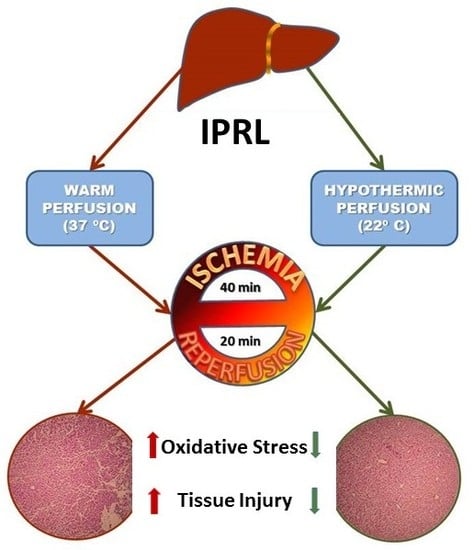
Preconditioning-Like Properties of Short-Term Hypothermia in Isolated Perfused Rat Liver (IPRL) System
Abstract
:1. Introduction
2. Results
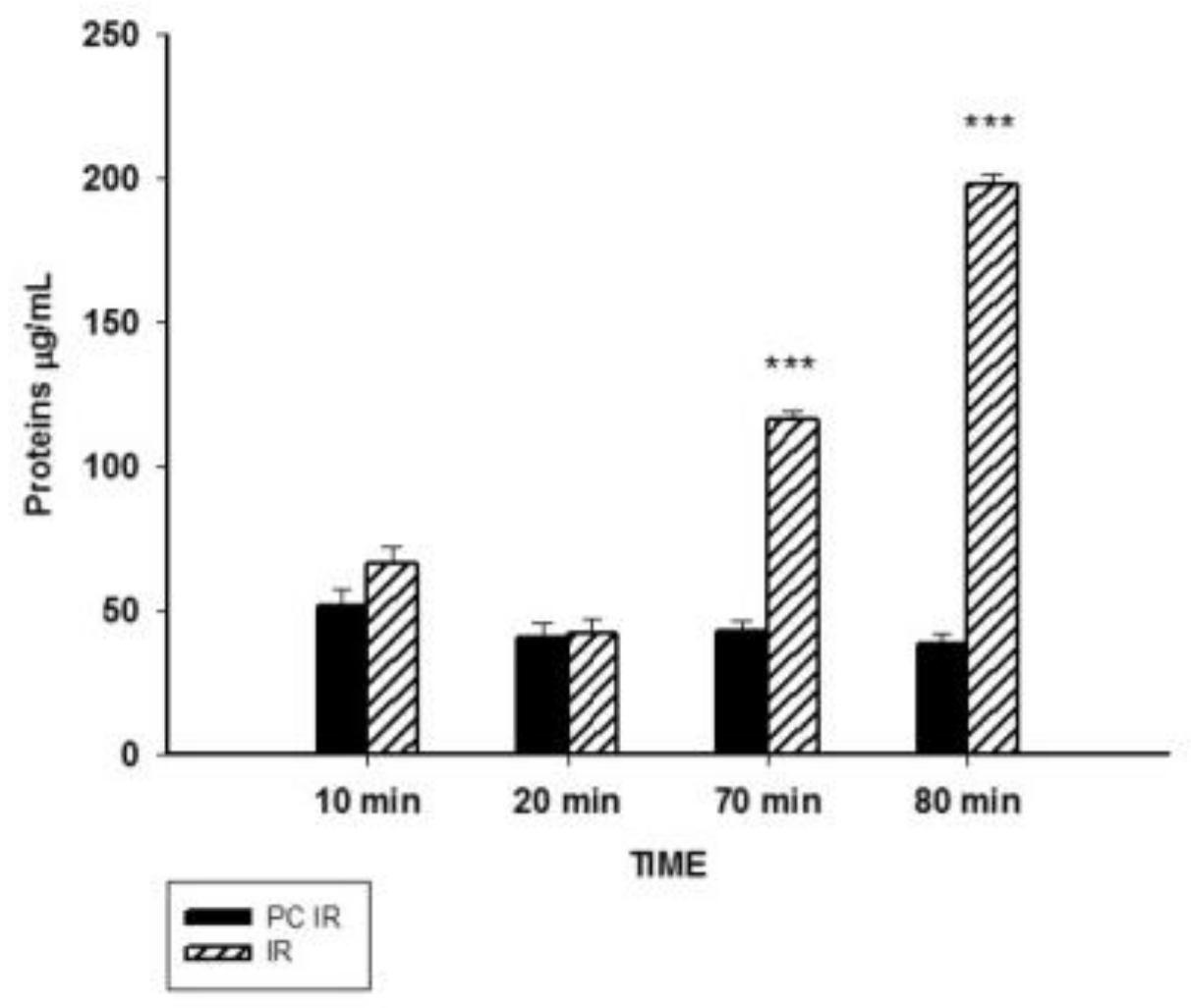
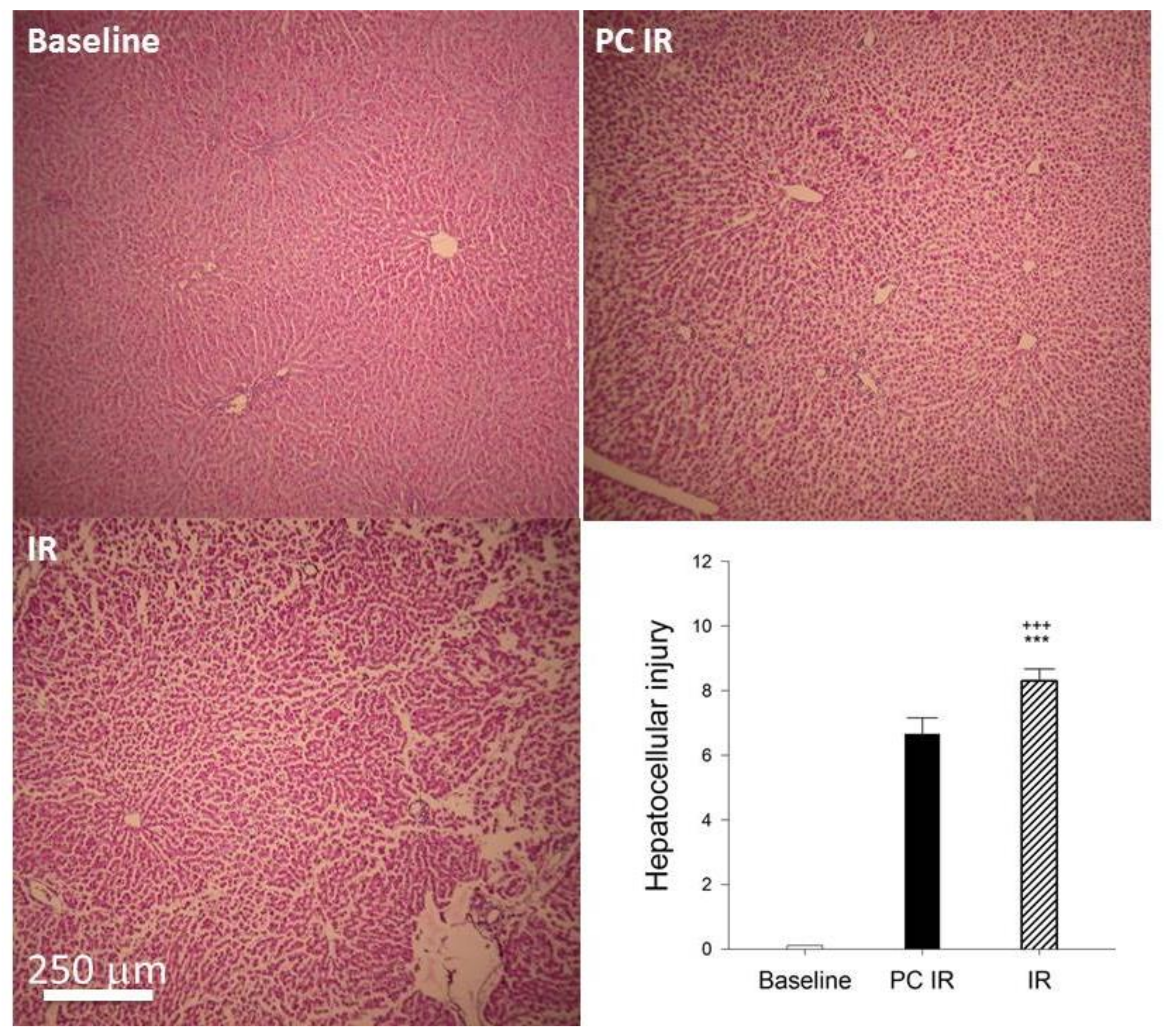
2.1. Effect of Hypothermic Perfusion on Cellular Integrity
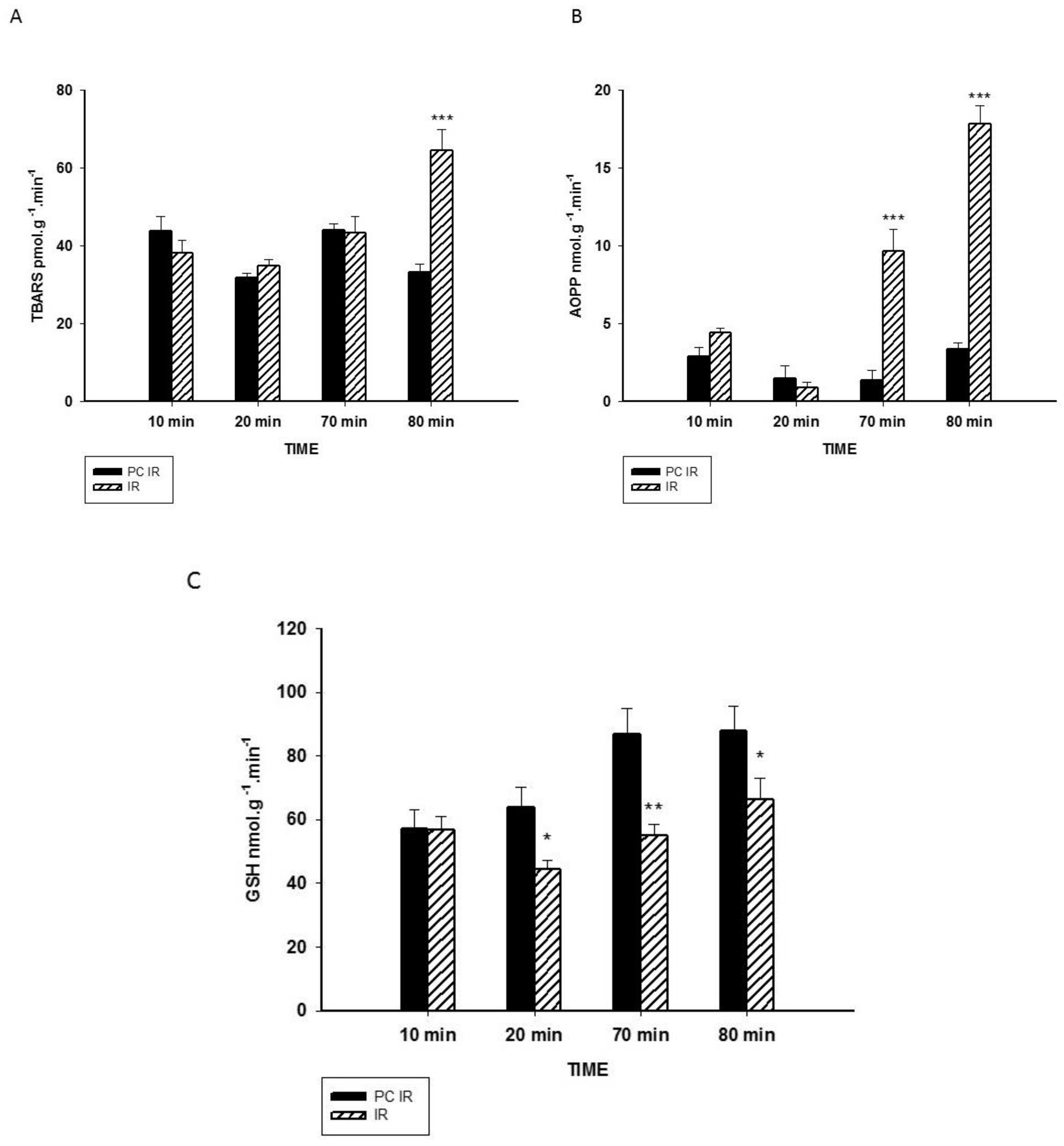
2.2. Effect of Hypothermic Perfusion on Biomarkers of Oxidative Stress in the Perfusate
2.3. Effect of Hypothermic Preconditioning on Oxidant/Antioxidant Parameters in Liver after Ex Vivo Warm Ischemia and Reperfusion Perfusion
3. Discussion
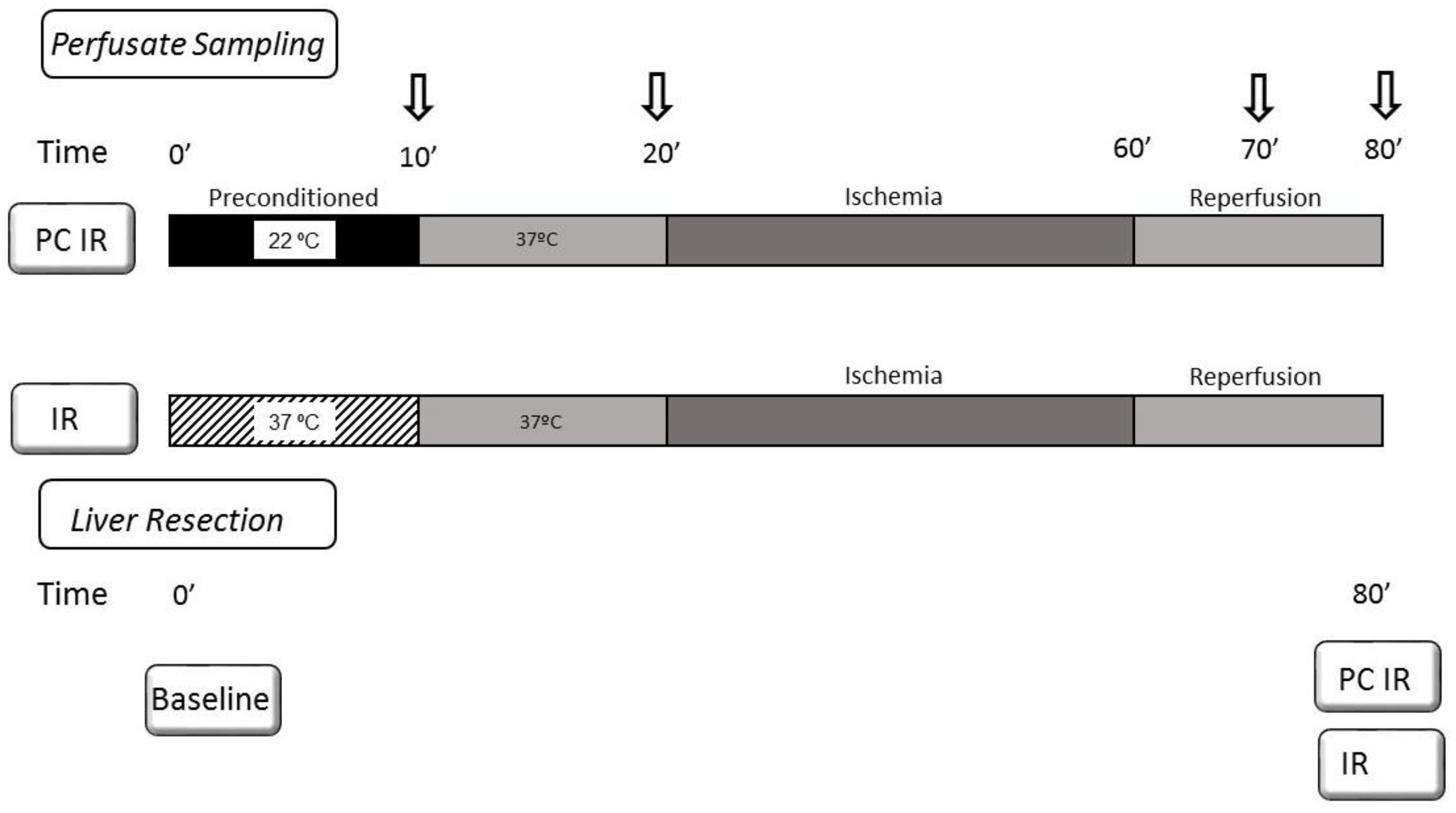
4. Materials and Methods
4.1. Animals and Liver Isolation and Perfusion
4.2. Biochemical Assays in the Perfusate
4.3. Oxidant and Antioxidant Assays in Liver
4.4. Histology
4.5. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iniguez, M.; Dotor, J.; Feijoo, E.; Goni, S.; Prieto, J.; Berasain, C.; Avila, M.A. Novel pharmacologic strategies to protect the liver from ischemia-reperfusion injury. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 9–18. [Google Scholar] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissa-Altés, S.; Mahfoudh-Boussaid, A.; Roselló-Catafau, J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin. Pharmacother. 2010, 11, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, W.J. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Krug, A.; Krysiak, M.; Dünschede, F.; Seifert, J.K.; Junginger, T. Detection of mitochondrial electron chain carrier redox status by transhepatic light intensity during rat liver reperfusion. Cryobiology 2003, 47, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Panisello-roselló, A.; Lopez, A.; Benítez, C.C.; Folch-puy, E.; García-gil, A.; Carbonell, T.; Adam, R.; Roselló-catafau, J.; Zaouali, M.A.; et al. Relevance of proteolysis and proteasome activation in fatty liver graft preservation : An Institut Georges Lopez-1 vs University of Wisconsin appraisal. World J. Gastroenterol. 2017, 23, 4211–4221. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Gonzalez, H.D.; Davidson, B.R. Current protective strategies in liver surgery. World J. Gastroenterol. 2010, 16, 6098–6103. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Carbonell, T.; Bardag-Gorce, F.; Oliva, J.; Pantazi, E.; Ben Abdennebi, H.; Rosello-Catafau, J. Bortezomid, a Reversible Proteasome Inhibitor, Improves the Steatotic Liver Graft Preservation. Am. J. Transplant. 2012, 12, 454–455. [Google Scholar]
- Boncompagni, E.; Zaouali, M.A.; Padrissa-Altes, S.; Ben Abdennebi, H.; Reiter, R.J.; Freitas, I.; Vairetti, M.; Rimola, A.; Rosello-Catafau, J. Melatonin and Trimetazidine in IGL-1 Solution: New Insights for Fatty Liver Preservation. Liver Transplant. 2010, 16, S173–S173. [Google Scholar]
- Zaouali, M.A.; Bardag-Gorce, F.; Carbonell, T.; Oliva, J.; Pantazi, E.; Bejaoui, M.; Abdennebi, H.B.; Rimola, A.; Rosello-Catafau, J. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Exp. Mol. Pathol. 2013, 94, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Serafín, A.; Roselló-Catafau, J.; Prats, N.; Gelpí, E.; Rodés, J.; Peralta, C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology 2004, 39, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Hotter, G.; Closa, D.; Gelpi, E.; Bulbena, O.; RoselloCatafau, J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: Role of nitric oxide and adenosine. Hepatology 1997, 25, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; Calvo, M.; Folch-Puy, E.; Serafin, A.; Pasut, G.; Panisello, A.; Adam, R.; Rosello-Catafau, J. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 9096549. [Google Scholar] [CrossRef] [PubMed]
- Selten, J.; Schlegel, A.; de Jonge, J.; Dutkowski, P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract. Res. Clin. Gastroenterol. 2017, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Avruch, J.H.; Weeder, P.D.; Sridharan, G.V.; Uygun, B.E.; Karimian, N.G.; Porte, R.J.; Markmann, J.F.; Yeh, H.; Uygun, K. Functional Human Liver Preservation and Recovery by Means of Subnormothermic Machine Perfusion. J. Vis. Exp. 2015, 98, 52777. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Zideman, D.A.; Biarent, D.; Bossaert, L.L.; Deakin, C.; Koster, R.W.; Wyllie, J.; Böttiger, B. European Resuscitation Council Guidelines for Resuscitation. Resuscitation 2010, 81, 1305–1352. [Google Scholar]
- Erecinska, M.; Thoresen, M.; Silver, I.A. Effects of hypothermia on energy metabolism in mammalian central nervous system. J. Cereb. Blood Flow Metab. 2003, 23, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Bayir, H.; Adelson, P.D.; Wisniewski, S.R.; Shore, P.; Lai, Y.; Brown, D.; Janesko-Feldman, K.L.; Kagan, V.E.; Kochanek, P.M. Therapeutic Hypothermia Preserves Antioxidant Defenses after Severe Traumatic Brain Injury in Infants and Children. Crit. Care Med. 2009, 37, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Tissier, R.; Chenoune, M.; Pons, S.; Zini, R.; Darbera, L.; Lidouren, F.; Ghaleh, B.; Berdeaux, A.; Morin, D. Mild hypothermia reduces per-ischemic reactive oxygen species production and preserves mitochondrial respiratory complexes. Resuscitation 2013, 84, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, I.; Clarke, S.J.; Lin, H.; Parker, J.; Suleiman, M.S.; Halestrap, A.P. Temperature preconditioning of isolated rat hearts—A potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J. Physiol. 2007, 581, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Rizzo, V.; Boncompagni, E.; Bianchi, A.; Gringeri, E.; Neri, D.; Richelmi, P.; Freitas, I.; Cillo, U.; Vairetti, M. Machine perfusion at 20 °C reduces preservation damage to livers from non-heart beating donors. Cryobiology 2011, 62, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, T.; Alva, N.; Sanchez-Nunõ, S.; Dewey, S.; Gomes, A.V. Subnormothermic Perfusion in the Isolated Rat Liver Preserves the Antioxidant Glutathione and Enhances the Function of the Ubiquitin Proteasome System. Oxid. Med. Cell. Longev. 2016, 2016, 9324692. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Glutathione and apoptosis. Free Radic. Res. 2008, 42, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. Interference of plasmatic reduced glutathione and hemolysis on glutathione disulfide levels in human blood. Free Radic. Res. 2004, 38, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Becker, L.B. State of the Art in Therapeutic Hypothermia. Ann. Rev. Med. 2011, 62, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Cheng, G.Q.; Ma, S.M.; Yang, Y.; Shao, X.M.; Zhou, W.-H. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem. Int. 2011, 58, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Wang, X.Y.; Xu, F.L.; Qiu, L.; Cheng, X.Y.; Simbruner, G.; Blomgren, K. Intraischemic mild hypothermia prevents neuronal cell death and tissue loss after neonatal cerebral hypoxia-ischemia. Eur. J. Neurosci. 2006, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, O.; Friberg, H. Therapeutic hypothermia for comatose survivors after near-hanging—A retrospective analysis. Resuscitation 2009, 80, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Sharp, W.W.; Wojcik, K.R.; Li, C.Q.; Han, M.; Chang, W.T.; Ramachandran, S.; Li, J.; Hamann, K.J.; Hoek, T.L.V. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am. J. Physiol. Circ. Physiol. 2010, 298, H2164–H2173. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.L.; Kloner, R.A. Mild Hypothermia as a Cardioprotective Approach for Acute Myocardial Infarction: Laboratory to Clinical Application. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Richelmi, P.; Bertè, F.; Currin, R.T.; Lemasters, J.J.; Imberti, R. Role of pH in protection by low sodium against hypoxic injury in isolated perfused rat livers. J. Hepatol. 2006, 44, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Khalilov, R.A.; Dzhafarova, A.M.; Khizrieva, S.I. Effect of Hypothermia on Kinetic Characteristics of Lactate Dehydrogenase in Rat Brain under Conditions of Global Ischemia and Reperfusion. Bull. Exp. Biol. Med. 2017, 163, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Alia, M.; Horcajo, C.; Bravo, L.; Goya, L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr. Res. 2003, 23, 1251–1267. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Alva, N.; Azuara, D.; Palomeque, J.; Carbonell, T. Deep hypothermia protects against acute hypoxia in vivo in rats: A mechanism related to the attenuation of oxidative stress. Exp. Physiol. 2013, 98, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bell, H.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid. Med. Cell. Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Bessems, M.; T’ Hart, N.A.; Tolba, R.; Doorschodt, B.M.; Leuvenink, H.G.D.; Ploeg, R.J.; Minor, T.; Van Gulik, T.M. The isolated perfused rat liver: Standardization of a time-honoured model. Lab. Anim. 2006, 40, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.; Zhang, L.; Hussain, A.; Braet, F.; Ramzan, I. Assessment and histological analysis of the IPRL technique for sequential in situ liver biopsy. Comp. Hepatol. 2011, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yagi, K. Assays for blood plasme or serum. Methods Enzymol. 1976, 105, 328–331. [Google Scholar]
- Hu, M.L. Measurement of Protein Thiol-Groups and Glutathione in Plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [PubMed]
- Aydın, S.; Atukeren, P.; Çakatay, U.; Uzun, H.; Altuğ, T. Gender-dependent oxidative variations in liver of aged rats. Biogerontology 2010, 11, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of malondialdehide precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]

| Fresh Resected Baseline | Hypothermia PC IR | Normothermia IR | |
|---|---|---|---|
| AOPP µmol/g wet weight | 6.86 ± 0.19 | 4.33 ± 0.76 + | 6.36 ± 0.49 * |
| TBARS nmol/g wet weight | 37.35 ± 1.32 | 37.22 ± 5.15 | 61.40 ± 9.45 *+ |
| NOx µmol/g wet weight | 0.16 ± 0.02 | 0.23 ± 0.06 | 0.43 ± 0.07 *++ |
| GSH + GSSG µmol/g wet weight | 1.66 ± 0.10 | 1.22 ± 0.25 | 0.79 ± 0.12 + |
| GSH µmol/g wet weight | 1.32 ± 0.07 | 1.11 ± 0.22 | 0.50 ± 0.07 **++ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alva, N.; Bardallo, R.G.; Basanta, D.; Palomeque, J.; Carbonell, T. Preconditioning-Like Properties of Short-Term Hypothermia in Isolated Perfused Rat Liver (IPRL) System. Int. J. Mol. Sci. 2018, 19, 1023. https://doi.org/10.3390/ijms19041023
Alva N, Bardallo RG, Basanta D, Palomeque J, Carbonell T. Preconditioning-Like Properties of Short-Term Hypothermia in Isolated Perfused Rat Liver (IPRL) System. International Journal of Molecular Sciences. 2018; 19(4):1023. https://doi.org/10.3390/ijms19041023
Chicago/Turabian StyleAlva, Norma, Raquel G. Bardallo, David Basanta, Jesús Palomeque, and Teresa Carbonell. 2018. "Preconditioning-Like Properties of Short-Term Hypothermia in Isolated Perfused Rat Liver (IPRL) System" International Journal of Molecular Sciences 19, no. 4: 1023. https://doi.org/10.3390/ijms19041023