Altered Purinergic Receptor Sensitivity in Type 2 Diabetes-Associated Endothelial Dysfunction and Up4A-Mediated Vascular Contraction
Abstract
:1. Introduction
2. Results
2.1. Characterization of Endothelial Function in Diabetic Rats
2.2. Effects of the Non-Specific P1R and P2R Antagonists on Endothelial Function in Aortas and Mesenteric Arteries
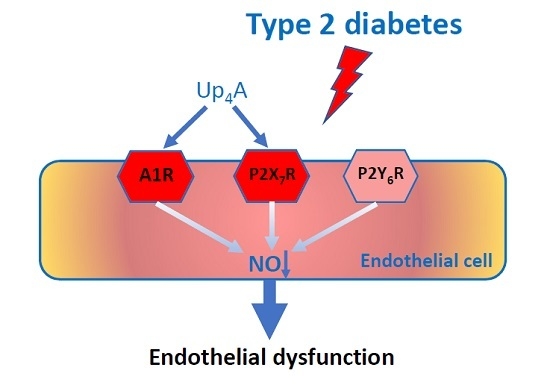
2.3. Effects of the Specific Antagonists for A1R, P2X7R, and P2Y6R on Endothelial Function in Aortas
2.4. Effects of Up4A on Vascular Function in Aortas and Mesenteric Arteries
2.5. Effects of the Non-Specific P1R and P2R Antagonists on Up4A-Mediated Vascular Contraction in Aortas
2.6. Effects of the Specific Antagonists for A1R, P2X7R and P2Y6R on Up4A-Mediated Vascular Contraction in Aortas
2.7. Protein Expression of A1R, P2X7R, and P2Y6R in Aortas from WT and GK Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparations and Wire Myograph Protocols
4.3. Western Blotting
4.4. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| DPCPX | dipropylcyclopentylxanthine |
| EDR | endothelium-dependent relaxation |
| EIDR | endothelium-independent relaxation |
| GK | Goto Kakizaki |
| L-NAME | N(G)-Nitro-L-arginine methyl ester |
| OLETF | Otsuka Long-Evans Tokushima fatty |
| PE | phenylephrine |
| PPADS | pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) |
| PR | purinergic receptor |
| ROS | reactive oxygen species |
| SNP | sodium nitroprusside |
| T2D | type 2 diabetes |
| Up4A | uridine adenosine tetraphosphate |
| WT | Wistar |
References
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Labazi, H.; Trask, A.J. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol. Res. 2017, 123, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalziel, H.H.; Westfall, D.P. Receptors for adenine nucleotides and nucleosides: Subclassification, distribution, and molecular characterization. Pharmacol. Rev. 1994, 46, 449–466. [Google Scholar]
- Zhou, Z.; Merkus, D.; Cheng, C.; Duckers, H.J.; Jan Danser, A.H.; Duncker, D.J. Uridine adenosine tetraphosphate is a novel vasodilator in the coronary microcirculation which acts through purinergic P1 but not P2 receptors. Pharmacol. Res. 2013, 67, 10–17. [Google Scholar] [CrossRef]
- Burnstock, G.; Novak, I. Purinergic signalling and diabetes. Purinergic Signal. 2013, 9, 307–324. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Sorop, O.; de Beer, V.J.; Heinonen, I.; Cheng, C.; Jan Danser, A.H.; Duncker, D.J.; Merkus, D. Altered purinergic signaling in uridine adenosine tetraphosphate-induced coronary relaxation in swine with metabolic derangement. Purinergic Signal. 2017, 13, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Kobayashi, S.; Ando, M.; Iguchi, M.; Takayanagi, K.; Kojima, M.; Taguchi, K.; Kobayashi, T. Alteration of Vascular Responsiveness to Uridine Adenosine Tetraphosphate in Aortas Isolated from Male Diabetic Otsuka Long-Evans Tokushima Fatty Rats: The Involvement of Prostanoids. Int. J. Mol. Sci. 2017, 18, 2378. [Google Scholar] [CrossRef]
- Matsumoto, T.; Watanabe, S.; Kawamura, R.; Taguchi, K.; Kobayashi, T. Enhanced uridine adenosine tetraphosphate-induced contraction in renal artery from type 2 diabetic Goto-Kakizaki rats due to activated cyclooxygenase/thromboxane receptor axis. Pflugers Arch. 2014, 466, 331–342. [Google Scholar] [CrossRef]
- Ishida, K.; Matsumoto, T.; Taguchi, K.; Kamata, K.; Kobayashi, T. Mechanisms underlying altered extracellular nucleotide-induced contractions in mesenteric arteries from rats in later-stage type 2 diabetes: Effect of ANG II type 1 receptor antagonism. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1850–H1861. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsumoto, T.; Taguchi, K.; Kamata, K.; Kobayashi, T. Mechanisms underlying reduced P2Y(1) -receptor-mediated relaxation in superior mesenteric arteries from long-term streptozotocin-induced diabetic rats. Acta Physiol. (Oxf) 2013, 207, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Thaning, P.; Bune, L.T.; Hellsten, Y.; Pilegaard, H.; Saltin, B.; Rosenmeier, J.B. Attenuated purinergic receptor function in patients with type 2 diabetes. Diabetes 2010, 59, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, V.; Tolle, M.; Vanholder, R.; Schonfelder, G.; van der Giet, M.; Henning, L.; Schluter, H.; Paul, M.; Zidek, W.; Jankowski, J. Uridine adenosine tetraphosphate: A novel endothelium- derived vasoconstrictive factor. Nat. Med. 2005, 11, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Goulopoulou, S.; Taguchi, K.; Tostes, R.C.; Kobayashi, T. Constrictor prostanoids and uridine adenosine tetraphosphate: Vascular mediators and therapeutic targets in hypertension and diabetes. Br. J. Parmacol. 2015, 172, 3980–4001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, C.; Tilley, S.L.; Mustafa, S.J. Mechanisms underlying uridine adenosine tetraphosphate-induced vascular contraction in mouse aorta: Role of thromboxane and purinergic receptors. Vascul. Pharmacol. 2015, 73, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Yadav, V.R.; Sun, C.; Teng, B.; Mustafa, J.S. Impaired Aortic Contractility to Uridine Adenosine Tetraphosphate in Angiotensin II-Induced Hypertensive Mice: Receptor Desensitization? Am. J. Hypertens. 2017, 30, 304–312. [Google Scholar] [CrossRef]
- Linder, A.E.; Tumbri, M.; Linder, F.F.; Webb, R.C.; Leite, R. Uridine adenosine tetraphosphate induces contraction and relaxation in rat aorta. Vascul. Pharmacol. 2008, 48, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Teng, B.; Labazi, H.; Sun, C.; Yang, Y.; Zeng, X.; Mustafa, S.J.; Zhou, Z. Divergent coronary flow responses to uridine adenosine tetraphosphate in atherosclerotic ApoE knockout mice. Purinergic Signal. 2017, 13, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, A.; Kovamees, O.; Checa, A.; Wheelock, C.E.; von Heijne, M.; Alvarsson, M.; Pernow, J. Arginase inhibition improves endothelial function in patients with type 2 diabetes mellitus despite intensive glucose-lowering therapy. J. Intern. Med. 2018, 284, 388–398. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahoran, S.; Kovamees, O.; Nordin, F.; Uribe Gonzalez, A.E.; Alvarsson, M.; Ostenson, C.G.; Andersson, D.C.; et al. Erythrocytes From Patients With Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of Glycemic Variability on Chromatin Remodeling, Oxidative Stress, and Endothelial Dysfunction in Patients With Type 2 Diabetes and With Target HbA1c Levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Nguyen Dinh Cat, A.; Alves-Lopes, R.; Harvey, K.Y.; da Costa, R.M.; Lobato, N.S.; Montezano, A.C.; de Oliveira, A.M.; Touyz, R.M.; Tostes, R.C. Chemerin receptor blockade improves vascular function in diabetic obese mice via redox-sensitive- and Akt-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Goulopoulou, S.; Hannan, J.L.; Matsumoto, T.; Ogbi, S.; Ergul, A.; Webb, R.C. Reduced vascular responses to soluble guanylyl cyclase but increased sensitivity to sildenafil in female rats with type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H297–H304. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Kobayashi, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Losartan improves aortic endothelium-dependent relaxation via proline-rich tyrosine kinase 2/Src/Akt pathway in type 2 diabetic Goto-Kakizaki rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2383–H2394. [Google Scholar] [CrossRef] [Green Version]
- Fotino, C.; Dal Ben, D.; Adinolfi, E. Emerging Roles of Purinergic Signaling in Diabetes. Med. Chem. 2018, 14, 428–438. [Google Scholar] [CrossRef]
- Matsumoto, T.; Watanabe, S.; Ando, M.; Yamada, K.; Iguchi, M.; Taguchi, K.; Kobayashi, T. Diabetes and Age-Related Differences in Vascular Function of Renal Artery: Possible Involvement of Endoplasmic Reticulum Stress. Rejuvenation Res. 2016, 19, 41–52. [Google Scholar] [CrossRef]
- Zhou, Z.; Lankhuizen, I.M.; van Beusekom, H.M.; Cheng, C.; Duncker, D.J.; Merkus, D. Uridine Adenosine Tetraphosphate-Induced Coronary Relaxation Is Blunted in Swine With Pressure Overload: A Role for Vasoconstrictor Prostanoids. Front Pharmacol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Labazi, H.; Teng, B.; Mustafa, S.J. Functional changes in vascular reactivity to adenosine receptor activation in type I diabetic mice. Eur. J. Pharmacol. 2018, 820, 191–197. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and pyrimidine receptors. Cell Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef]
- Konishi, C.; Naito, Y.; Ohara, N. Age-related changes in adenosine 5′-triphosphate-induced constriction of isolated, perfused mesenteric arteries of rats. Life Sci. 1999, 64, 1265–1273. [Google Scholar] [CrossRef]
- Burnstock, G. Control of vascular tone by purines and pyrimidines. Br J Pharmacol 2010, 161, 527–529. [Google Scholar] [CrossRef] [Green Version]
- Ralevic, V.; Burnstock, G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect 2003, 16, 133–140. [Google Scholar] [CrossRef]
- Headrick, J.P.; Ashton, K.J.; Rose′meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Kreft, E.; Kowalski, R.; Jankowski, M.; Szczepanska-Konkel, M. Renal vasculature reactivity to agonist of P2X7 receptor is increased in streptozotocin-induced diabetes. Pharmacol. Rep. 2016, 68, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Oku, H.; Komori, A.; Ikeda, T. Effect of P2X7 receptor activation on the retinal blood velocity of diabetic rabbits. Arch. Ophthalmol. 2006, 124, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Haanes, K.A.; Spray, S.; Syberg, S.; Jorgensen, N.R.; Robaye, B.; Boeynaems, J.M.; Edvinsson, L. New insights on pyrimidine signalling within the arterial vasculature - Different roles for P2Y2 and P2Y6 receptors in large and small coronary arteries of the mouse. J. Mol. Cell Cardiol. 2016, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Matsumoto, T.; Ando, M.; Iguchi, M.; Watanabe, S.; Taguchi, K.; Kobayashi, T. UDP-induced relaxation is enhanced in aorta from female obese Otsuka Long-Evans Tokushima Fatty rats. Purinergic Signal. 2018, 14, 91–96. [Google Scholar] [CrossRef]
- Zhou, Z.; Chrifi, I.; Xu, Y.; Pernow, J.; Duncker, D.J.; Merkus, D.; Cheng, C. Uridine adenosine tetraphosphate acts as a proangiogenic factor in vitro through purinergic P2Y receptors. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H299–H309. [Google Scholar] [CrossRef]
- Chettimada, S.; Ata, H.; Rawat, D.K.; Gulati, S.; Kahn, A.G.; Edwards, J.G.; Gupte, S.A. Contractile protein expression is upregulated by reactive oxygen species in aorta of Goto-Kakizaki rat. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H214–H224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathanoori, R.; Sward, K.; Olde, B.; Erlinge, D. The ATP Receptors P2X7 and P2X4 Modulate High Glucose and Palmitate-Induced Inflammatory Responses in Endothelial Cells. PLoS ONE 2015, 10, e0125111. [Google Scholar]
- Sichardt, K.; Nieber, K. Adenosine A(1) receptor: Functional receptor-receptor interactions in the brain. Purinergic Signal. 2007, 3, 285–298. [Google Scholar] [CrossRef]
- Ostenson, C.G.; Khan, A.; Abdel-Halim, S.M.; Guenifi, A.; Suzuki, K.; Goto, Y.; Efendic, S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia 1993, 36, 3–8. [Google Scholar] [CrossRef]
- Gunduz, D.; Aslam, M.; Krieger, U.; Becker, L.; Grebe, M.; Arshad, M.; Sedding, D.G.; Hartel, F.V.; Abdallah, Y.; Piper, H.M.; et al. Opposing effects of ATP and adenosine on barrier function of rat coronary microvasculature. J. Mol. Cell Cardiol. 2012, 52, 962–970. [Google Scholar] [CrossRef]
- Abbas, Z.S.B.; Latif, M.L.; Dovlatova, N.; Fox, S.C.; Heptinstall, S.; Dunn, W.R.; Ralevic, V. UDP-sugars activate P2Y14 receptors to mediate vasoconstriction of the porcine coronary artery. Vascul. Pharmacol. 2018, 103–105, 36–46. [Google Scholar] [CrossRef]
- Grbovic, L.; Radenkovic, M. Analysis of adenosine vascular effect in isolated rat aorta: Possible role of Na+/K+-ATPase. Pharmacol. Toxicol. 2003, 92, 265–271. [Google Scholar] [CrossRef]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdi, A.; Jiao, T.; Tratsiakovich, Y.; Yang, J.; Östenson, C.-G.; Pernow, J.; Zhou, Z. Altered Purinergic Receptor Sensitivity in Type 2 Diabetes-Associated Endothelial Dysfunction and Up4A-Mediated Vascular Contraction. Int. J. Mol. Sci. 2018, 19, 3942. https://doi.org/10.3390/ijms19123942
Mahdi A, Jiao T, Tratsiakovich Y, Yang J, Östenson C-G, Pernow J, Zhou Z. Altered Purinergic Receptor Sensitivity in Type 2 Diabetes-Associated Endothelial Dysfunction and Up4A-Mediated Vascular Contraction. International Journal of Molecular Sciences. 2018; 19(12):3942. https://doi.org/10.3390/ijms19123942
Chicago/Turabian StyleMahdi, Ali, Tong Jiao, Yahor Tratsiakovich, Jiangning Yang, Claes-Göran Östenson, John Pernow, and Zhichao Zhou. 2018. "Altered Purinergic Receptor Sensitivity in Type 2 Diabetes-Associated Endothelial Dysfunction and Up4A-Mediated Vascular Contraction" International Journal of Molecular Sciences 19, no. 12: 3942. https://doi.org/10.3390/ijms19123942