Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1
Abstract
:1. Introduction
2. Results
2.1. Effects of TJ-90 on CBA and CBF
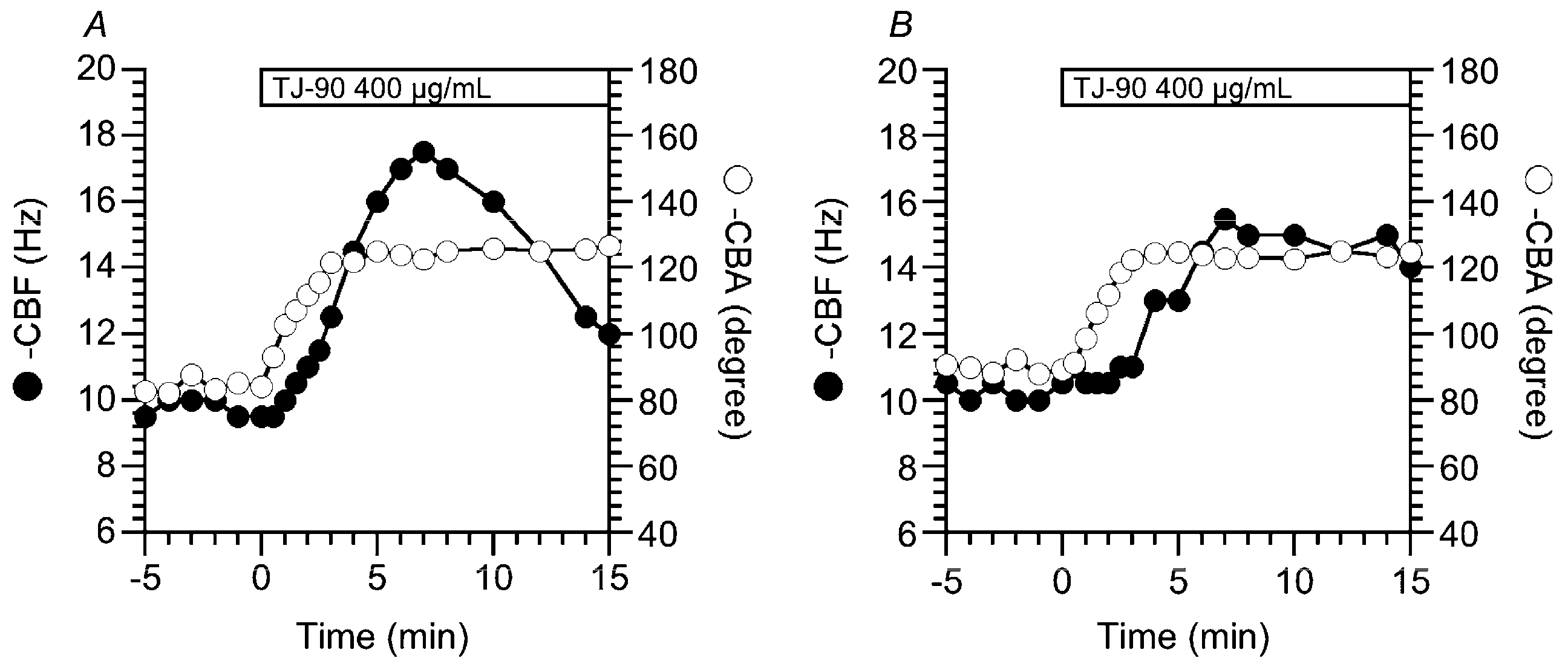
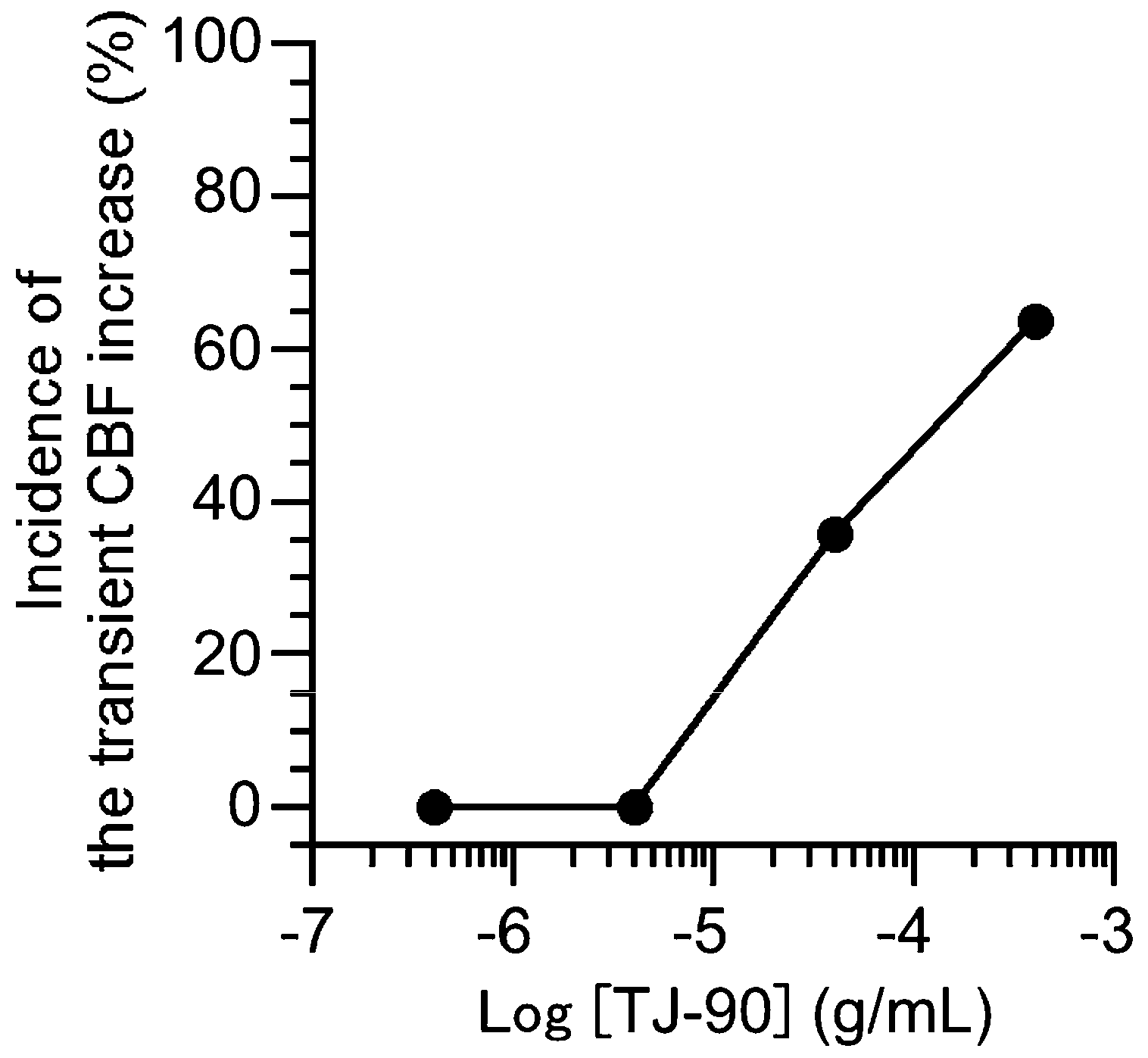
2.1.1. Two Types of CBF Increase Stimulated by TJ-90
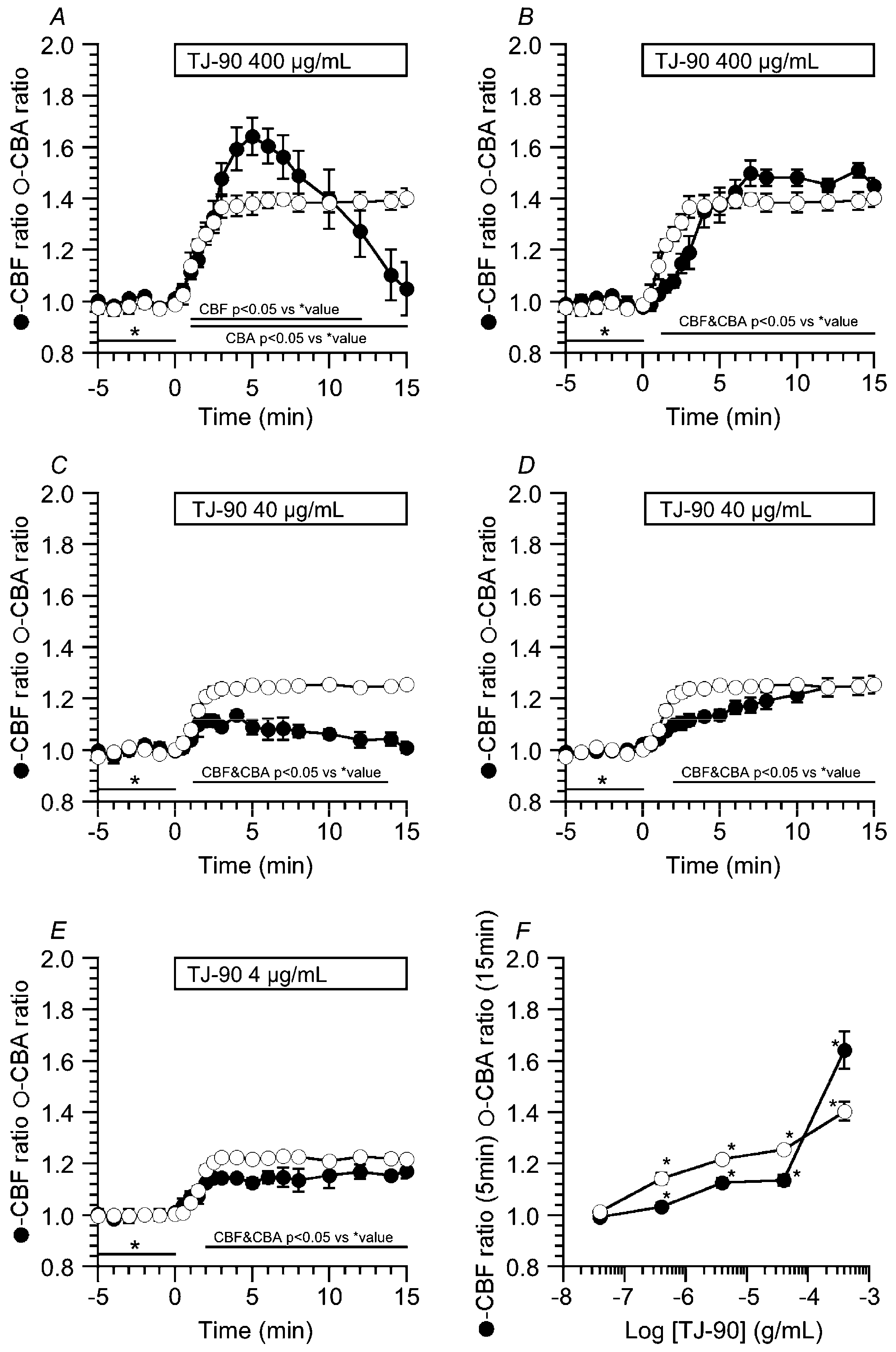
2.1.2. Concentration Effects of TJ-90 on the CBF Increase and CBA Increase
2.1.3. Effects of a PKA Inhibitor (PKI-A) on CBA Increase and CBF Increase Stimulated by TJ-90
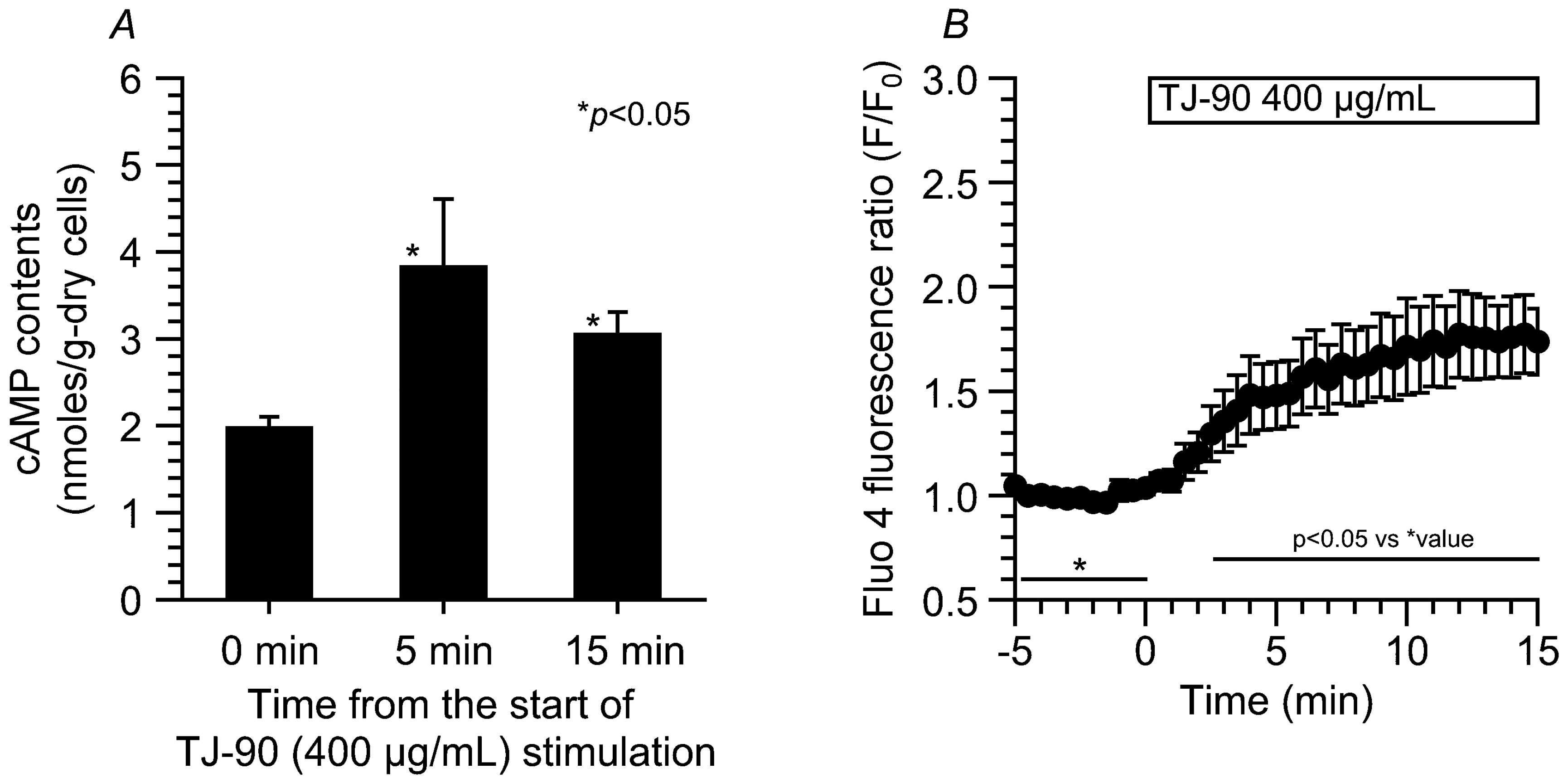
2.2. cAMP Contents and [Ca2+]i
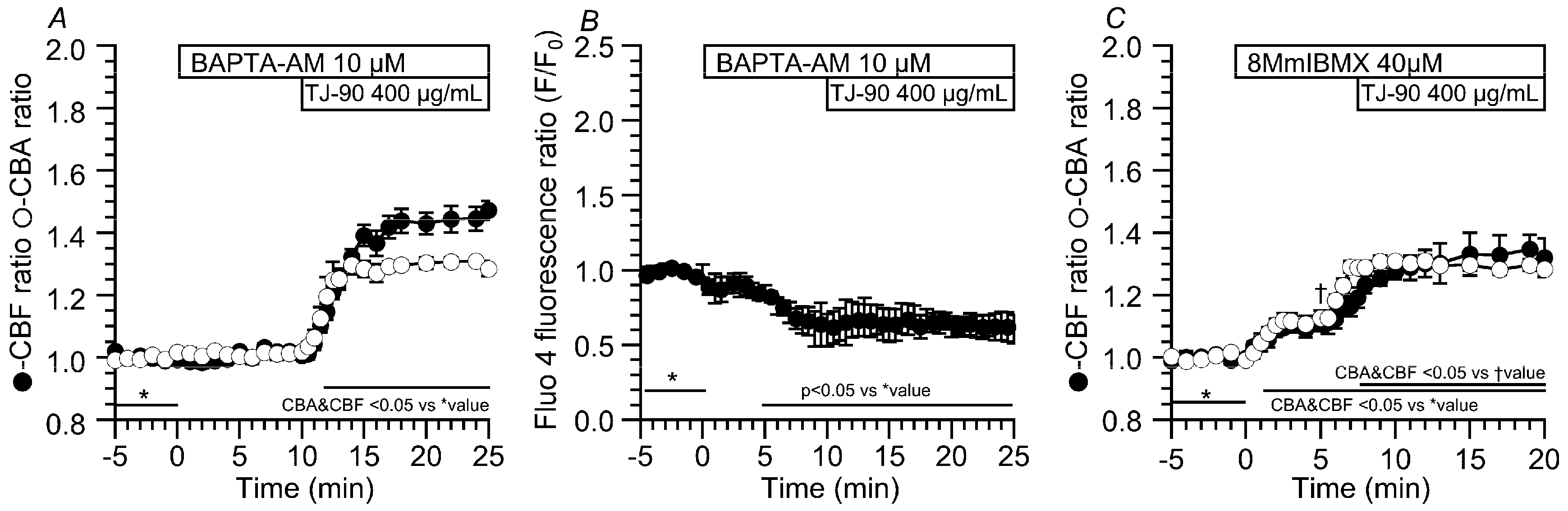
2.3. Effects of BAPTA-AM and 8MmIBMX on the TJ-90-Induced Transient CBF Increase
2.4. Effects of Procaterol and Ionomycin on Increases in CBF and CBA
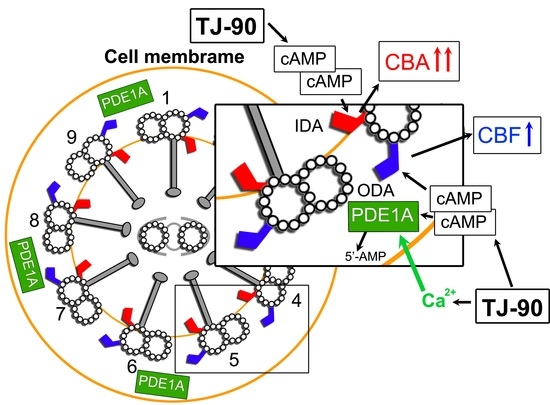
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Solution and Chemicals
4.3. Cell Preparations
4.4. CBA and CBF Measurements
4.5. Measurement of cAMP Contents
4.6. Measurement of the Cell Volume
4.7. Measurement of [Ca2+]i
4.8. Administration of TJ-90 for Examining the Chronic Effects on CBA and CBF
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Wanner, A.; Salathe, M.; O’riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Shellard, R.; Dhillon, D.P.; Winter, J.; Mehta, A. Asynmmetric interactions between phosphorylation pathways regulating ciliary beat frequency in human nasal respiratory epithelium in vitro. J. Physiol. 1996, 496, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Wang, Q.; Satoh, N.; Yoshida, S.; Akaike, T.; Sekizawa, K.; Maeda, H.; Sasaki, H. Effects of quing Fei Tang (TJ-90) on aspiration pneumonia in mice. Phytomedicine 1999, 6, 95–101. [Google Scholar] [CrossRef]
- Mantani, N.; Kasahara, Y.; Kamata, T.; Sekiya, N.; Shimada, Y.; Usuda, K.; Sakakibara, I.; Hattori, N.; Terasawa, K. Effects of Seihai-to, a Kampo medicine, in relapsing aspiration pneumonia—An open-label pilot study. Phytomedicine 2002, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Ebina, T.; Ishida, N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol. Immunol. 1982, 26, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, I.; Mizoguchi, Y.; Tsutsui, H.; Ichikawa, Y.; Kawada, N.; Kioka, K.; Morisawa, S.; Yamamoto, Y. Effects of gomishin A on IL4 and 6 production from human peripheral blood mononuclear cells. J. Tradit. Med. 1990, 7, 190–194. [Google Scholar]
- Chiyotani, A.; Tamaoki, J.; Tagaya, E.; Konno, K. Effects of Sei-hai-to on iontransport in airway epithelial cells. Jpn. J. Allergol. 1994, 43, 1210–1214. [Google Scholar]
- Brokaw, C.J. Control of flagellar bending: A new agenda based on dynein diversity. Cell Motil. Cytoskelet. 1994, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskelet. 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Komatani-Tamiya, N.; Daikoku, E.; Takemura, Y.; Shimamoto, C.; Nakano, T.; Iwasaki, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. Cell Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Tanaka, S.; Hosogi, S.; Nakahari, T.; Marunaka, Y. Activation of ciliary beating by carbocistein via modulation of [Cl−]i and pHi in bronchiolar ciliary cells in mice. J. Physiol. Sci. 2016, 66, S86. [Google Scholar]
- Ikeuchi, Y.; Kogiso, H.; Tanaka, S.; Hosogi, S.; Nakahari, T.; Marunaka, Y. Carbocistein-activated bronchiolar ciliary beating via Cl−- and pH-mediated pathways in mice. J. Physiol. Sci. 2017, 67, S137. [Google Scholar]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Nakahari, T.; Marunaka, Y. Ciliary beat frequency modulated by PDE1A activity in procaterol stimulated mouse bronchiole. J. Physiol. Sci. 2016, 66, S86. [Google Scholar]
- Kogiso, H.; Ikeuchi, Y.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Nakahari, T.; Marunaka, Y. Ca2+-regulation of cAMP-activated ciliary beating mediated via PDE1 in mouse bronchiolar cilia. J. Physiol. Sci. 2017, 67, S176. [Google Scholar]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.; Inui, T.; Marunaka, Y.; et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflügers Arch. Eur. J. Physiol. 2017, 469, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Inui, T.; Marunaka, Y.; Nakahari, T. [Ca2+]i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; Melvin, J.E. Activation of salivary secretion: Coupling of cell volume and [Ca2+]i in single cells. Science 1989, 244, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Chiyotani, A.; Tamaoki, J.; Yamayaki, I.; Sakai, N.; Tagaya, E.; Konno, K. Effect of Hochu-ekki-to (Buzhongyiqitang) on ciliary motility of airway epithelial cells as assessed by the microphoto oscillation technique. J. Jpn. Soc. Respir. Endoscopy 1995, 17, 385–389. [Google Scholar]
- Li, W.-E.; Chen, W.; Ma, Y.-F.; Tuo, Q.-R.; Luo, X.-J.; Zhang, T.; Sai, W.-B.; Liu, J.; Shen, J.; Liu, Z.-G.; et al. Methods to measure and analyze ciliary beat activity: Ca2+ influx-mediated cilia mechanosensitivity. Pflügers Arch. Eur. J. Physiol. 2012, 464, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Shiima-Kinoshita, C.; Min, K.-Y.; Hanafusa, T.; Mori, H.; Nakahari, T. β2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: Potentiation by isosmotic cell shrinkage. J. Physiol. 2004, 554, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Yang, G.; Song, Y.; Li, F.; Lin, N. Application of isoabsorption plots generated by high-performance liquid chromatography with diode array detection to the development of multicomponent quantitative analysis of traditional herbal medicines. J. Sep. Sci. 2014, 37, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, W.; Wang, M.; Wu, Y.; Zhang, Z.; Shen, S.; Jiang, J. Simultaneous determination of multiple bioactive components of Bu-zhong-yi-qi-tang in rat tissue by LC-MS/MS: Application to a tissue distribution study. J. Chromotogr. B 2017, 1044–1045, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Butenko, I.G.; Gladtchenko, S.V.; Galushko, S.V. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavonoid baicalein from Scutellaria baicalensis GIORGI roots. Agents Act. 1993, 39, C49–C51. [Google Scholar] [CrossRef]
- Nozaki, I.; Kato-Motozaki, Y.; Ikeda, T.; Takahashi, K.; Tagami, A.; Ishida, C.; Komai, K. Aspiration pneumonia and bronchopneumonia in progressive supranuclear palsy treated with quing fei tang: Two case reports. J. Med. Case Rep. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-R.; Liang, K.-L.; Huang, T.-P.; Fan, J.-Y.; Chang, T.-T.; Sun, M.-F. Characteristics of traditional Chinese medicine use for children with allergic rhinitis: A nationwide population-based study. Int. J. Pediat. Otorhinolaryngol. 2015, 79, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Nakahari, T.; Marunaka, Y. Measurement of [Cl−]i unaffected by the cell volume change using MQAE-based two-photon microscopy in airway ciliary cells of mice. J. Physiol. Sci. 2018, 68, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogiso, H.; Ikeuchi, Y.; Sumiya, M.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Marunaka, Y.; Nakahari, T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. Int. J. Mol. Sci. 2018, 19, 658. https://doi.org/10.3390/ijms19030658
Kogiso H, Ikeuchi Y, Sumiya M, Hosogi S, Tanaka S, Shimamoto C, Inui T, Marunaka Y, Nakahari T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. International Journal of Molecular Sciences. 2018; 19(3):658. https://doi.org/10.3390/ijms19030658
Chicago/Turabian StyleKogiso, Haruka, Yukiko Ikeuchi, Masako Sumiya, Shigekuni Hosogi, Saori Tanaka, Chikao Shimamoto, Toshio Inui, Yoshinori Marunaka, and Takashi Nakahari. 2018. "Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1" International Journal of Molecular Sciences 19, no. 3: 658. https://doi.org/10.3390/ijms19030658
APA StyleKogiso, H., Ikeuchi, Y., Sumiya, M., Hosogi, S., Tanaka, S., Shimamoto, C., Inui, T., Marunaka, Y., & Nakahari, T. (2018). Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. International Journal of Molecular Sciences, 19(3), 658. https://doi.org/10.3390/ijms19030658