Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges
Abstract
:1. Introduction
2. Results
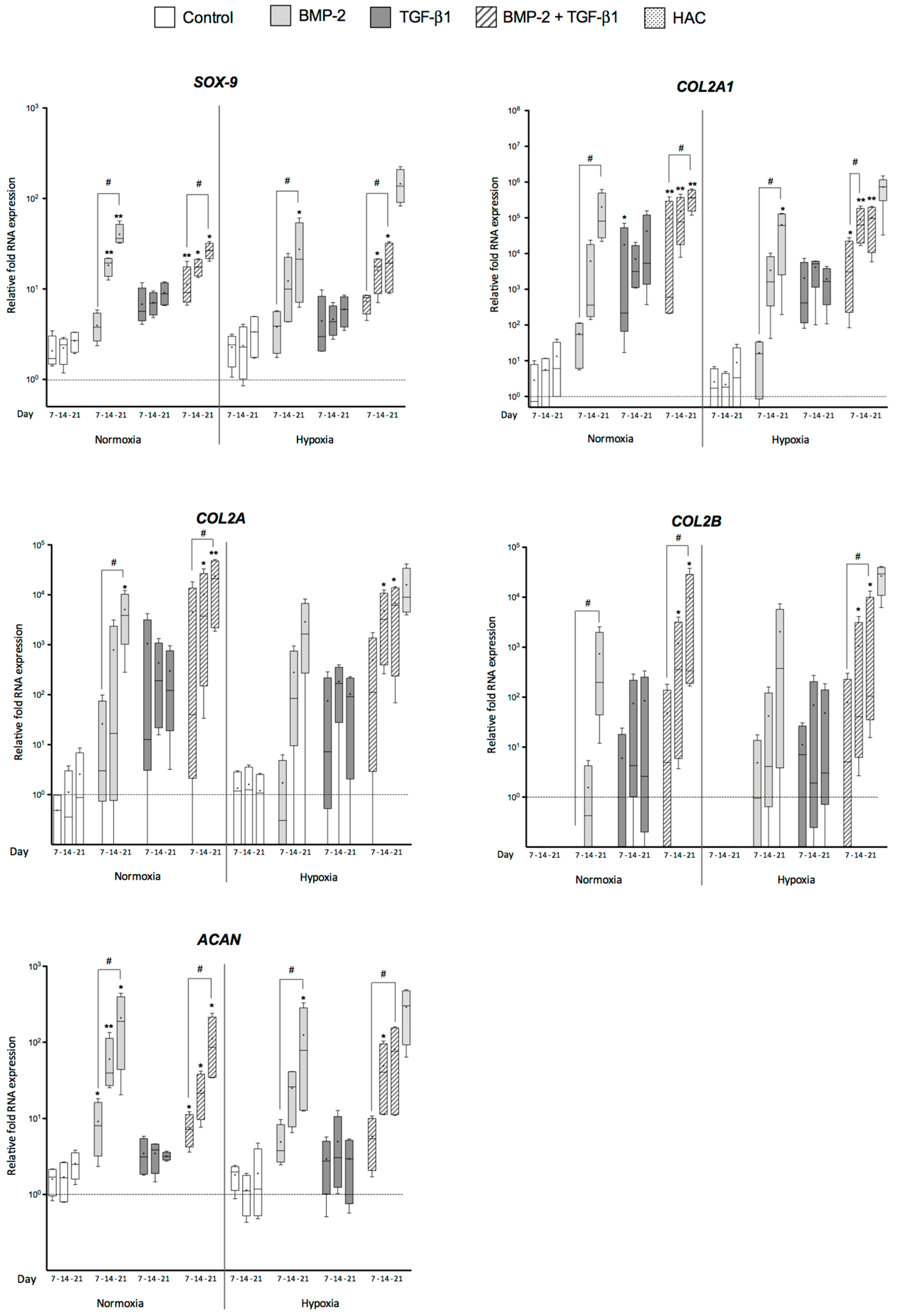
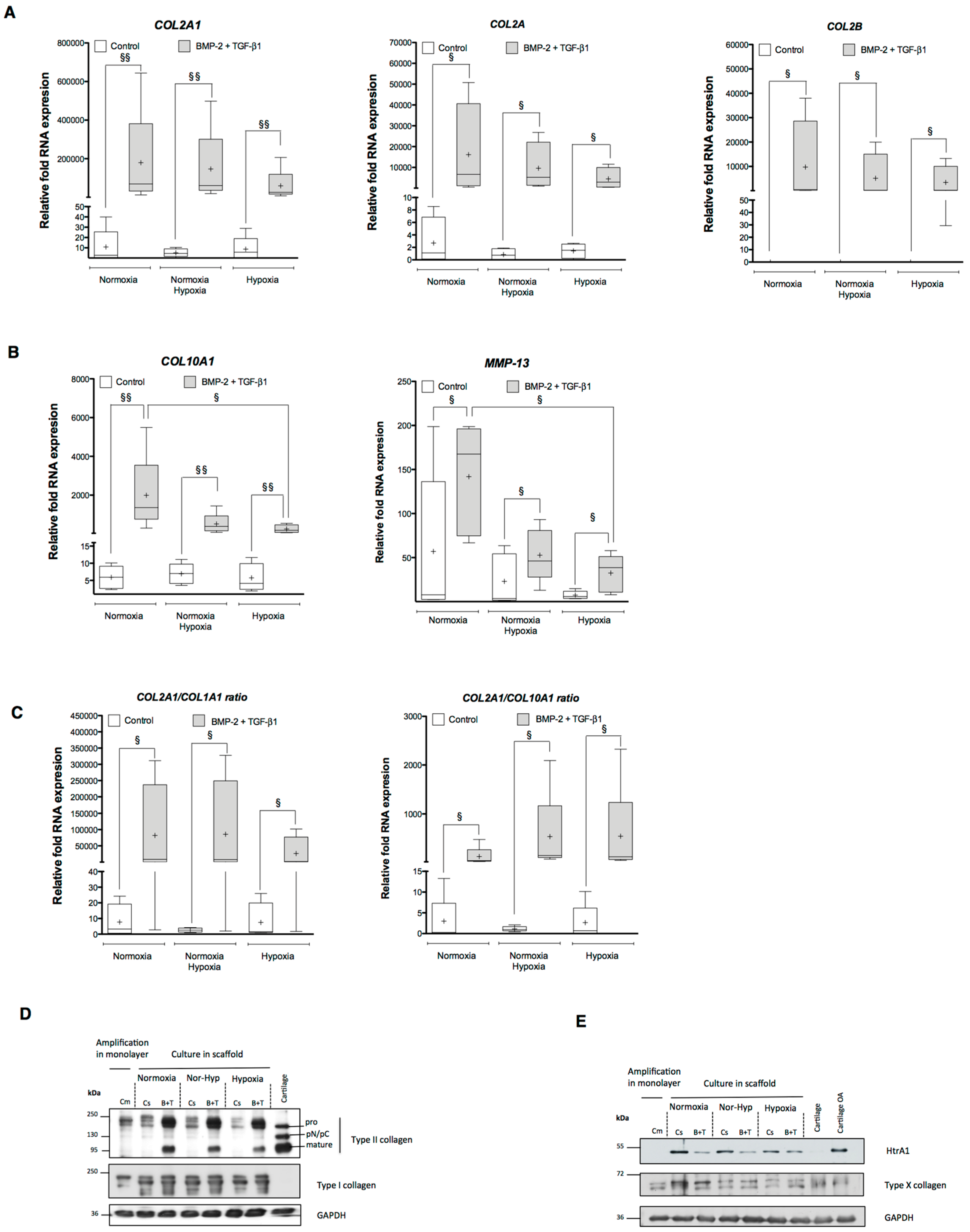
2.1. Effects of Bone Morphogenetic Protein 2 (BMP-2) and/or Transforming Growth Factor-β (TGF-β1) on the Amounts of mRNA Encoding Cartilage-Specific Markers during Chondrogenesis of Human Umbilical Cord Mesenchymal Stem Cells (hUCB-MSCs)
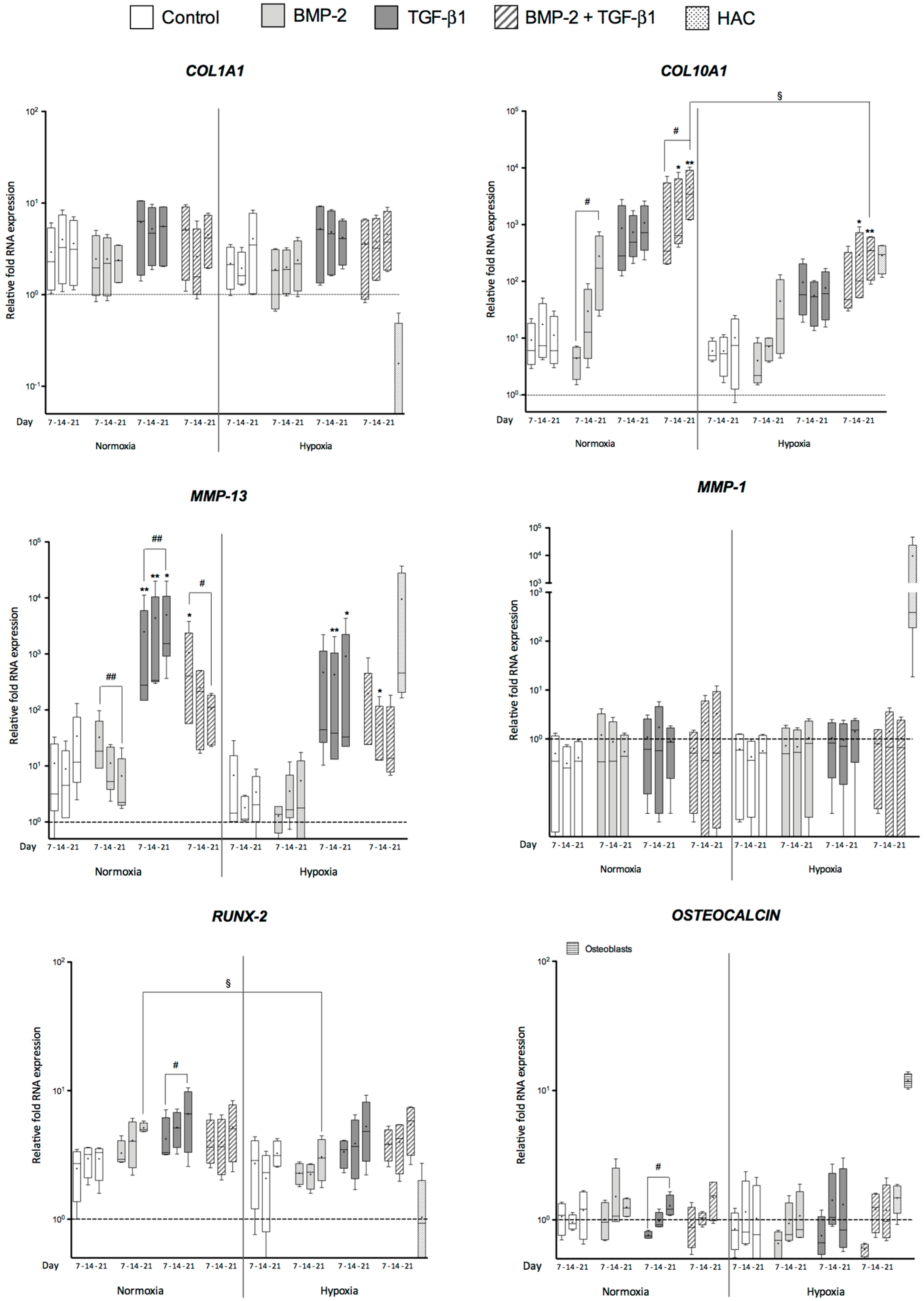
2.2. Effects of BMP-2 and/or TGF-β1 on the Levels of mRNA Encoding Non-Cartilage Markers during Chondrogenic Commitment of hUCB-MSCs
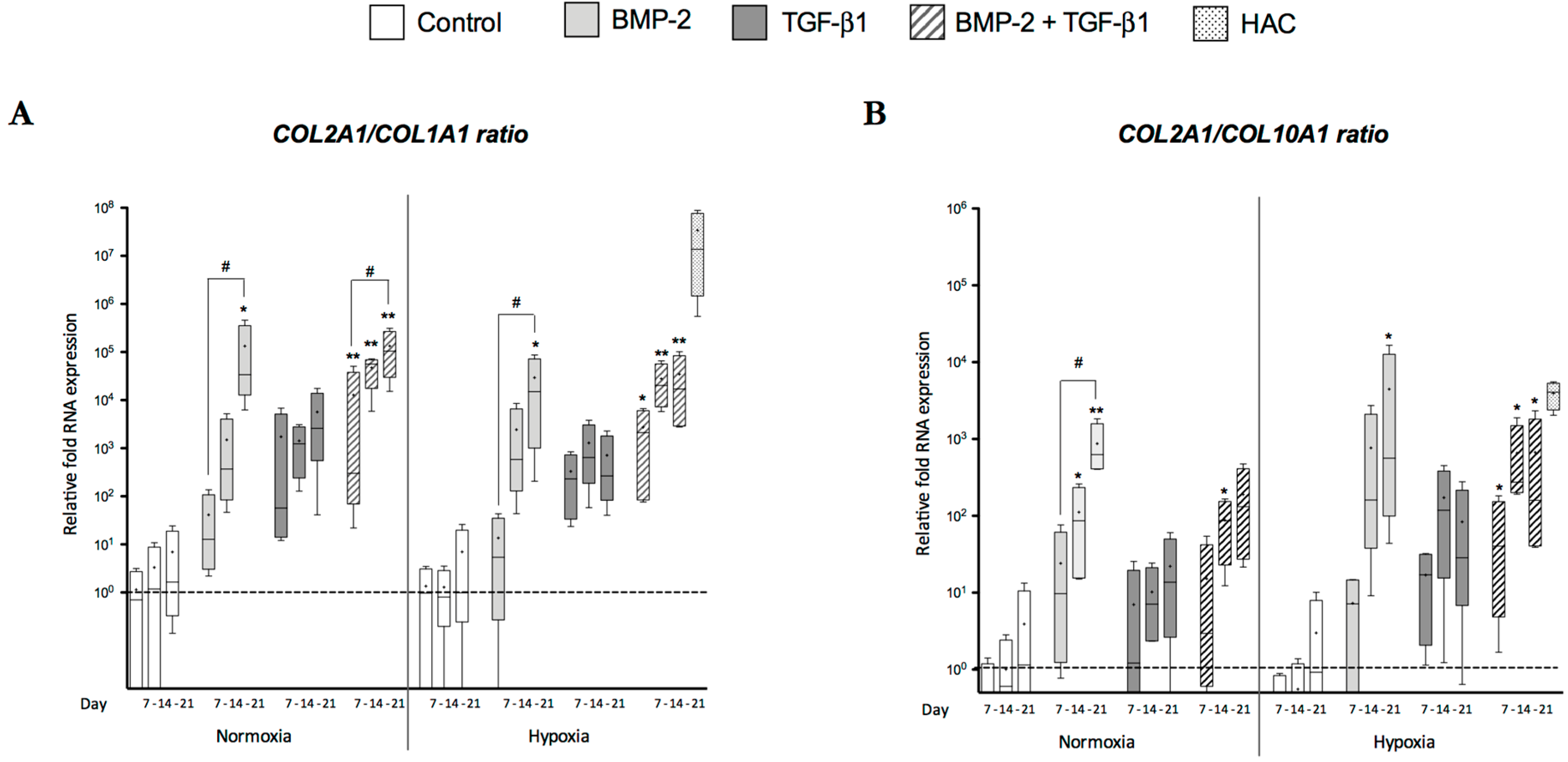
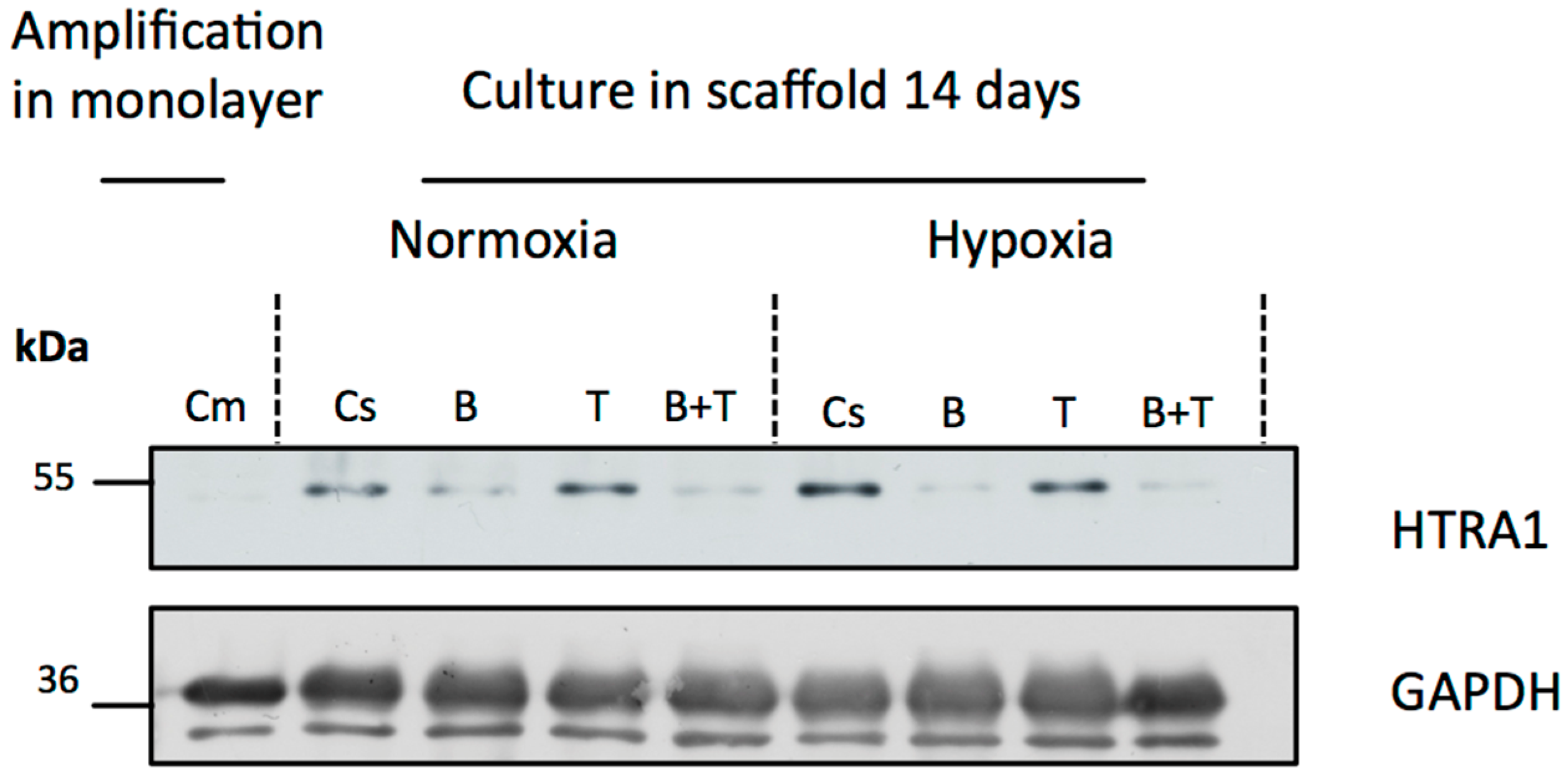
2.3. Newly Synthesized Extracellular Matrix (ECM) Proteins Expressed by hUCB-MSCs Differentiating into Chondrocytes
2.4. Modification of Oxygen Tension Conditions during Differentiation towards the Chondrocyte Lineage Improves the Chondrocyte Phenotype
3. Discussion
3.1. Cartilage-Specific Markers during Chondrogenesis of hUCB-MSCs
3.2. Atypical Markers of Cartilage during Chondrogenesis of hUCB-MSCs
3.3. Effects of Oxygen Tension during hUCB-MSCs Differentiation
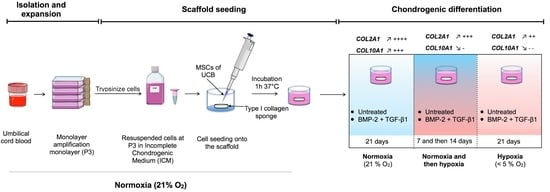
4. Materials and Methods
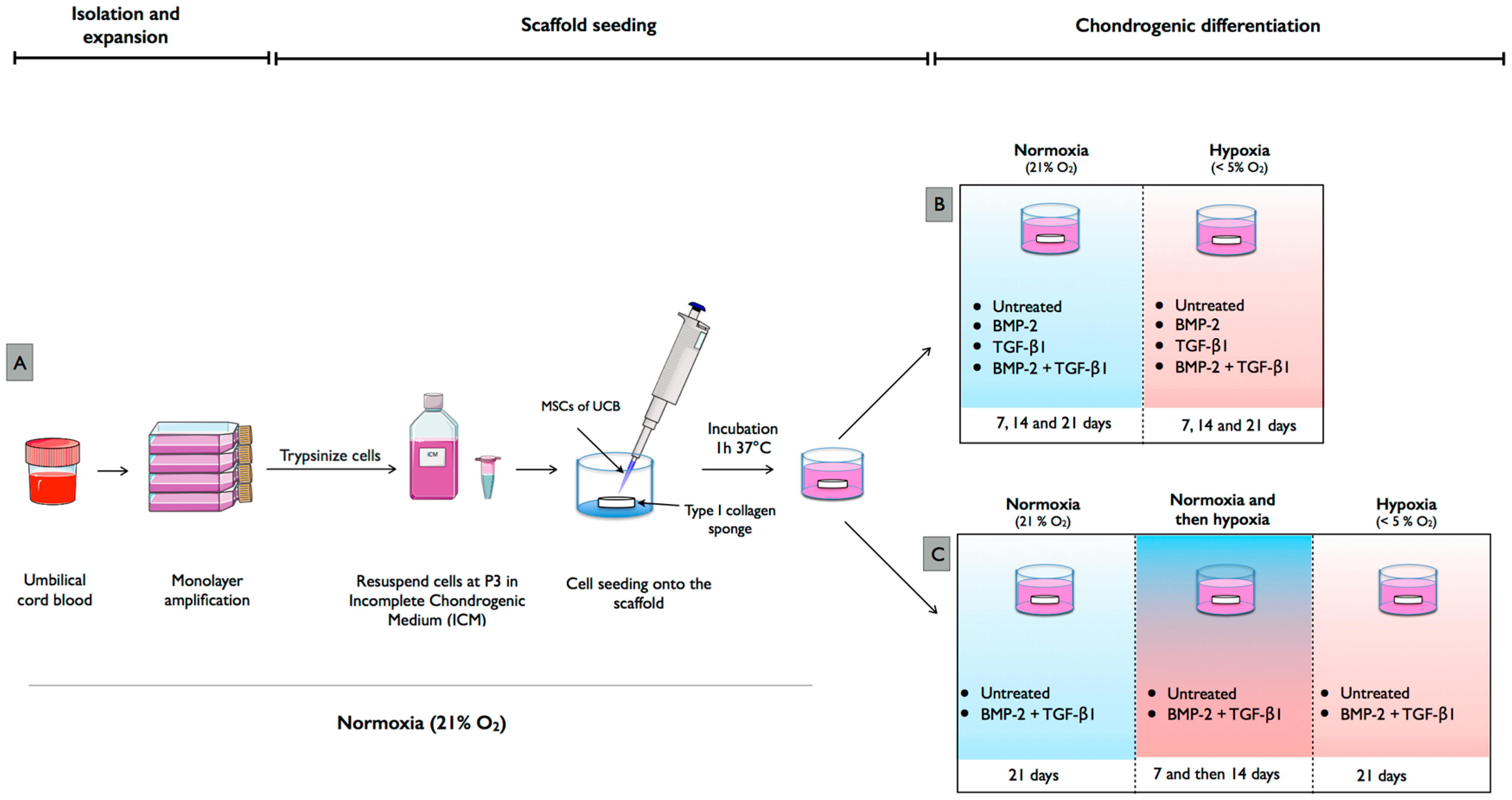
4.1. Isolation and Culture of hUCB-MSCs
4.2. Human Articular Chondrocytes
4.3. Chondrogenic Differentiation of hUCB-MSCs in Normoxia and Hypoxia
4.4. Chondrogenic Differentiation with Modification of Oxygen Tension
4.5. RNA Extraction and RT-PCR
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef] [PubMed]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kanawa, M.; Igarashi, A.; Ronald, V.S.; Higashi, Y.; Kurihara, H.; Sugiyama, M.; Saskianti, T.; Pan, H.; Kato, Y. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy 2013, 15, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.S.; Eichler, H.H.; Stoeve, J.J.; Klüter, H.H.; Bieback, K.K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leduc, T.; Hervieu, M.; Legendre, F.; Bouyoucef, M.; Gruchy, N.; Poulain, L.; de Vienne, C.; Herlicoviez, M.; Demoor, M.; Galéra, P. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci. Rep. 2016, 6, 32786. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Chen, W.H.; Lin, T.C.; Hwang, S.M.; Zeng, R.; Hsu, W.C.; Chiang, Y.M.; Liu, M.C.; Williams, D.F.; Deng, W.P. Preferential therapy for osteoarthritis by cord blood MSCs through regulation of chondrogenic cytokines. Biomaterials 2013, 34, 4739–4748. [Google Scholar] [CrossRef] [PubMed]
- Pievani, A.; Scagliotti, V.; Russo, F.M.; Azario, I.; Rambaldi, B.; Sacchetti, B.; Marzorati, S.; Erba, E.; Giudici, G.; Riminucci, M.; et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy 2014, 16, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-β-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef] [PubMed]
- Freyria, A.M.; Ronzière, M.C.; Cortial, D.; Galois, L.; Hartmann, D.; Herbage, D.; Mallein-Gerin, F. Comparative phenotypic analysis of articular chondrocytes cultured within type I or type II collagen scaffolds. Tissue Eng. Part A 2009, 15, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Mayer, N.; Aubert-Foucher, E.; Chajra, H.; Perrier-Groult, E.; Lafont, J.; Piperno, M.; Damour, O.; Mallein-Gerin, F. Cartilage-characteristic matrix reconstruction by sequential addition of soluble factors during expansion of human articular chondrocytes and their cultivation in collagen sponges. Tissue Eng. Part C Methods 2012, 18, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Ollitrault, D.; Hervieu, M.; Baugé, C.; Maneix, L.; Goux, D.; Chajra, H.; Mallein-Gerin, F.; Boumédiene, K.; Galéra, P.; et al. Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C Methods 2013, 19, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Bénateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2014, 21, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Hennig, T.; Lorenz, H.; Thiel, A.; Goetzke, K.; Dickhut, A.; Geiger, F.; Richter, W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGF-β receptor and BMP profile and is overcome by BMP-6. J. Cell. Physiol. 2007, 211, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Khorsandi, L.; Atashi, A.; Nejaddehbashi, F. Chondrogenic differentiation of human umbilical cord blood-derived unrestricted somatic stem cells on a 3D β-tricalcium phosphate-alginate-gelatin scaffold. Cell J. 2014, 16, 43–52. [Google Scholar] [PubMed]
- Co, C.; Vickaryous, M.K.; Koch, T.G. Membrane culture and reduced oxygen tension enhances cartilage matrix formation from equine cord blood mesenchymal stromal cells in vitro. Osteoarthr. Cartil. 2014, 22, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Song, M.; Ha, C.-W.; Kim, J.-A.; Lee, C.H.; Park, Y.-B. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res. Ther. 2014, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Song, M.; Lee, C.H.; Kim, J.A.; Ha, C.W. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J. Orthop. Res. 2015, 33, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; He, X.; Xu, Y.; Yang, Z.; Chen, L.; Zhou, S.; Yang, Y.; Zhou, Z.; Sheng, W.; et al. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury 2013, 44, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Deng, N.; Zhao, X.; Liu, Y.; Wang, X.; Zhang, H. Transfection of hBMP-2 into mesenchymal stem cells derived from human umbilical cord blood and bone marrow induces cell differentiation into chondrocytes. Minerva Med. 2014, 105, 283–288. [Google Scholar] [PubMed]
- De Mara, C.S.; Duarte, A.S.S.; Sartori-Cintra, A.R.; Luzo, A.C.M.; Saad, S.T.O.; Coimbra, I.B. Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatol. Int. 2013, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 417, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1139–C1146. [Google Scholar] [CrossRef] [PubMed]
- Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Fuentes-Boquete, I.; Díaz-Prado, S.; Blanco, F.J. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013, 2013, 232896. [Google Scholar] [CrossRef] [PubMed]
- Gawlitta, D.; van Rijen, M.H.P.; Schrijver, E.J.M.; Alblas, J.; Dhert, W.J.A. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng. Part A 2012, 18, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Buchheiser, A.; Houben, A.P.; Bosch, J.; Marbach, J.; Liedtke, S.; Kögler, G. Oxygen tension modifies the “stemness” of human cord blood-derived stem cells. Cytotherapy 2012, 14, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Behringer, R.R.; de Crombrugghe, B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 2001, 9, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leduc, T. Cellules Souches Mésenchymateuses Humaines de Sang de Cordon Ombilical, Chondrogènese et Ingénierie du Cartilage Articulaire. Ph.D. Thesis, Université de Caen/Basse Normandie, Caen, France, December 2014. [Google Scholar]
- Duval, E.; Baugé, C.; Andriamanalijaona, R.; Bénateau, H.; Leclercq, S.; Dutoit, S.; Poulain, L.; Galéra, P.; Boumédiene, K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 2012, 33, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Mendler, M.; Eich-Bender, S.G.; Vaughan, L.; Winterhalter, K.H.; Bruckner, P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J. Cell Biol. 1989, 108, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, T.-J.; Park, J.; Hwang, J.-E.; Jin, M.; Jang, H.-K.; Hwang, N.S.; Kim, B.S. Modulation of BMP-2-induced chondrogenic versus osteogenic differentiation of human mesenchymal stem cells by cell-specific extracellular matrices. Tissue Eng. Part A 2013, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Solchaga, L.A.; Caplan, A.I.; Hering, T.M.; Goldberg, V.M.; Yoo, J.U.; Johnstone, B. BMP-2 induction and TGF-β 1 modulation of rat periosteal cell chondrogenesis. J. Cell. Biochem. 2001, 81, 284–294. [Google Scholar] [CrossRef]
- Fan, J.; Park, H.; Tan, S.; Lee, M. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2. PLoS ONE 2013, 8, e72474. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Opran, A.; Wozney, J.; Csimma, C.; Lilly, L.; Riedel, G.E. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin. Orthop. Relat. Res. 2002, 395, 110–120. [Google Scholar] [CrossRef]
- Toh, W.S.; Liu, H.; Heng, B.C.; Rufaihah, A.J.; Ye, C.P.; Cao, T. Combined effects of TGFβ1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors 2005, 23, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, A.T.; Niemeyer, P.; Kaschte, K.; Muller, L.; Finkenzeller, G.; Hartl, D.; Sudkamp, N.P.; Schmal, H. Differential effects of BMP-2 and TGF-β1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007, 40, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wei, A.; Tao, H.; Diwan, A.D.; Ma, D.D.F. BMP-2 enhances TGF-β3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng. Part A 2009, 15, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Shintani, N.; Siebenrock, K.A.; Hunziker, E.B. TGF-ß1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS ONE 2013, 8, e53086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, B.; Ringe, J.; Häupl, T.; Notter, M.; Manz, R.; Burmester, G.-R.; Sittinger, M.; Kaps, C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation 2003, 71, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Stachura, D.; Roughley, P.; Antoniou, J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. 2006, 24, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Rowland, C.R.; Lennon, D.P.; Caplan, A.I.; Guilak, F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: Induction by growth factors and cartilage-derived matrix. Tissue Eng. Part A 2010, 16, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, G.D.; Liu, W.; Zhang, W.J.; Cui, L.; Liu, X.; Liu, T.Y.; Cao, Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials 2008, 29, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Proffen, B.; Kunz, M.; Hendrich, C.; Ghivizzani, S.C.; Nöth, U.; Rethwilm, A.; Eulert, J.; Evans, C.H. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res. Ther. 2009, 11, R148. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Narusawa, K.; Kobayashi, S.; Shiraki, M.; Horie-Inoue, K.; Sasaki, N.; Hosoi, T.; Ouchi, Y.; Nakamura, T.; Inoue, S. Association of HTRA1 promoter polymorphism with spinal disc degeneration in Japanese women. J. Bone Miner. Metab. 2010, 28, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Golshirazian, I.; Asbury, B.J.; Li, Y. Induction of high temperature requirement A1, a serine protease, by TGF-β1 in articular chondrocytes of mouse models of OA. Histol. Histopathol. 2014, 29, 609–618. [Google Scholar] [PubMed]
- Bau, B.; Gebhard, P.M.; Haag, J.; Knorr, T.; Bartnik, E.; Aigner, T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.W.; Henderson, M.L.; Stockdale, C.E.; Farrell, J.T.; Kooyman, D.L.; Bridgewater, L.C.; Seegmiller, R.E. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: A new model of osteoarthritis. Osteoarthr. Cartil. 2012, 20, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, R.B.; Østrup, E.; Zhang, X.; Mikkelsen, T.S.; Brinchmann, J.E. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS ONE 2014, 9, e96615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Palomäki, S.; Pietilä, M.; Laitinen, S.; Pesälä, J.; Sormunen, R.; Lehenkari, P.; Koivunen, P. HIF-1α is Upregulated in Human Mesenchymal Stem Cells. Stem Cells 2013, 31, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Cho, H.; Johnstone, B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res. Ther. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Hirao, M.; Tamai, N.; Tsumaki, N.; Yoshikawa, H.; Myoui, A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J. Biol. Chem. 2006, 281, 31079–31092. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T.; Heppenstall, R.B. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J. Bone Jt. Surg. Am. 1971, 53, 719–728. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; De Vienne, C.; Herlicoviez, M.; Galéra, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. https://doi.org/10.3390/ijms18091933
Gómez-Leduc T, Desancé M, Hervieu M, Legendre F, Ollitrault D, De Vienne C, Herlicoviez M, Galéra P, Demoor M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. International Journal of Molecular Sciences. 2017; 18(9):1933. https://doi.org/10.3390/ijms18091933
Chicago/Turabian StyleGómez-Leduc, Tangni, Mélanie Desancé, Magalie Hervieu, Florence Legendre, David Ollitrault, Claire De Vienne, Michel Herlicoviez, Philippe Galéra, and Magali Demoor. 2017. "Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges" International Journal of Molecular Sciences 18, no. 9: 1933. https://doi.org/10.3390/ijms18091933