Effects of Honokiol on CYP450 Activity and Transporter mRNA Expression in Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Method Validation
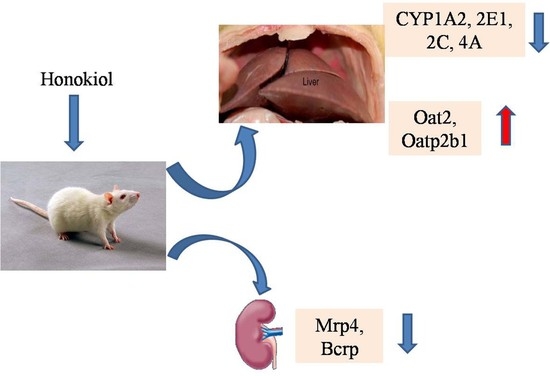
2.2. Effect of Hon on Hepatic CYP Activity
2.3. Effect of T2DM on the mRNA Expression of Related Hepatic and Renal Transporters
2.4. Effect of Hon on the mRNA Expression of Hepatic and Renal Transporters
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Type 2 Diabetic Rats Model
4.3. Animals Grouping and Treatment Protocol
4.4. Assay for CYP450 Activity
4.5. LC/MS/MS Analysis
4.6. Assay for mRNA Expression of Transporters
4.7. Data Analysis
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Stumvoll, M.; Goldstein, B.J.; Van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.X. Alteration of related transporters and its application significance in common intestinal disease, liver disease, renal disease and diabetes. Acta Pharm. Sin. 2015, 50, 127–132. [Google Scholar]
- Hu, M.Y.; Liu, C.; Zhang, M.; Hu, N.; Liu, L.; Liu, X.D. Alteration of cytochrome P450s activity under diabetic conditions and its impact on the development of diabetes mellitus. J. China Pharm. Univ. 2014, 45, 153–160. [Google Scholar]
- Cheng, P.Y.; Morgan, E.T. Hepatic cytochrome P450 regulation in disease states. Curr. Drug Metab. 2001, 2, 165–183. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, J.K.; Li, F.; Li, R.G.; Zhan, G.Q.; Li, G.; Du, W.X.; Tan, H.B. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2015, 21, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.P.; Kumar, R.D.; Mehta, S.; Gupta, R.; Sharma, B.; Watal, G. Effect of trichosanthesdioica on oxidative stress and CYP450 gene expression levels in experimentally induced diabetic rats. Cell Mol. Biol. 2011, 57, 31–39. [Google Scholar]
- Gawrońska-Szklarz, B.; Musiał, D.H.; Pawlik, A.; Paprota, B. Effect of experimental diabetes on pharmacokinetic parameters of lidocaine and MEGX in rats. Pol. J. Pharmacol. 2003, 55, 619–624. [Google Scholar] [PubMed]
- Sindhu, R.K.; Koo, J.R.; Sindhu, K.K.; Ehdaie, A.; Farmand, F.; Roberts, C.K. Differential regulation of hepatic cytochrome P450 monooxygenases in streptozotocin-induced diabetic rats. Free Radic. Res. 2006, 40, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, L.; Li, J.; Mei, D.; Duan, R.; Hu, N.; Guo, H.; Zhong, Z.; Liu, X. Combined contributions of impaired hepatic CYP2C11 and intestinal breast cancer resistance protein activities and expression to increased oral glibenclamide exposure in rats with streptozotoein-induced diabetes mellitus. Drug Metab. Dispos. 2012, 40, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Pass, G.J.; Becker, W.; Kluge, R.; Linnartz, K.; Plum, L.; Giesen, K.; Joost, H.G. Effect of hyperinsulinemia and type 2 diabetes-like hyperglycemia on expression of hepatic cytochrome p450 and glutathione s-transferase isoforms in a New Zealand obese-derived mouse backcross population. J. Pharmacol. Exp. Ther. 2002, 302, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Shimada, T.; Toda, T.; Igeta, S.; Suzuki, W.; Ikarashi, N.; Ochiai, W.; Ito, K.; Abruada, M.; Sugiyama, K. Altered expression of CYP in TSOD mice: A model of type 2 diabetes and obesity. Xenobiotica 2009, 9, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.L.; Jiang, Y.; Zhang, T.; Zhang, E.Y.; Smith, B.J. Expression and functional analysis of hepatic cytochromes P450, nuclear receptors, and membrane transporters in 10-and 25-week-old db/db mice. Drug Metab. Dispos. 2010, 38, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Aleksunes, L.M.; Manautou, J.E.; Cherrington, N.J.; Scheffer, G.L.; Yamasaki, H.; Slitt, A.L. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol. Pharm. 2008, 5, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.T.; Aleksunes, L.M.; Sawant, S.P.; Dnyanmote, A.V.; Mehendale, H.M.; Manautou, J.E. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab. Lett. 2008, 2, 11–17. [Google Scholar] [PubMed]
- Wu, F.; Zhang, W.; Li, L.; Zhang, F.; Shao, X.; Zhou, J.; Li, H. Inhibitory effects of honokiol on lipopolysaccharide-induced cellular responses and signaling events in human renal mesangial cells. Eur. J. Pharmacol. 2011, 654, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Hueng, D.Y.; Huang, H.Y.; Chen, J.Y.; Chen, Y. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 2016, 7, 29116–29130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, M.; Su, N.; Zhang, Z.; Zhao, H.; Yu, H.; Xu, Y. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol. Med. Rep. 2016, 13, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huo, M.; Jia, Y.; Xu, A. KRT6B, a key mediator of notch signaling in honokiol-induced human hepatoma cell apoptosis. Int. J. Clin. Exp. Med. 2015, 8, 16880–16889. [Google Scholar] [PubMed]
- Wang, J.J.; Zhao, R.; Liang, J.C.; Chen, Y. Antidiabetic and antioxidative effects of honokiol on diabetic rats induced by high-fat diet and streptozotocin. CHM 2014, 6, 42–46. [Google Scholar]
- Jeong, H.U.; Kong, T.Y.; Kwon, S.S.; Hong, S.W.; Yeon, S.H.; Choi, J.H.; Lee, J.Y.; Cho, Y.Y.; Lee, H.S. Effect of honokiol on cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in human liver microsomes. Molecules 2013, 18, 10681–10693. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xiao, J.; Chen, Y.; Han, F.M. Inhibition of magonolol and honokiol on cytochrome P450 enzyme in rat and human liver microsomes. CHM 2015, 7, 167–172. [Google Scholar]
- Quezada, C.; Alarcón, S.; Cárcamo, J.G.; Yanez, A.; Casanello, P.; Sobrevia, L.; San Martin, R. Increased expression of the multidrug resistance-associated protein 1 (MRP1) in kidney glomeruli of streptozotocin-induced diabetic rats. Biol. Chem. 2011, 392, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Goodwin, E.; Channer, K.S.; Jones, T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, viscera/adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2005, 154, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.J.; Allum, A.; Jones, S.; Sutherland, W.H.; Willians, A.M. The effect of hormone replacement therapy on cardiovascular risk factors in type 2 diabetes: A randomized controlled trial. Arch. Intern. Med. 2001, 161, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Kemohan, A.F.; Sattar, N.; Hilditch, T.; Cleland, S.J.; Small, M.; Lumsden, M.A.; Connell, J.M.; Petrie, J.R. Effects of low-dose continuous combined hormone replacement therapy on glucose homeostasis and markem of cardiovascular risk in women with type 2 diabetes. Clin. Endocrinol. 2007, 66, 27–34. [Google Scholar]
- Erikstrup, C.; Mortensen, O.H.; Nielsen, A.R.; Fischer, C.P.; Plomgaard, P.; Petersen, A.M.; Krogh-Madsen, R.; Lindegaard, B.; Erhardt, J.G.; Ullum, H.; et al. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Aghasi, M.; Vafa, M.; Heydari, I.; Hosseini, S.; Shidfar, S. Effects of combination of zinc and vitamin A supplementation on serum fasting blood sugar, insulin, apoprotein B and apoprotein A-I in patients with type I diabetes. Int. J. Food Sci. Nutr. 2010, 61, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.F. The CYP2 family: Models, mutants and interactions. Xenobiotica 1998, 28, 617–661. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Chacos, N.; Werringloer, J.; Prough, R.A.; Estabrook, R.W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 5362–5366. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.R.; Pascoe, N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc. Natl. Acad. Sci. USA 1981, 78, 7375–7378. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Gorin, Y.; Fagg, B.M.; Maalouf, R.; Barnes, J.L.; Block, K.; Abboud, H.E. Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 2009, 58, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Cheng, J.; Deng, H.; Kemp, P.; Ishizuka, T.; Nasjletti, A.; Schwartzman, M.L. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 2007, 50, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kroetz, D.L.; Xu, F. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.H.; Jang, S.; Gonzalez, F.J.; Song, B.J. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Armoni, M.; Harel, C.; Karnieli, E.; Pessin, J.E. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E532–E539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, D.; Dong, W.; Zhang, L.; Zhang, X.; Quan, X.; Ma, C.; Lian, H.; Zhang, L. Expression of CYP2E1 increases oxidative stress and induces apoptosis of cardiomyocytes in transgenic mice. FEBS J. 2011, 278, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, E.; Chen, P.; Morgan, K.; French, S.W.; Morgan, T.R. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P450 2E1 transgenic mouse model of non-alcoholic fatty liver. J. Gastroenterol. Hepatol. 2010, 25, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Park, E.C.; Kim, S.I.; Hong, Y.; Hwang, J.W.; Cho, G.S.; Cha, H.N.; Han, J.K.; Yun, C.H.; Park, S.Y.; Jang, J.S.; et al. Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology 2014, 147, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Jerabek, P.; Martinek, V.; Stiborova, M. Theoretical investigation of differences in nitroreduction of aristolochic acid Ⅰ by cytochromes P4501A1, 1A2 and1B1. Neuro Endocrinol. Lett. 2012, 33, 25–32. [Google Scholar] [PubMed]
- Engst, W.; Landsiedel, R.; Hermersdorfer, H.; Doehmer, J.; Glatt, H. Benzylic hydroxylation of 1-methylpyrene and 1-ethylpyrene by human and rat cytochromesP450 individuallyexpressed in V79 Chinese hamster cells. Carcinogenesis 1999, 20, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; EL-Maraghy, S.A.; Teleb, Z.A.; Shaheen, A.A. Pretreatment with turmeric modulates the inhibitory influence of cisplatin and paclitaxel on CYP2E1 and CYP3A1/2 in osolated rat hepatic microsomes. Chem. Biol. Interact. 2014, 220, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kandem, L.K.; Flovkhart, D.A.; Desta, Z. In vitro cytochrome P450-mediated metabolism of exemetane. Drug Metab. Dispos. 2011, 9, 98–105. [Google Scholar] [CrossRef] [PubMed]
| Group | Enzyme Activity (ng/min/mg Protein) | |||||
|---|---|---|---|---|---|---|
| CYP1A2 | CYP2B | CYP2E1 | CYP3A | CYP2C | CYP4A | |
| NC | 40.9 ± 4.8 | 6.96 ± 0.54 | 132.7 ± 33.1 | 3789.6 ± 733.5 | 59.9 ± 11.5 | 458.0 ± 50.3 |
| DC | 96.6 ± 12.6 ** | 7.20 ± 1.27 | 278.0 ± 48.6 ** | 3760.8 ± 588.1 | 111.5 ± 27.0 * | 1167.4 ± 109.7 ** |
| Hon25 | 84.0 ± 18.6 # | 7.46 ± 1.29 | 248.8 ± 28.5 | 3979.7 ± 263.9 | 102.2 ± 19.9 | 1170.7 ± 221.2 |
| Hon50 | 82.0 ± 9.7 # | 7.25 ± 1.96 | 195.5 ± 50.8 # | 3324.2 ± 1116.1 | 99.2 ± 10.4 | 1091.4 ± 82.7 |
| Hon100 | 74.7 ± 16.6 ## | 7.13 ± 0.97 | 151.2 ± 45.1 ## | 2983.8 ± 959.6 # | 98.2 ± 10.2 | 929.3 ± 262.7 ## |
| Gene | Fold Response | |||
|---|---|---|---|---|
| Liver | Kidney | |||
| NC | DC | NC | DC | |
| Oat2 | 1.00 ± 0.32 | 0.64 ± 0.14 ** | 1.00 ± 0.25 | 1.56 ± 0.48 * |
| Oct1 | 1.00 ± 0.67 | 0.93 ± 0.27 | 1.00 ± 0.33 | 0.79 ± 0.14 * |
| Octn1 | 1.00 ± 0.46 | 1.60 ± 0.71 | 1.00 ± 0.30 | 1.13 ± 0.49 |
| Octn2 | 1.00 ± 0.67 | 3.24 ± 0.44 * | 1.00 ± 0.17 | 0.54 ± 0.12 ** |
| Oatp2b1 | 1.00 ± 0.45 | 0.64 ± 0.21 ** | 1.00 ± 0.14 | 0.78 ± 0.16 * |
| Oatp1a5 | 1.00 ± 0.46 | 0.47 ± 0.14 * | 1.00 ± 0.56 | 0.50 ± 0.16 * |
| Oatp3a1 | 1.00 ± 0.78 | 1.65 ± 0.15 * | 1.00 ± 0.34 | 1.27 ± 0.63 |
| Oatp1a1 | 1.00 ± 0.64 | 1.34 ± 0.06 * | 1.00 ± 0.47 | 0.85 ± 0.58 |
| Oatp1a4 | 1.00 ± 0.34 | 1.43 ± 0.18 | 1.00 ± 0.73 | 0.62 ± 0.43 |
| Mdr2 | 1.00 ± 0.62 | 1.81 ± 0.15 * | 1.00 ± 0.33 | 1.38 ± 0.22 * |
| Ntcp | 1.00 ± 0.45 | 1.31 ± 0.69 | 1.00 ± 0.63 | 1.76 ± 0.22 * |
| Mate1 | / | / | 1.00 ± 0.32 | 0.87 ± 0.48 |
| Gene | Fold Response | ||||
|---|---|---|---|---|---|
| NC | DC | Hon25 | Hon50 | Hon100 | |
| Oat2 | 1.00 ± 0.11 | 0.57 ± 0.24 ** | 0.61 ± 0.68 | 0.61 ± 0.47 | 0.87 ± 0.38 # |
| Octn2 | 1.00 ± 0.56 | 2.71 ± 1.04 * | 2.36 ± 0.32 | 2.06 ± 0.31 | 2.82 ± 0.36 |
| Oatp2b1 | 1.00 ± 0.27 | 0.61 ± 0.41 ** | 0.71 ± 0.85 | 0.74 ± 0.35 | 0.94 ± 0.41 # |
| Oatp1a5 | 1.00 ± 0.05 | 0.33 ± 0.29 * | 0.22 ± 0.41 | 0.17 ± 0.45 | 0.10 ± 0.28 |
| Mrp5 | 1.00 ± 0.44 | 1.26 ± 0.43 | 0.94 ± 0.16 | 1.09 ± 0.60 | 1.01 ± 0.60 |
| Gene | Fold Response | ||||
|---|---|---|---|---|---|
| NC | DC | Hon25 | Hon50 | Hon100 | |
| Oat2 | 1.00 ± 0.53 | 2.70 ± 0.52 ** | 2.43 ± 0.96 | 2.62 ± 0.82 | 2.71 ± 0.69 |
| Octn2 | 1.00 ± 0.55 | 0.59 ± 0.46 * | 0.50 ± 0.37 | 0.59 ± 0.49 | 0.57 ± 0.29 |
| Oatp2b1 | 1.00 ± 0.33 | 0.70 ± 0.46 * | 0.77 ± 0.34 | 0.84 ± 0.58 | 0.71 ± 0.16 |
| Oatp1a5 | 1.00 ± 0.21 | 0.61 ± 0.15 * | 0.57 ± 0.41 | 0.94 ± 0.16 | 0.26 ± 0.20 |
| Mrp4 | 1.00 ± 0.55 | 1.73 ± 0.56 * | 1.37 ± 0.59 | 1.12 ± 0.73 # | 1.22 ± 0.23 |
| Bcrp | 1.00 ± 0.21 | 2.23 ± 0.81 ** | 1.69 ± 0.59 | 0.85 ± 0.22 | 0.92 ± 0.14 # |
| Metabolite/IS | Mobile Phase | Flow Rate (mL/min) | Detection |
|---|---|---|---|
| acetaminophen/ 6β-hydroxytestosterone | A: 0. 1% formic acid in water; B: acetonitrile (0–5 min, 15–85% B; 5–6 min, 85% B; 6–6.01 min, 15% B; 6–11 min, 15% B) | 0.2 | MRM+: 152. 0→110. 1/305. 0→269. 0 |
| 6-hydroxychlorzoxazone/ 4′-hydroxytolbutamide | A: 0. 1% formic acid in water; B: acetonitrile (0–5 min, 20–70% B; 5–6 min, 70% B; 6–6.01 min, 20% B; 6–11min, 20% B) | 0.2 | MRM−: 184. 0→120. 1/285. 0→186. 1 |
| hydroxybupropion/acetaminophen | A: 0. 1% formic acid in water; B: acetonitrile (0–5 min, 15–85% B; 5–6 min, 85% B; 6–6.01 min, 15% B; 6–11 min, 15% B) | 0.2 | MRM+: 256. 0→238. 0/152. 0→110. 1 |
| 6β-hydroxytestosterone/ acetaminophen | A: 0. 1% formic acid in water; B: acetonitrile (0–5 min, 15–85% B; 5–6 min, 85% B; 6–6.01 min, 15% B; 6–11 min, 15% B) | 0.2 | MRM+: 305. 0→269. 0/152. 0→110. 1 |
| 4′-hydroxytolbutamide/6-hydroxychlorzoxazone | A: 0. 1% formic acid in water; B: acetonitrile (0–5 min, 20–70% B; 5–6 min, 70% B; 6–6.01 min, 20% B; 6–11 min, 20% B) | 0.2 | MRM−: 285. 0→186. 1/184. 0→120. 1 |
| 12-hydroxylauric acid/ 6-hydroxychiorzoxazone | A water; B: methanol (0–4 min, 40–60% B; 4–5 min, 60–90% B; 5–9 min, 90% B; 9–9.01 min, 40% B; 9–14 min, 40% B) | 0.2 | SIM−: 215. 0/184. 0 |
| Gene | Forward Primer 5′ to 3′ | Reverse Primer 5′ to 3′ |
|---|---|---|
| Oat2 | GCTGCATGATGGTGTGGTTT | CGGCGCACAAGGAAGTAGAC |
| Oct1 | TGGCCGTAAGCTCTGTCTCT | TCAAGGTATAGCCGGACACC |
| Octn1 | TGATGTCTGTGATGCTGTGG | ATATATCTCCGACGCAGGGTTC |
| Octn2 | AACAATGGCAAATCCAAAGC | CATCCGTGAGCATGTGAGAC |
| Oatp2b1 | CCGCTACGACCACAGCA | CCAAGACCTTCTGCCTGA |
| Oatp1a5 | CGCTTTGATAGACAGAACCT | AGTAGCAGCATGAAACGACA |
| Oatp3a1 | TTCTGCTCCTTCGTTTGTT | GGTTTTTGATGTAGCGTTT |
| Oatp1a1 | TCAACCAAAGCACAAAGCAG | CCTAGGCATAGGCATTTGGA |
| Oatp1a4 | ATGGCCTGGCATACATGTCA | GGGAACTGGAATGTCCTCGTA |
| Mdr2 | ACTGTCCGGAATGCAGATGTC | TCTTTATCAGCTCACTGTGGCTT |
| Ntcp | ATGCCCTTCTCTGGCTTTCT | GCTCCATGGTTCTGATGGTT |
| Mate1 | CTCTTCATCAACACCGAGCA | ACCCATCACCCCAAGATGTA |
| Mrp4 | AATGGACACTGAACTAGCAGAATCTG | CCCGGATTTTCTGTTGTATTAACTC |
| Mrp5 | CCACCATCCATGCCTATAACAA | CCCCGTGGTGGTGATCAG |
| Bcrp | CAGCAGGTTACCACTGTGAG | TTCCCCTCTGTTTAACATTACA |
| Gapdh | ATGGGAAGCTGGTCATCAAC | GTGGTTCACACCCATCACAA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhai, T.; Chen, Y. Effects of Honokiol on CYP450 Activity and Transporter mRNA Expression in Type 2 Diabetic Rats. Int. J. Mol. Sci. 2018, 19, 815. https://doi.org/10.3390/ijms19030815
Wang J, Zhai T, Chen Y. Effects of Honokiol on CYP450 Activity and Transporter mRNA Expression in Type 2 Diabetic Rats. International Journal of Molecular Sciences. 2018; 19(3):815. https://doi.org/10.3390/ijms19030815
Chicago/Turabian StyleWang, Junjun, Ting Zhai, and Yong Chen. 2018. "Effects of Honokiol on CYP450 Activity and Transporter mRNA Expression in Type 2 Diabetic Rats" International Journal of Molecular Sciences 19, no. 3: 815. https://doi.org/10.3390/ijms19030815