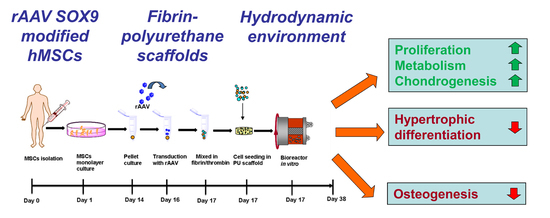
Improved Chondrogenic Differentiation of rAAV SOX9-Modified Human MSCs Seeded in Fibrin-Polyurethane Scaffolds in a Hydrodynamic Environment
Abstract
:1. Introduction
2. Results
2.1. rAAV-Mediated SOX9 Overexpression in Human Mesenchymal Stem Cells Seeded in Polyurethane Scaffolds in a Hydrodynamic Environment
2.2. Effects of SOX9 Overexpression upon the Biological and Chondrogenic Activities of hMSCs Seeded in PU Scaffolds in a Hydrodynamic Environment
2.3. Effects of SOX9 Overexpression upon the Hypertrophic and Osteogenic Activities of hMSCs Seeded in PU Scaffolds in a Hydrodynamic Environment
2.4. Real-Time RT-PCR Analyses in rAAV-Mediated SOX9-Overexpressing hMSCs Seeded in PU Scaffolds in a Hydrodynamic Environment
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Plasmids and rAAV Vectors
4.4. rAAV-Mediated Gene Transfer and Hydrodynamic Bioreactor Culture
4.5. Histology, Immunocytochemistry, and Immunohistochemistry
4.6. Histomorphometry
4.7. Biochemical Assays
4.8. Real-Time RT-PCR Analyses
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.; Shintani, N. An educational review of cartilage repair: Precepts & practice–myths & misconceptions–progress & prospects. Osteoarthritis Cartilage 2015, 23, 334–350. [Google Scholar] [PubMed]
- Safran, M.R.; Seiber, K. The evidence for surgical repair of articular cartilage in the knee. J. Am. Acad. Orthop. Surg. 2010, 18, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H.; Guilak, F.; Saris, D.B.; Stoddart, M.J.; Koon Wong, M.; Roughley, P. A vision on the future of articular cartilage repair. Eur. Cell Mater. 2014, 27, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Joint Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Okabe, T.; Horibe, S.; Mitsuoka, T.; Saito, M.; Koyama, T.; Nawata, M.; Tensho, K.; Kato, H.; Uematsu, K.; et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.D.; Kopesky, P.W.; Evans, C.H.; Grodzinsky, A.J.; McIlwraith, C.W.; Frisbie, D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J. Orthop. Res. 2008, 26, 322–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, D.; Kujat, R.; Zellner, J.; Angele, M.K.; Nerlich, M.; Mayr, E.; Angele, P. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology 2006, 43, 431–443. [Google Scholar] [PubMed]
- Kupcsik, L.; Stoddart, M.J.; Li, Z.; Benneker, L.M.; Alini, M. Improving chondrogenesis: Potential and limitations of sox9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kupcsik, L.; Yao, S.J.; Alini, M.; Stoddart, M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng. Part A 2009, 15, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.J.; Alini, M.; Archer, C.W.; Stoddart, M.J. Chondrogenesis of human bone marrow-derived mesenchymal stem cells is modulated by complex mechanical stimulation and adenoviral-mediated overexpression of bone morphogenetic protein 2. Tissue Eng. Part A 2013, 19, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kaul, G.; Zurakowski, D.; Vunjak-Novakovic, G.; Cucchiarini, M. Cartilage constructs engineered from chondrocytes overexpressing igf-i improve the repair of osteochondral defects in a rabbit model. Eur. Cell Mater. 2013, 25, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Padera, R.; Seidel, J.; Langer, R.; Freed, L.E.; Trippel, S.B.; Vunjak-Novakovic, G. Gene transfer of a human insulin-like growth factor i cdna enhances tissue engineering of cartilage. Hum. Gene Ther. 2002, 13, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Madry, H.; Cucchiarini, M. Current progress in stem cell-based gene therapy for articular cartilage repair. Curr. Stem Cell Res. Ther. 2015, 10, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Orth, P.; Madry, H. Direct raav sox9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J. Mol. Med. 2013, 91, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions between sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.Y.; Gao, B.; Leung, K.K.; Melhado, I.G.; Wynn, S.L.; Au, T.Y.; Dung, N.W.; Lau, J.Y.; Mak, A.C.; Chan, D.; et al. Sox9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 2011, 7, e1002356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchiarini, M.; Ekici, M.; Schetting, S.; Kohn, D.; Madry, H. Metabolic activities and chondrogenic differentiation of human mesenchymal stem cells following recombinant adeno-associated virus-mediated gene transfer and overexpression of fibroblast growth factor 2. Tissue Eng. Part A 2011, 17, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-beta via recombinant adeno-associated viral vectors. Hum. Gene Ther. 2014, 25, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M. Influence of insulin-like growth factor i overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Pagnotto, M.R.; Wang, Z.; Karpie, J.C.; Ferretti, M.; Xiao, X.; Chu, C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007, 14, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Ekici, M.; Madry, H.; Schmitt, G.; Kohn, D.; Cucchiarini, M. Sox9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res. Ther. 2012, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Schmitt, G.; Monge-Marcet, A.; Lopez-Chicon, P.; Mata, A.; Semino, C.; Madry, H.; Cucchiarini, M. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of raav vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Grad, S.; Gorna, K.; Gogolewski, S.; Goessl, A.; Alini, M. Fibrin-polyurethane composites for articular cartilage tissue engineering: A preliminary analysis. Tissue Eng. 2005, 11, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Wezel, A.; Madry, H.; Cucchiarini, M. Raav-mediated overexpression of tgf-beta stably restructures human osteoarthritic articular cartilage in situ. J. Transl. Med. 2013, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Babister, J.C.; Tare, R.S.; Green, D.W.; Inglis, S.; Mann, S.; Oreffo, R.O. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials 2008, 29, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Kitoh, H.; Sugiura, F.; Ishiguro, N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2003, 301, 338–343. [Google Scholar] [CrossRef]
- Cao, L.; Yang, F.; Liu, G.; Yu, D.; Li, H.; Fan, Q.; Gan, Y.; Tang, T.; Dai, K. The promotion of cartilage defect repair using adenovirus mediated sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011, 32, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Guo, X.M.; Tan, H.S.; Hui, J.H.; Lim, B.; Lee, E.H. Zinc-finger protein 145, acting as an upstream regulator of sox9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011, 63, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, D.C.; Bai, J.Y.; Kang, N.; Feng, J.Y.; Yang, Z.Q. Overexpression of sox9 gene by the lentiviral vector in rabbit bone marrow mesenchymal stem cells for promoting the repair of cartilage defect. Zhongguo Gu Shang 2015, 28, 433–440. [Google Scholar] [PubMed]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yu, L.; Lim, C.G.; Goodley, A.S.; Xiao, X.; Placone, J.K.; Ferlin, K.M.; Nguyen, B.B.; Hsieh, A.H.; Fisher, J.P. Effect of dynamic culture and periodic compression on human mesenchymal stem cell proliferation and chondrogenesis. Ann. Biomed. Eng. 2016, 44, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Belkin, N.S.; Milby, A.H.; Henning, E.A.; Bostrom, M.; Kim, M.; Pfeifer, C.; Meloni, G.; Dodge, G.R.; Burdick, J.A.; et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: Implications for tissue engineering. Tissue Eng. Part A 2015, 21, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.; Dearman, B.L.; Wagstaff, M.J.; Greenwood, J.E. A novel biodegradable polyurethane matrix for auricular cartilage repair: An in vitro and in vivo study. J. Burn Care Res. 2016, 37, e353–e364. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [PubMed]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [PubMed]
- Freed, L.E.; Vunjak-Novakovic, G. Cultivation of cell-polymer tissue constructs in simulated microgravity. Biotechnol. Bioeng. 1995, 46, 306–313. [Google Scholar] [CrossRef] [PubMed]






| Parameter | rAAV-lacZ | rAAV-FLAG-hsox9 |
|---|---|---|
| SOX9 immunostaining | 0.8 (0.5) | 2.8 (0.5) * |
| Toluidine blue staining | 1.8 (0.5) | 2.5 (0.6) * |
| Aggrecan immunostaining | 0.8 (0.3) | 2.1 (0.6) * |
| Type-II collagen immunostaining | 0.9 (0.1) | 2.4 (0.2) * |
| Type-I collagen immunostaining | 2.4 (0.5) | 0.6 (0.2) * |
| Type-X collagen immunostaining | 4.0 (0.1) | 1.3 (0.5) * |
| Alizarin red staining | 3.3 (0.5) | 1.1 (0.5)* |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, J.K.; Gardner, O.; Rey-Rico, A.; Eglin, D.; Alini, M.; Stoddart, M.J.; Cucchiarini, M.; Madry, H. Improved Chondrogenic Differentiation of rAAV SOX9-Modified Human MSCs Seeded in Fibrin-Polyurethane Scaffolds in a Hydrodynamic Environment. Int. J. Mol. Sci. 2018, 19, 2635. https://doi.org/10.3390/ijms19092635
Venkatesan JK, Gardner O, Rey-Rico A, Eglin D, Alini M, Stoddart MJ, Cucchiarini M, Madry H. Improved Chondrogenic Differentiation of rAAV SOX9-Modified Human MSCs Seeded in Fibrin-Polyurethane Scaffolds in a Hydrodynamic Environment. International Journal of Molecular Sciences. 2018; 19(9):2635. https://doi.org/10.3390/ijms19092635
Chicago/Turabian StyleVenkatesan, Jagadeesh K., Oliver Gardner, Ana Rey-Rico, David Eglin, Mauro Alini, Martin J. Stoddart, Magali Cucchiarini, and Henning Madry. 2018. "Improved Chondrogenic Differentiation of rAAV SOX9-Modified Human MSCs Seeded in Fibrin-Polyurethane Scaffolds in a Hydrodynamic Environment" International Journal of Molecular Sciences 19, no. 9: 2635. https://doi.org/10.3390/ijms19092635