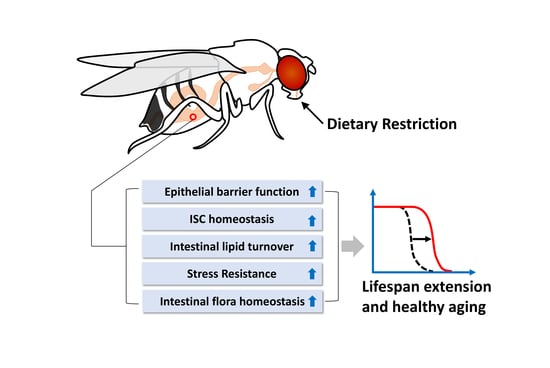
Drosophila Gut—A Nexus Between Dietary Restriction and Lifespan
Abstract
:1. Introduction
2. Gut function During DR-Induced Longevity
2.1. Epithelial Homeostasis with Aging
2.1.1. DR and DR Mimetics Improve Gut Epithelial Homeostasis
2.1.2. Pathways
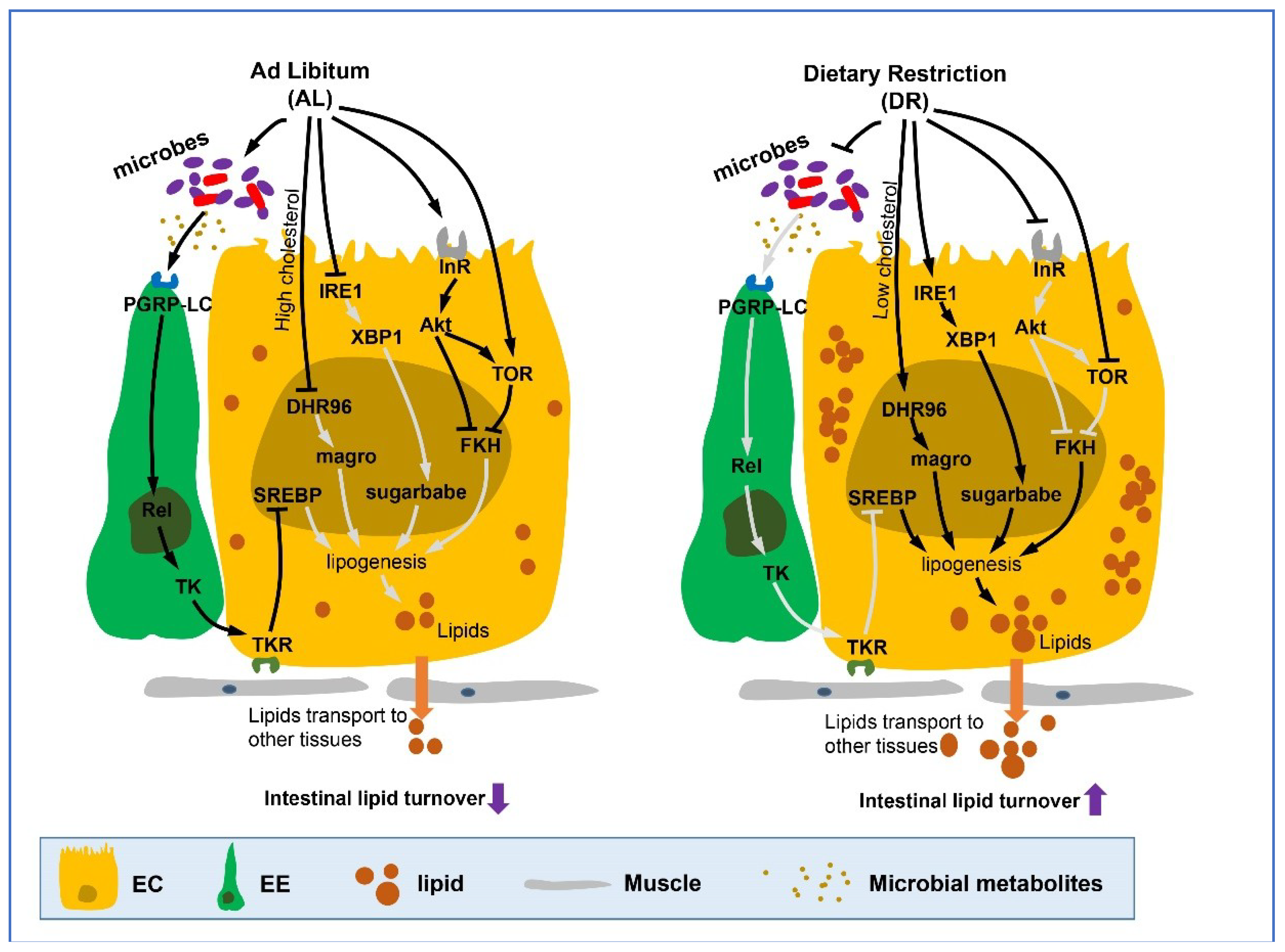
2.2. Intestinal Lipid Homeostasis
2.2.1. DR Maintains Intestinal Lipid Homeostasis
2.3. DR Improves the Intestinal Oxidative Stress Resistance
2.4. How Gut-Other Organs Communication Contributes to the Benefits of DR
2.4.1. Gut-Brain
2.4.2. Gut-Fat Body
3. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| AMPs | Antimicrobial peptides |
| Atg1 | Autophagy-specific gene 1 |
| CCK | Cholecystokinin |
| DMC | 2,5-dimethyl-celecoxib |
| DR | Dietary restriction |
| EB | Enteroblast |
| EC | Enterocyte |
| EE | Enteroendocrine cell |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| FFA | Free-fatty acid |
| Fkh | Forkhead |
| FOXO | Forkhead boxO |
| GLP | Glucagon-like peptide |
| HSC | Hematopoietic stem cell |
| IBD | Inflammatory bowel disease |
| IIS | Insulin/IGF-1 signaling pathway |
| Ilps | Insulin-like peptides |
| IMD | Immune Deficiency |
| InR | Insulin/IGF-1 like tyrosine kinase receptor |
| IPC | Insulin producing cell |
| ISC | Intestinal stem cell |
| JNK | Jun N-terminal kinase |
| mTOR | Mechanistic target of rapamycin |
| PGRP | Peptidoglycan-recognition protein |
| PI3K | Phosphatidylinositol 3-kinase |
| PKA | Protein kinase A |
| ROS | Reactive oxygen species |
| SREBP | Sterol regulatory element-binding protein |
| TAG | Triacylglycerol |
| TCJ | Tricellular junction |
| TGF-β | Transforming growth factor β |
| TK | Tachykinin |
| TSC2 | Tuberous sclerosis complex protein 2 |
References
- S Hikida, R. Aging changes in satellite cells and their functions. Curr. Aging Sci. 2011, 4, 279–297. [Google Scholar] [CrossRef]
- Neves, J.; Demaria, M.; Campisi, J.; Jasper, H. Of flies, mice, and men: Evolutionarily conserved tissue damage responses and aging. Dev. Cell 2015, 32, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Resnik-Docampo, M.; Koehler, C.L.; Clark, R.I.; Schinaman, J.M.; Sauer, V.; Wong, D.M.; Lewis, S.; D’Alterio, C.; Walker, D.W.; Jones, D.L. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat. Cell Boil. 2016, 19, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Bandura, J.; Zhang, P.; Jin, Y.; Reuter, H.; Edgar, B.A. Egfr-dependent tor-independent endocycles support Drosophila gut epithelial regeneration. Nat. Commun. 2017, 8, 15125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, L.H. Organ-to-organ communication: A Drosophila gastrointestinal tract perspective. Front. Cell Dev. Boil. 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Murillo, F.H.; Bellver-Pérez, A.; Gianotten, W.L. Sexuality and cancer in the aged/aging population. In Cancer, Intimacy and Sexuality; Springer: New York, NY, USA, 2017; pp. 257–266. [Google Scholar]
- Paneni, F.; Cañestro, C.D.; Libby, P.; Lüscher, T.F.; Camici, G.G. The aging cardiovascular system: Understanding it at the cellular and clinical levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.J.-K.; Jasper, H. Epithelia: Understanding the cell biology of intestinal barrier dysfunction. Curr. Boil. 2017, 27, R185–R187. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.D.; Weber, D.; Day, N.; Vitelli, C.; Crippen, D.; Herndon, L.A.; Hall, D.H.; Melov, S. Loss of intestinal nuclei and intestinal integrity in aging C. Elegans. Aging Cell 2011, 10, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef] [PubMed]
- Steegenga, W.T.; de Wit, N.J.; Boekschoten, M.V.; IJssennagger, N.; Lute, C.; Keshtkar, S.; Bromhaar, M.M.G.; Kampman, E.; de Groot, L.C.; Muller, M. Structural, functional and molecular analysis of the effects of aging in the small intestine and colon of C57BL/6J mice. BMC Med Genom. 2012, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Davis, A.; Brass, K.; Dendinger, M.; Barner, R.; Gharaibeh, R.; Fodor, A.; Kavanagh, K. Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J. Nutr. Health Aging 2017, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. The current future understanding of inflammatory bowel disease. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Longo, V. Dietary restriction with and without caloric restriction for healthy aging. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- gStrilbytska, O.M.; Semaniuk, U.V.; Storey, K.B.; Edgar, B.A.; Lushchak, V. Activation of the TOR/MYC signaling axis in intestinal stem and progenitor cells affects longevity, stress resistance and metabolism in Drosophila. Comp. Biochem. Physiol. Part B Biochem. Mol. Boil. 2017, 203, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Luis, N.M.; Wang, L.; Ortega, M.; Deng, H.; Katewa, S.D.; Li, P.W.-L.; Karpac, J.; Jasper, H.; Kapahi, P. Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep. 2016, 17, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Akagi, K.; Bose, N.; Rakshit, K.; Camarella, T.; Zheng, X.; Hall, D.; Davis, S.; Nelson, C.S.; Brem, R.B. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab. 2016, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Droujinine, I.A.; Perrimon, N. Interorgan communication pathways in physiology: Focus on Drosophila. Annu. Rev. Genet. 2016, 50, 539–570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Y.; Jasper, H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe 2016, 19, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta 2018, 1864, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Jasper, H. Exploring the physiology and pathology of aging in the intestine of Drosophila melanogaster. Invertebr. Reprod. Dev. 2015, 59, 51–58. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.E.; Soliman, S.S.; Li, X.; Bilder, D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 2011, 147, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Karpac, J.; Supoyo, S.; DeGennaro, M.; Lehmann, R.; Jasper, H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010, 6, e1001159. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Hochmuth, C.E.; Jasper, H. Maintaining tissue homeostasis: Dynamic control of somatic stem cell activity. Cell Stem Cell 2011, 9, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Micchelli, C.A.; Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, B.; Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2006, 439, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Gervais, L.; Bardin, A.J. Tissue homeostasis and aging: New insight from the fly intestine. Curr. Opin. Cell Boil. 2017, 48, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lucchetta, E.; Rafel, N.; Ohlstein, B. Maintenance of the adult Drosophila intestine: All roads lead to homeostasis. Curr. Opin. Genet. Dev. 2016, 40, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gaur, U.; Yang, M. Intestinal homeostasis and longevity: Drosophila gut feeling. Adv. Exp. Med. Boil. 2018, 1086, 157–168. [Google Scholar]
- Biteau, B.; Hochmuth, C.E.; Jasper, H. Jnk activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 2008, 3, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lian, T.; Fan, X.; Song, C.; Gaur, U.; Mao, X.; Yang, D.; Piper, M.D.; Yang, M. 2,5-dimethyl-celecoxib extends Drosophila life span via a mechanism that requires insulin and target of rapamycin signaling. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 72, 1334–1341. [Google Scholar]
- Fan, X.; Liang, Q.; Lian, T.; Wu, Q.; Gaur, U.; Li, D.; Yang, D.; Mao, X.; Jin, Z.; Li, Y. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget 2015, 6, 35274–35283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jasper, H. Gastrointestinal stem cells in health and disease: From flies to humans. Dis. Model. Mech. 2016, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Rauchhaus, M.; Anker, S.D.; von Haehling, S. The emerging role of the gut in chronic heart failure. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Shea-Donohue, T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; William, W.J. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, U.; Fan, X.; Yang, M. Rapamycin slows down gut aging. Aging (Albany NY) 2016, 8, 833. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.C.; Khericha, M.; Dobson, A.J.; Bolukbasi, E.; Rattanavirotkul, N.; Partridge, L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 2016, 5, e10956. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Jasper, H. You are what you eat: Linking high-fat diet to stem cell dysfunction and tumorigenesis. Cell Stem Cell 2016, 18, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991, 325, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Kapuria, S.; Karpac, J.; Biteau, B.; Hwangbo, D.; Jasper, H. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 2012, 8, e1003045. [Google Scholar] [CrossRef] [PubMed]
- Post, S.; Tatar, M. Nutritional geometric profiles of insulin/IGF expression in Drosophila melanogaster. PLoS ONE 2016, 11, e0155628. [Google Scholar] [CrossRef] [PubMed]
- Essers, P.; Tain, L.S.; Nespital, T.; Goncalves, J.; Froehlich, J.; Partridge, L. Reduced insulin/insulin-like growth factor signaling decreases translation in Drosophila and mice. Sci. Rep. 2016, 6, 30290. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of chico, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Tain, L.S.; Sehlke, R.; Jain, C.; Chokkalingam, M.; Nagaraj, N.; Essers, P.; Rassner, M.; Gronke, S.; Froelich, J.; Dieterich, C.; et al. A proteomic atlas of insulin signalling reveals tissue-specific mechanisms of longevity assurance. Mol. Syst. Biol. 2017, 13, 939. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, E.; Khericha, M.; Regan, J.C.; Ivanov, D.K.; Adcott, J.; Dyson, M.C.; Nespital, T.; Thornton, J.M.; Alic, N.; Partridge, L. Intestinal fork head regulates nutrient absorption and promotes longevity. Cell Rep. 2017, 21, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. Daf-16: An HNF-3/forkhead family member that can function to double the life-span of caenorhabditis elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. Elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.; Alic, N.; Foley, A.; Cabecinha, M.; Hoddinott, M.P.; Partridge, L. The ras-erk-ets-signaling pathway is a drug target for longevity. Cell 2015, 162, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, M.E.; Goss, M.; Partridge, L. Role of dfoxo in lifespan extension by dietary restriction in Drosophila melanogaster: Not required, but its activity modulates the response. Aging Cell 2008, 7, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C. Foxos are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Bryder, D.; Zahn, J.M.; Ahlenius, H.; Sonu, R.; Wagers, A.J.; Weissman, I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 2005, 102, 9194–9199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F.; Takubo, K.; Yamazaki, S.; Matsuoka, S.; Miyamoto, T.; Ito, K.; Ohmura, M. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007, 1, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.H.; Lucchetta, E.; Ohlstein, B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 18702–18707. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.; He, X.; Blanc, E.; Bolukbasi, E.; Feseha, Y.; Yang, M.; Piper, M. Ageing, tor and amino acid restriction: A cross-tissue transcriptional network connects gata factors to Drosophila longevity. bioRxiv 2016. [CrossRef]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Fernandez, E.; Flurkey, K.; Han, M.; Javors, M.A.; Li, X.; Nadon, N.L.; Nelson, J.F. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 2014, 13, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Müller, F. Genetics: Influence of tor kinase on lifespan in C. Elegans. Nature 2003, 426, 620. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.A.; Guarente, L. Genetic links between diet and lifespan: Shared mechanisms from yeast to humans. Nat. Rev. Genet. 2007, 8, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.; Dillin, A. Aging and survival: The genetics of life span extension by dietary restriction. Annu. Rev Biochem. 2008, 77, 727–754. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. Mtor signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Jasper, H.; Ho, T.T.; Passegué, E. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat. Cell Boil. 2016, 18, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Sun, P.; Lin, G.; Xi, R. TSC1/2 regulates intestinal stem cell maintenance and lineage differentiation through RHEB–TORC1–S6K but independently of nutritional status or notch regulation. J. Cell Sci. 2013, 126, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.H.; Katajisto, P.; Lamming, D.W.; Gültekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M. MTORC1 in the paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, M.; Guarente, L. Mtorc1 and sirt1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Lemaitre, B. Gut homeostasis in a microbial world: Insights from Drosophila melanogaster. Nat. Rev. Microbiol. 2013, 11, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.; Bülow, M.H.; Pesch, Y.-Y.; Loch, G.; Hoch, M. Forkhead, a new cross regulator of metabolism and innate immunity downstream of tor in Drosophila. J. Insect Physiol. 2014, 69, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 2009, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Kim, S.-H.; Lee, H.-Y.; Bai, J.Y.; Nam, Y.-D.; Bae, J.-W.; Lee, D.G.; Shin, S.C.; Ha, E.-M.; Lee, W.-J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 2008, 319, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Loch, G.; Zinke, I.; Mori, T.; Carrera, P.; Schroer, J.; Takeyama, H.; Hoch, M. Antimicrobial peptides extend lifespan in Drosophila. PLoS ONE 2017, 12, e0176689. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Fukagawa, K.; Iwakiri, R.; Fujimoto, K.; Akashi, K.; Tso, P. Increased lipid absorption and transport in the small intestine of zucker obese rats. J. Clin. Biochem. Nutr. 2009, 45, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Warnakula, S.; Hsieh, J.; Adeli, K.; Hussain, M.M.; Tso, P.; Proctor, S.D. New insights into how the intestine can regulate lipid homeostasis and impact vascular disease: Frontiers for new pharmaceutical therapies to lower cardiovascular disease risk. Can. J. Cardiol. 2011, 27, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J.; Pajukanta, P. A treasure trove for lipoprotein biology. Nat. Genet. 2008, 40, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, A.E.; Brufau, G.; Groen, A.K. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 2010, 21, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Veenstra, J.A.; Perrimon, N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014, 9, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Grönke, S.; Stubbs, T.M.; Ficz, G.; Hendrich, O.; Krueger, F.; Andrews, S.; Zhang, Q.; Wakelam, M.J.; Beyer, A. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Boil. 2017, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Demontis, F.; Kolipinski, M.; Hubbard, A.; Gill, M.S.; Perrimon, N.; Melov, S.; Kapahi, P. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012, 16, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.H.; Thummel, C.S. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila lipa homolog magro. Cell Metab. 2012, 15, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Karpac, J.; Biteau, B.; Jasper, H. Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell Rep. 2013, 4, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, Z.E.; Pickering, J.; Eskiw, C.H. Better living through chemistry: Caloric restriction (CR) and CR mimetics alter genome function to promote increased health and lifespan. Front. Genet. 2016, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Hahn, S.; Butschkau, A.; Lange, S.; Wree, A.; Vollmar, B. Lifelong caloric restriction reprograms hepatic fat metabolism in mice. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Weindruch, R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 2010, 21, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.H.; Thummel, C.S. The dhr96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009, 10, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Horner, M.A.; Pardee, K.; Liu, S.; King-Jones, K.; Lajoie, G.; Edwards, A.; Krause, H.M.; Thummel, C.S. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009, 23, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Bujold, M.; Gopalakrishnan, A.; Nally, E.; King-Jones, K. Nuclear receptor DHR96 acts as a sentinel for low cholesterol concentrations in Drosophila melanogaster. Mol. Cell. Boil. 2010, 30, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Reiher, W.; Shirras, C.; Kahnt, J.; Baumeister, S.; Isaac, R.E.; Wegener, C. Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 2011, 10, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A.; Sellami, A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008, 334, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Siviter, R.J.; Coast, G.M.; Winther, Å.M.; Nachman, R.J.; Taylor, C.A.; Shirras, A.D.; Coates, D.; Isaac, R.E.; Nässel, D.R. Expression and functional characterization of aDrosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J. Boil. Chem. 2000, 275, 23273–23280. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, D.R.; Johnson, B.; Presnell, J.S.; Catignas, K.K.; Zapotoczny, G. Peristalsis in the junction region of the Drosophila larval midgut is modulated by DH31 expressing enteroendocrine cells. BMC Physiol. 2010, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Birse, R.T.; Johnson, E.C.; Taghert, P.H.; Nässel, D.R. Widely distributed Drosophila G-protein-coupled receptor (CG7887) is activated by endogenous tachykinin-related peptides. J. Neurobiol. 2006, 66, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Birse, R.T.; Nachman, R.J.; Fichna, J.; Janecka, A.; Broeck, J.V.; Nässel, D.R. Characterization and distribution of nkd, a receptor for Drosophila tachykinin-related peptide 6. Peptides 2009, 30, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kamareddine, L.; Robins, W.P.; Berkey, C.D.; Mekalanos, J.J.; Watnick, P.I. The Drosophila immune deficiency pathway modulates enteroendocrine function and host metabolism. Cell Metab. 2018, 28, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Boil. 2007, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Volmer, R.; Ron, D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Boil. 2015, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Shan, B.; Liu, Y.; Deng, Y.; Yan, C.; Wu, Y.; Mao, T.; Qiu, Y.; Zhou, Y.; Jiang, S. Hepatic ire1 [alpha] regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARα axis signalling. Nat. Commun. 2014, 5, 3528. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; He, Y.; Chen, H.; Wang, C.; Zenno, A.; Shi, H.; Yang, X.; Zhang, X.; Qi, L. The ire1α-xbp1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009, 9, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The effects of dietary restriction on oxidative stress in rodents. Free. Radic. Boil. Med. 2014, 66, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wang, M.C.; Bohmann, D. JNK protects Drosophila from oxidative stress by trancriptionally activating autophagy. Mech. Dev. 2009, 126, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, X.; Ryoo, H.D.; Jasper, H. Integration of uprer and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 2014, 10, e1004568. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Poidevin, M.; Lemaitre, B. The Drosophila MAPK p38c regulates oxidative stress and lipid homeostasis in the intestine. PLoS Genet. 2014, 10, e1004659. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free. Radic. Boil. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Kim, S.-H.; Kim, E.-K.; Ha, E.-M.; You, H.; Kim, B.; Kim, M.-J.; Kwon, Y.; Ryu, J.-H.; Lee, W.-J. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 2013, 153, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ip, Y.T. Pathogenic stimulation of intestinal stem cell response in Drosophila. J. Cell. Physiol. 2009, 220, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Trindade de Paula, M.; Poetini Silva, M.R.; Machado Araujo, S.; Cardoso Bortolotto, V.; Barreto Meichtry, L.; Zemolin, A.P.P.; Wallau, G.L.; Jesse, C.R.; Franco, J.L.; Posser, T. High-fat diet induces oxidative stress and MPK2 and HSP83 gene expression in Drosophila melanogaster. Oxidative Med. Cell. Longev. 2016, 2016, 4018157. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Le Pécheur, M.; Tanguay, R.M. Drosophila melanogaster mitochondrial HSP22: A role in resistance to oxidative stress, aging and the mitochondrial unfolding protein response. Biogerontology 2016, 17, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Luo, J.; He, L.; Montell, C.; Perrimon, N. Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca2+ signaling in the Drosophila midgut. eLife 2017, 6, e22441. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. The interplay between respiratory supercomplexes and ros in aging. Antioxidants Redox Signal. 2015, 23, 208–238. [Google Scholar] [CrossRef] [PubMed]
- Gelino, S.; Chang, J.T.; Kumsta, C.; She, X.; Davis, A.; Nguyen, C.; Panowski, S.; Hansen, M. Intestinal autophagy improves healthspan and longevity in C. Elegans during dietary restriction. PLoS Genet. 2016, 12, e1006135. [Google Scholar]
- Ikeda, Y.; Shirakabe, A.; Brady, C.; Zablocki, D.; Ohishi, M.; Sadoshima, J. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J. Mol. Cell. Cardiol. 2015, 78, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. N. Y. Acad. Sci. 2008, 1147, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Galikova, M.; Klepsatel, P. Obesity and aging in the Drosophila model. Int. J. Mol. Sci. 2018, 19, 1896. [Google Scholar] [CrossRef] [PubMed]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Boil. 2001, 11, 213–221. [Google Scholar] [CrossRef]
- Broughton, S.J.; Slack, C.; Alic, N.; Metaxakis, A.; Bass, T.M.; Driege, Y.; Partridge, L. Dilp-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 2010, 9, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Yamamoto, R.; Buch, S.; Pankratz, M.; Tatar, M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell 2008, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Rana, A.; Rera, M.; Graniel, J.; Walker, D.W. Ampk modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014, 8, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.; Foley, A.; Partridge, L. Activation of ampk by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS ONE 2012, 7, e47699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B. An obligatory role for neurotensin in high fat diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Drayon, V.; Poidevin, M.; Boneca, I.G.; Narbonne-Reveau, K.; Royet, J.; Charroux, B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 2012, 12, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.C.; Welchman, D.P.; Poidevin, M.; Lemaitre, B. Negative regulation by amidase pgrps shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 2011, 35, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Zheng, Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell 2014, 159, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.S.B.; Schüpfer, F.; Lemaitre, B. Transforming growth factor β/activin signaling functions as a sugar-sensing feedback loop to regulate digestive enzyme expression. Cell Rep. 2014, 9, 336–348. [Google Scholar]
- Sousa-Pinto, B.; Gonçalves, L.; Rodrigues, A.; Tomada, I.; Almeida, H.; Neves, D.; Gouveia, A. Expression of TGF-beta in different adipose tissue depots is singularly regulated by a high-fat diet and energy restriction. In IJUP 2015 Book of Abstracts of the 8th Meeting of Young Researchers of University of Porto/Livro de resumos do 8.° Encontro de Investigação Jovem da U. Porto; University of Porto: Porto, Portugal, 2015. [Google Scholar]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2011, 4, 21–30. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, T.; Wu, Q.; Hodge, B.A.; Wilson, K.A.; Yu, G.; Yang, M. Drosophila Gut—A Nexus Between Dietary Restriction and Lifespan. Int. J. Mol. Sci. 2018, 19, 3810. https://doi.org/10.3390/ijms19123810
Lian T, Wu Q, Hodge BA, Wilson KA, Yu G, Yang M. Drosophila Gut—A Nexus Between Dietary Restriction and Lifespan. International Journal of Molecular Sciences. 2018; 19(12):3810. https://doi.org/10.3390/ijms19123810
Chicago/Turabian StyleLian, Ting, Qi Wu, Brian A. Hodge, Kenneth A. Wilson, Guixiang Yu, and Mingyao Yang. 2018. "Drosophila Gut—A Nexus Between Dietary Restriction and Lifespan" International Journal of Molecular Sciences 19, no. 12: 3810. https://doi.org/10.3390/ijms19123810