C8:0 | 0.066 ± 0.001 ab | 0.051 ± 0.002 a | 0.052 ± 0.003 a | 0.093 ± 0.005 c | 0.110 ± 0.009 d | 0.066 ± 0.005 ab | 0.116 ± 0.005 d | 0.070 ± 0.001 b | 0.075 ± 0.003 b |
| C10:0 | 0.015 ± 0.001 ab | 0.014 ± 0.001 ab | 0.013 ± 0.001 ab | 0.049 ± 0.001 cd | 0.055 ± 0.000 d | 0.042 ± 0.003 c | 0.067 ± 0.002 e | 0.010 ± 0.000 a | 0.019 ± 0.002 b |
| C12:0 | 0.019 ± 0.001 b | 0.035 ± 0.004 d | 0.034 ± 0.003 d | 0.029 ± 0.001 cd | 0.029 ± 0.002 cd | 0.024 ± 0.002 bc | 0.050 ± 0.000 e | 0.010 ± 0.000 a | 0.006 ± 0.000 a |
| C14:0 | 0.077 ± 0.002 e | 0.076 ± 0.003 e | 0.059 ± 0.002 d | 0.033 ± 0.002 bc | 0.031 ± 0.001 b | 0.042 ± 0.003 c | 0.083 ± 0.003 e | 0.017 ± 0.002 a | 0.011 ± 0.001 a |
| C16:0 | 0.391 ± 0.036 a | 0.410 ± 0.051 a | 0.467 ± 0.029 a | 0.773 ± 0.016 c | 0.720 ± 0.009 c | 0.578 ± 0.022 b | 0.777 ± 0.045 c | 0.443 ± 0.017 a | 0.471 ± 0.005 a |
| C18:0 | 0.050 ± 0.000 ab | 0.050 ± 0.000 ab | 0.050 ± 0.000 ab | 0.097 ± 0.005 c | 0.098 ± 0.006 c | 0.095 ± 0.003 c | 0.109 ± 0.011 c | 0.065 ± 0.004 b | 0.037 ± 0.002 a |
| C18:1n9 | 0.388 ± 0.008 ab | 0.363 ± 0.023 ab | 0.425 ± 0.023 b | 0.632 ± 0.040 c | 0.610 ± 0.036 c | 0.639 ± 0.052 c | 0.950 ± 0.028 d | 0.447 ± 0.019 b | 0.336 ± 0.015 a |
| C18:3n3 | 1.417 ± 0.042 b | 1.503 ± 0.082 bc | 1.417 ± 0.057 b | 1.750 ± 0.022 d | 1.707 ± 0.039 cd | 1.471 ± 0.111 b | 1.758 ± 0.082 d | 1.718 ± 0.045 d | 1.204 ± 0.046 a |
| C18:2n6c | 0.698 ± 0.056 ab | 0.782 ± 0.057 abc | 0.775 ± 0.063 abc | 0.877 ± 0.025 bcd | 0.930 ± 0.014 cd | 0.792 ± 0.049 abcd | 0.970 ± 0.078 d | 0.787 ± 0.041 abcd | 0.657 ± 0.018 a |
| Sums, Ratios and Indexes of Fatty Acids | |||||||||
| SFA | 0.694 ± 0.027 a | 0.696 ± 0.045 a | 0.739 ± 0.027 a | 1.158 ± 0.009 c | 1.116 ± 0.011 c | 0.904 ± 0.017 b | 1.345 ± 0.033 d | 0.655 ± 0.011 a | 0.673 ± 0.009 a |
| MUFA | 0.388 ± 0.008 ab | 0.363 ± 0.023 ab | 0.425 ± 0.0023 b | 0.632 ± 0.040 c | 0.610 ± 0.036 c | 0.639 ± 0.043 c | 0.0950 ± 0.028 d | 0.447 ± 0.019 b | 0.328 ± 0.024 a |
| PUFA | 2.116 ± 0.096 ab | 2.285 ± 0.127 bc | 2.192 ± 0.053 bc | 2.627 ± 0.033 d | 2.637 ± 0.037 d | 2.272 ± 0.105 bc | 2.728 ± 0.162 d | 2.504 ± 0.050 cd | 1.861 ± 0.060 a |
| NI | 0.106 ± 0.003 a | 0.123 ± 0.010 abc | 0.123 ± 0.005 abc | 0.193 ± 0.005 de | 0.223 ± 0.017 ef | 0.160 ± 0.02 cd | 0.245 ± 0.007 f | 0.112 ± 0.004 ab | 0.155 ± 0.023 bcd |
| TFA | 3.304 ± 0.114 ab | 3.468 ± 0.160 bc | 3.479 ± 0.047 bc | 4.610 ± 0.012 e | 4.586 ± 0.018 e | 3.975 ± 0.070 d | 5.268 ± 0.200 f | 3.719 ± 0.076 cd | 3.009 ± 0.013 a |
| PUFA:SFA | 3.049 ± 0.080 de | 3.234 ± 0.051 d | 2.970 ± 0.159 de | 2.268 ± 0.016 ab | 2.362 ± 0.013 ab | 2.029 ± 0.124 bc | 2.514 ± 0.130 a | 3.823 ± 0.046 f | 2.768 ± 0.124 cd |
| n6:n3 | 0.492 ± 0.028 a | 0.520 ± 0.028 a | 0.549 ± 0.062 a | 0.287 ± 0.002 a | 0.545 ± 0.017 a | 0.551 ± 0.045 a | 0.539 ± 0.068 a | 0.458 ± 0.030 a | 0.546 ± 0.015 a |
| AI | 0.287 ± 0.002 cd | 0.283 ± 0.002 c | 0.281 ± 0.014 c | 0.287 ± 0.002 cd | 0.268 ± 0.003 bc | 0.315 ± 0.013 bc | 0.264 ± 0.014 d | 0.176 ± 0.006 a | 0.239 ± 0.008 b |
| TI | 0.076 ± 0.004 ab | 0.076 ± 0.004 ab | 0.086 ± 0.009 bc | 0.113 ± 0.001 d | 0.110 ± 0.001 d | 0.120 ± 0.007 cd | 0.102 ± 0.010 d | 0.066 ± 0.004 a | 0.087 ± 0.003 bc |
| Extracts 2 | Antioxidant Activities (Mean ± SD) 1 | |||
|---|---|---|---|---|
| ABTS | DPPH | BCB | FRP | |
| S1 | 2.12 ± 0.03 f | 2.52 ± 0.06 g | 0.93 ± 0.06 f | 1.82 ± 0.14 h |
| S2 | 2.59 ± 0.16 e | 1.94 ± 0.17 h | 1.22 ± 0.14 f | 2.22 ± 0.20 g |
| S3 | 1.23 ± 0.13 h | 4.46 ± 0.35 f | 2.47 ± 0.11 d | 3.36 ± 0.16 f |
| S4 | 0.91 ± 0.05 i | 5.58 ± 0.17 d | 2.71 ± 0.19 d | 3.08 ± 0.05 f |
| S5 | 1.58 ± 0.15 g | 5.15 ± 0.07 e | 1.98 ± 0.20 e | 3.73 ± 0.17 e |
| S6 | 4.92 ± 0.18 b | 6.56 ± 0.27 c | 6.71 ± 0.34 a | 8.77 ± 0.23 a |
| S7 | 5.73 ± 0.16 a | 7.99 ± 0.21 a | 5.18 ± 0.40 b | 7.20 ± 0.17 b |
| S8 | 3.37 ± 0.34 d | 7.45 ± 0.11 b | 4.05 ± 0.29 c | 5.15 ± 0.09 c |
| S9 | 4.06 ± 0.07 c | 6.66 ± 0.28 c | 4.89 ± 0.16 b | 4.53 ± 0.18 d |
| BHA | 0.09 ± 0.01 | 1.25 ± 0.02 | 1.23 ± 0.03 | 1.45 ± 0.03 |
| Extract | Enzyme Inhibitory Activities (Mean ± SD) 1 | ||||||
|---|---|---|---|---|---|---|---|
| α-AMY 2 | AChE 2 | TYR 2 | LOX 2 | LIP 2 | HYAL 3 | AHA 3 | |
| S1 | 1015.94 ± 12.16 a | 827.48 ± 21.94 b | 1140.44 ± 58.88 a | 312.74 ± 12.64 a | 4.16 ± 0.08 a | 17.33 ± 0.76 a | 74.13 ± 1.76 d |
| S2 | 910.79 ± 12.37 b | 967.53 ± 17.57 a | 999.16 ± 9.98 b | 285.12 ± 11.77 b | 3.57 ± 0.16 b | 15.67 ± 0.76 b | 73.11 ± 2.61 d |
| S3 | 488.02 ± 12.35 c | 150.12 ± 14.10 c | 627.09 ± 28.22 d | 92.99 ± 2.47 d | 2.66 ± 0.09 d | 13.00 ± 0.50 c | 78.29 ± 2.37 c |
| S4 | 412.89 ± 12.25 d | 89.66 ± 8.28 e | 701.02 ± 11.30 c | 116.91 ± 8.24 c | 3.28 ± 0.21 c | 14.00 ± 0.66 c | 85.83 ± 0.24 a |
| S5 | 372.08 ± 5.48 e | 121.50 ± 2.90 c | 519.60 ± 16.99 e | 84.38 ± 2.77 d | 2.33 ± 0.16 e | 14.17 ± 0.58 c | 81.91 ± 1.17 b |
| S6 | 116.17 ± 13.64 f | 71.52 ± 3.58 f | 212.69 ± 22.95 f | 23.62 ± 2.16 f | 0.81 ± 0.06 h | 11.33 ± 0.58 d | 61.44 ± 1.12 e |
| S7 | 50.81 ± 1.45 g | 3.93 ± 0.64 h | No activity | 13.51 ± 1.16 f | 1.34 ± 0.11 g | 7.00 ± 0.50 f | 43.85 ± 1.65 h |
| S8 | 20.52 ± 0.82 h | 38.38 ± 2.47 g | 54.14 ± 7.43 g | 54.95 ± 1.61 e | 1.89 ± 0.15 f | 9.25 ± 0.66 e | 51.31 ± 1.67 g |
| S9 | 16.44 ± 0.79 h | 26.85 ± 1.61 g | 45.88 ± 1.62 g | 45.20 ± 2.39 e | 1.56 ± 0.09 g | 10.25 ± 0.66 e | 55.70 ± 1.97 f |
| Control | 51.44 ± 0.81 Ascarbose | 0.005 ± 0.001 Eserine | 5.19 ± 0.29 Kojic acid | 61.92 ± 2.97 NDGA | 0.018 ± 0.0007 Orlistat | 98.85 ± 0.53 Epigall | 98.78 ± 0.53 Ascorbic acid |
| Pollen Extracts | Pollen Types * | Predominant Botanical Origin | Harvest Place/State |
|---|---|---|---|
| S1 | DP: Cocos nucifera (100%) | Cocos nucifera | Neópolis/Sergipe |
| S2 | DP: Cocos nucifera (100%) | Cocos nucifera | Maraú/Bahia |
| S3 | DP: Miconia spp. (97.1%) OIP: Cocos nucifera (2.9%) | Miconia spp. | Valença/Bahia |
| S4 | DP: Miconia spp. (98.5%) OIP: Cocos nucifera (1.5%) | Miconia spp. | Valença/Bahia |
| S5 | DP: Miconia spp. (97.2%) OIP: Cocos nucifera (2.8%) | Miconia spp. | Valença/Bahia |
| S6 | DP: Spondias spp. (95.8%) IIP: Cocos nucifera (4.2%) | Spondias spp. | Canavieiras/Bahia |
| S7 | DP: Myrcia spp. (60.0%) AP: Cocos nucifera (33.3%) IIP: Saccharum spp. (6.7%) | Multifloral | Ilhéus/Bahia |
| S8 | PD: Eucalyptus spp. (96.8%) OIP: Mikania spp. (2.2%), Cocos nucifera (1.0%) | Eucalyptus spp. | Teixeira de Freitas/Bahia |
| S9 | DP: Eucalyptus spp. (97.0%) OIP: Mimosa spp. (1.0%), Myrcia spp. (2.0%) | Eucalyptus spp. | Canavieiras/Bahia |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.S.; Chambó, E.D.; Costa, M.A.P.d.C.; Cavalcante da Silva, S.M.P.; Lopes de Carvalho, C.A.; M. Estevinho, L. Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. Int. J. Mol. Sci. 2017, 18, 921. https://doi.org/10.3390/ijms18050921
Araújo JS, Chambó ED, Costa MAPdC, Cavalcante da Silva SMP, Lopes de Carvalho CA, M. Estevinho L. Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. International Journal of Molecular Sciences. 2017; 18(5):921. https://doi.org/10.3390/ijms18050921
Chicago/Turabian StyleAraújo, Jucilene Silva, Emerson Dechechi Chambó, Maria Angélica Pereira de Carvalho Costa, Samira Maria Peixoto Cavalcante da Silva, Carlos Alfredo Lopes de Carvalho, and Leticia M. Estevinho. 2017. "Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins" International Journal of Molecular Sciences 18, no. 5: 921. https://doi.org/10.3390/ijms18050921
APA StyleAraújo, J. S., Chambó, E. D., Costa, M. A. P. d. C., Cavalcante da Silva, S. M. P., Lopes de Carvalho, C. A., & M. Estevinho, L. (2017). Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. International Journal of Molecular Sciences, 18(5), 921. https://doi.org/10.3390/ijms18050921






