The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Calcium Signaling in Cells
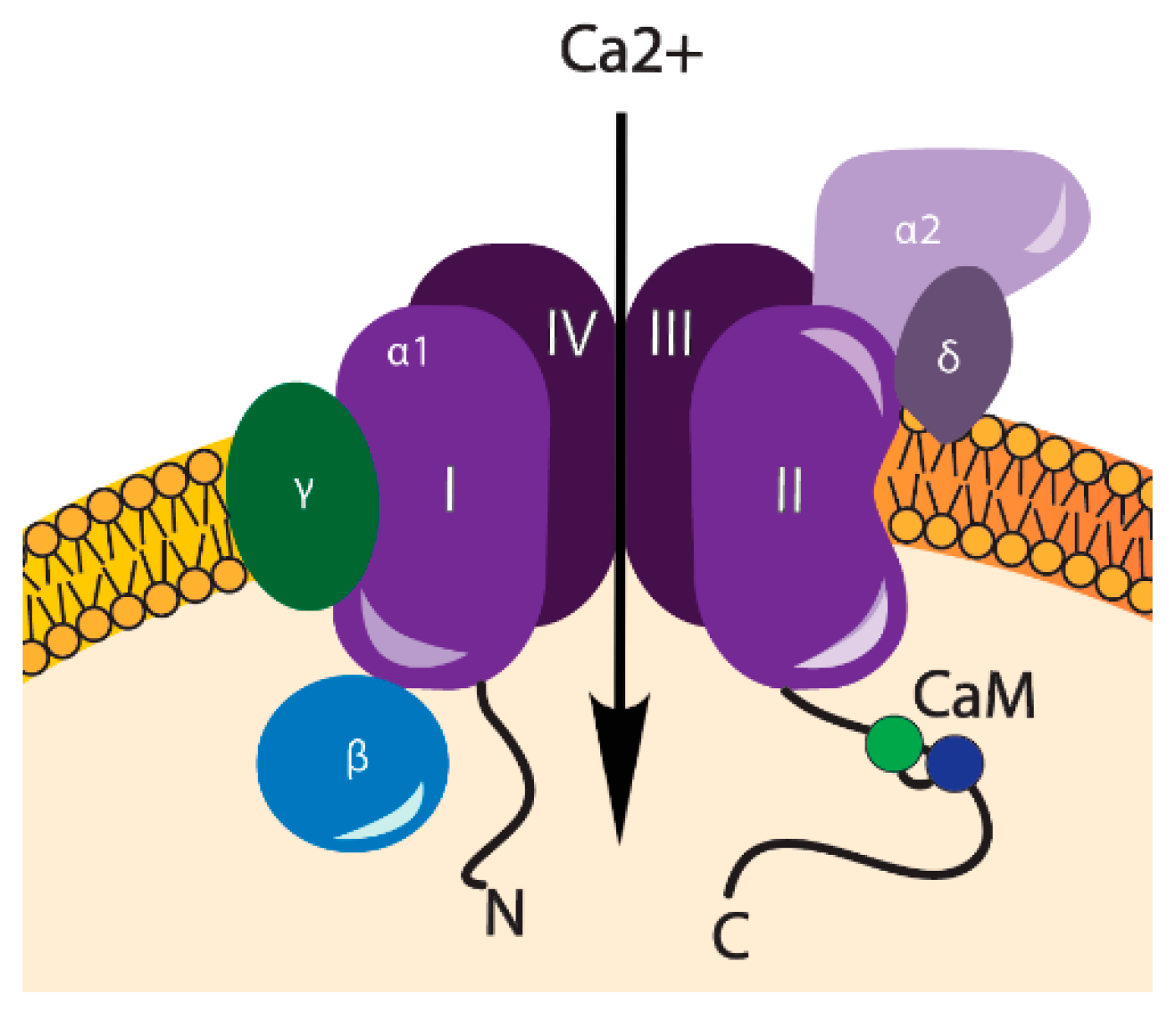
3. VOCCs and Their Regulation
4. Transient Receptor Potential Channels
5. Purinergic Receptors (P2X4)
6. Calcium Oscillations during Chondrogenic Differentiation of MSCs
7. Stimulation of Chondrogenic Differentiation with Electric Fields
8. Magnetic and Electromagnetic Fields Stimulate Chondrogenic Differentiation via Calcium Channels
9. Stimulation of Chondrogenic Differentiation by Mechanical Forces
10. Conclusions
Different Stimuli Result in Increased Intracellular Calcium Leading To Similar Effects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BK | Large Conductance Calcium-activated potassium channels |
| SK | Small Conductance Calcium-activated potassium channels |
| ECM | Extracellular matrix |
| ES | Electrical stimulation |
| ADSC | Adipose-derived stem cells |
| EMS | Electromagnetic stimulation |
| EMF | Electromagnetic field |
| GAG | Glycosaminoglycan |
| PEMF | Pulsed electromagnetic field |
| MF | Magnetic field |
| Ca2+ | Calcium ions |
| CaM | Calmodulin |
| HVA | High-voltage activated |
| InsP3R | Inositol 1,4,5-triphosphate receptor |
| KV | Voltage activated potassium channels |
| KATP | ATP Sensitive Potassium channels |
| LVA | Low-voltage activated |
| MSCs | Mesenchymal stem cells |
| Na+ | Sodium ions |
| OA | Osteoarthritis |
| P2X4 | Purinergic receptors, associated with mechanotransduction |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKC | Protein kinase C |
| RyR | Ryanodine receptor |
| TMs | Transmembrane domains |
| TRP | Transient receptor potential channels |
| VOCCs | Voltage-operated calcium channels |
References
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A. Cell sources for the regeneration of articular cartilage: The past, the horizon and the future. Int. J. Exp. Pathol. 2012, 93, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Danišovič, L.; Varga, I.; Polák, Š. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell 2012, 44, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Shoji, S.; Ichinose, S.; Yamagata, K.; Tagami, M.; Hiraoka, M. Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium 2002, 32, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Viti, F.; Landini, M.; Mezzelani, A.; Petecchia, L.; Milanesi, L.; Scaglione, S. Osteogenic differentiation of MSC through calcium signaling activation: Transcriptomics and functional analysis. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Rietdorf, K.; Hardy, H.; Dautova, Y.; Corps, E.; Pierro, C.; Stapleton, E.; Kang, E.; Proudfoot, D. Calcium Calcium signalling and regulation of cell function. eLS 2012. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Ye, B. Ca2+ oscillations and its transporters in mesenchymal stem cells. Physiol. Res. 2010, 59, 323–329. [Google Scholar] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gamal El-Din, T.M.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 2013, 505, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Snutch, T.P.; Peloquin, J.; Mathews, E.; Mcrory, J.E. Molecular Properties of Voltage-Gated Calcium Channels. In Voltage Gated Calcium Channels; Landes Bioscience: Austin, TX, USA, 2005; pp. 61–94. [Google Scholar]
- Sasaki, S.; Kanzaki, M.; Kaneko, T. Calcium influx through TRP channels induced by short-lived reactive species in plasma-irradiated solution. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Findeisen, F.; Minor, D.L. Calmodulin Interactions with Ca v 1 and Ca v 2 Voltage-Gated Calcium Channel IQ Domains. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1, pp. 1–18. ISBN 047086981X. [Google Scholar]
- Wakamori, M.; Imoto, K. Voltage-gated calcium channels. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: Boston, MA, USA, 2009; pp. 543–558. [Google Scholar]
- Simms, B.A.; Zamponi, G.W. Neuronal Voltage-Gated Calcium Channels: Structure, Function, and Dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Mcnulty, A.L.; Leddy, H.A.; Liedtke, W.; Guilak, F.; Care, P. TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, S.; Wakabayashi, M.; Ohno, T.; Amano, K.; Ooishi, R.; Sugahara, T.; Shiojiri, S.; Tashiro, K.; Suzuki, Y.; Nishimura, R.; et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J. Biol. Chem. 2007, 282, 32158–32167. [Google Scholar] [CrossRef] [PubMed]
- Torossian, F.; Bisson, A.; Vannier, J.-P.; Boyer, O.; Lamacz, M. TRPC expression in mesenchymal stem cells. Cell. Mol. Biol. Lett. 2010, 15, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G.; Boeynaems, J.-M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, J.; Matta, C.; Juhász, T.; Oláh, T.; Gönczi, M.; Szíjgyártó, Z.; Gergely, P.; Csernoch, L.; Zákány, R. Ionotropic purinergic receptor P2X4 is involved in the regulation of chondrogenesis in chicken micromass cell cultures. Cell Calcium 2009, 45, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, S.; Clauss, W.G.; Fronius, M. Laminar shear stress modulates the activity of heterologously expressed P2X4 receptors. Biochim. Biophys. Acta 2011, 1808, 2488–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahanich, I.; Graf, E.M.; Heubach, J.F.; Hempel, U.; Boxberger, S.; Ravens, U. Molecular and functional expression of voltage-operated calcium channels during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2005, 20, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Duriez, J.; Flautre, B.; Blary, M.C.; Hardouin, P. Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: A study in rabbit. Calcif. Tissue Int. 1993, 52, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Koori, K.; Maeda, H.; Fujii, S.; Tomokiyo, A.; Kawachi, G.; Hasegawa, D.; Hamano, S.; Sugii, H.; Wada, N.; Akamine, A. The roles of calcium-sensing receptor and calcium channel in osteogenic differentiation of undifferentiated periodontal ligament cells. Cell Tissue Res. 2014, 357, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A. narrative review. Jt. Bone Spine 2018. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.; Kelly, D.; Wagner, D. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. Eur. Cells Mater. 2014, 28, 358–371. [Google Scholar] [CrossRef]
- Matta, C.; Zakany, R. Calcium signalling in chondrogenesis: Implications for cartilage repair. Front. Biosci. (Schol. Ed.) 2013, 5, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Mobasheri, A. Regulation of chondrogenesis by protein kinase C: Emerging new roles in calcium signalling. Cell Signal. 2014, 26, 979–1000. [Google Scholar] [CrossRef] [PubMed]
- Valhmu, W.B.; Raia, F.J. Myo-Inositol 1,4,5-trisphosphate and Ca2+/calmodulin-dependent factors mediate transduction of compression-induced signals in bovine articular chondrocytes. Biochem. J. 2002, 361. [Google Scholar] [CrossRef]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Matta, C.; Oláh, T.; Juhász, T.; Takács, R.; Tóth, A.; Dienes, B.; Csernoch, L.; Zákány, R. Cell Calcium Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium 2013, 54, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-S.; Tzeng, B.-H.; Lee, K.-R.; Smith, R.J.H.; Campbell, K.P.; Chen, C.-C. Cav3.2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E1990–E1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Mayer-Wagner, S.; Passberger, A.; Sievers, B.; Aigner, J.; Summer, B.; Schiergens, T.S.; Jansson, V.; Müller, P.E. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics 2011, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Dong, L.; Zhang, B.; Qi, N. Effects of extremely low-frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagn. Biol. Med. 2010, 29, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Florin, C.; Muriel, G.; Eugenia, K.; Justin, T. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2017, 251, 221–228. [Google Scholar] [CrossRef]
- McCullen, S.D.; McQuilling, J.P.; Grossfeld, R.M.; Lubischer, J.L.; Clarke, L.I.; Loboa, E.G. Application of Low-Frequency Alternating Current Electric Fields via Interdigitated Electrodes: Effects on Cellular Viability, Cytoplasmic Calcium, and Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Tissue Eng. Part C Methods 2010, 16, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Liburdy, R.P.; Callahan, D.E.; Harland, J.; Dunham, E.; Sloma, T.R.; Yaswen, P. Experimental-Evidence for 60-Hz Magnetic-Fields Operating through the Signal-Transduction Cascade—Effects on Calcium Influx and C-Myc Messenger-Rna Induction. FEBS Lett. 1993, 334, 301–308. [Google Scholar] [CrossRef]
- Ross, C.L.; Siriwardane, M.; Christopher, D.; Brink, P.; Christ, G.J.; Harrison, B.S.; Brook, S. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015, 15, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fixler, D.; Yitzhaki, S.; Axelrod, A.; Zinman, T.; Shainberg, A. Correlation of magnetic AC field on cardiac myocyte Ca2+ transients at different magnetic DC levels. Bioelectromagnetics 2012, 33, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Tekieh, T.; Sasanpour, P.; Rafii-Tabar, H. Effects of electromagnetic field exposure on conduction and concentration of voltage gated calcium channels: A Brownian dynamics study. Brain Res. 2016, 1646, 560–569. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Hu, X.; Zhang, X.; Duan, X.; Yang, P.; Zhao, F.; Ao, Y. Effects of mechanical stress on chondrocyte phenotype and chondrocyte extracellular matrix expression. Sci. Rep. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Farrell, M.J.; Kim, M.; Mauck, R.L. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogels. Eur. Cells Mater. 2010, 19, 72–85. [Google Scholar] [CrossRef]
- Guilak, F.; Zell, R.A.; Erickson, G.R.; Grande, D.A.; Rubin, C.T.; McLeod, K.J.; Donahue, H.J. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J. Orthop. Res. 1999, 17, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; Van Der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Grad, S.; Wimmer, M.; Alini, M. The Influence of mechanical stimuli on articular cartilage tissue engineering. Tissue Eng. 2006, 2, 1–32. [Google Scholar]
- Liu, C.; Montell, C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, W. TRPV channels’ role in osmotransduction and mechanotransduction. Handb. Exp. Pharmacol. 2007, 179, 473–487. [Google Scholar] [CrossRef]

| VOCCs | Type: | α1 Subunits: | References: |
|---|---|---|---|
| LVA | T-type | CaV3.1, CaV3.2, CaV3.3 | [18,19] |
| HVA | L-type | CaV1.1, CaV1.2, CaV1.3, CaV1.4 | [20,9] |
| N-type | CaV2.1 | ||
| P/Q-type | CaV2.2 | ||
| R-type | CaV2.3 |
| Stimuli | Cell Type | Application | Effect | Channel/Inhibitor | Chondrogenic Response | References |
|---|---|---|---|---|---|---|
| Electric stimulation | ADSC | Electric stimulation, 1 KHz, 20 mv/cm, 20 min/day, 7 days | Increased expression of chondrogenic differentiation markers | VOCCs | positive | [41] |
| Electromagnetic stimulation | BMMSC | EMF, 15 Hz, 5 mT, 45 min every 8 h | Increased expression of chondrogenic differentiation markers | VOCCs | positive | [42] |
| BMMSC | PEMF, 15 Hz, 2 mT, 10 min, daily | Increased expression of chondrogenic differentiation markers | TRPs/10 μM Ruthenium Red; 100 µM 2-APB | positive | [10] | |
| BMMSC | EMF, 15 Hz, 5 mT | Increased expression of chondrogenic differentiation markers | Unclear | positive | [42] | |
| Magnetic field | BMMSC | MF, 50 Hz, 20 mT | Inhibited MSC growth | Unclear | negative | [43] |
| Mechanical forces | Bone marrow MSCs | Hydrostatic pressure, 10 MPa at a frequency of 1 Hz, 4 h/d, 5 d/week for 3 weeks | Enhanced chondrogenic gene expression | VOCCs/10 μM verapamil | positive | [34] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. https://doi.org/10.3390/ijms19102998
Uzieliene I, Bernotas P, Mobasheri A, Bernotiene E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2018; 19(10):2998. https://doi.org/10.3390/ijms19102998
Chicago/Turabian StyleUzieliene, Ilona, Paulius Bernotas, Ali Mobasheri, and Eiva Bernotiene. 2018. "The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells" International Journal of Molecular Sciences 19, no. 10: 2998. https://doi.org/10.3390/ijms19102998





