Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts
Abstract
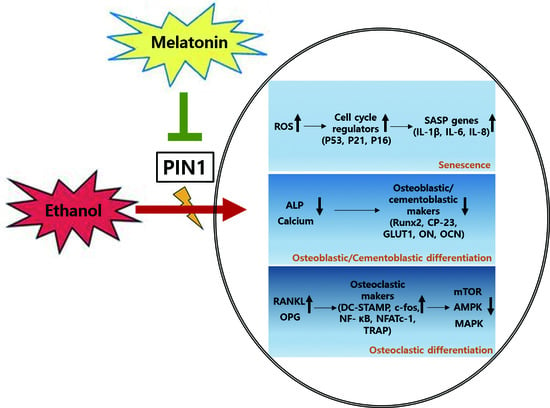
:1. Introduction
2. Results
2.1. EtOH Treatment Induces Cell Death and Features of Premature Senescence in PDLCs and Cementoblasts
2.2. Melatonin Reduces EtOH-Induced Cellular Senescence in PDLCs and Cementoblasts
2.3. Involvement of the PIN1 Pathway in the Anti-Senescence Effects of Melatonin
2.4. Melatonin Reverses EtOH-Suppressed Cementoblastic/Osteoblastic Differentiation
2.5. Melatonin Reverses EtOH-Induced Osteoclastic Differentiation
2.6. AMPK, mTOR and MAPK Signaling Cascades Are Involved in the Effects of Melatonin on EtOH-Mediated Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytotoxicity Assay
4.3. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
4.4. Reactive Oxygen Species (ROS) Detection
4.5. Cell Cycle Analysis
4.6. FITC-Annexin V/PI Double Staining
4.7. PIN1 siRNA Transfection
4.8. ALP Activity and Alizarin Red Staining
4.9. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.10. Western Blot Analysis
4.11. Preparation of Conditioned Medium
4.12. In Vitro Osteoclast Differentiation
4.13. Immunocytochemistry
4.14. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Kocher, T.; König, J.; Dzierzon, U.; Sawaf, H.; Plagmann, H.C. Disease progression in periodontally treated and untreated patients: A retrospective study. J. Clin. Periodontol. 2000, 27, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Seo, Y.H.; Bae, W.J.; Lee, H.S.; Choi, Y.C.; Kim, E.C. Milk activates the expression of cytokines via Nrf2/HO-1 pathway in human periodontal ligament cells. Dent. Traumatol. 2015, 31, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Park, K.H.; Kim, S.J.; Kang, Y.G.; Lee, Y.M.; Kim, E.C. Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin. Exp. Immunol. 2012, 168, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Pi, S.H.; Lee, Y.M.; Lee, S.I.; Kim, E.C. The anti-inflammatory role of heme oxygenase-1 in lipopolysaccharide and cytokine-stimulated inducible nitric oxide synthase and nitric oxide production in human periodontal ligament cells. J. Periodontol. 2009, 80, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, Y.S.; Lee, S.Y.; Bae, W.J.; Park, Y.D.; Hyun, Y.C.; Kang, K.; Kim, E.C. Expression of phospholipase D in periodontitis and its Role in the Inflammatory and osteoclastic response by nicotine- and Lipopolysaccharide-stimulated human periodontal ligament cells. J. Periodontol. 2015, 86, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, W.J.; Narayanan, A.S. Cementum and periodontal wound healing and regeneration. Crit. Rev. Oral Biol. Med. 2002, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Shimizu, N.; Yamaguchi, M.; Suzuki, H.; Takiguchi, H. Effect of ageing on functional changes of periodontal tissue cells. Ann. Periodontol. 1998, 3, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Yamaguchi, M.; Shimizu, N.; Abiko, Y. Mechanical stress enhances expression and production of plasminogen activatorin ageing human periodontal ligament cells. Mech. Ageing 2000, 112, 217–231. [Google Scholar] [CrossRef]
- Nishimura, F.; Terranova, V.P.; Braithwaite, M.; Orman, R.; Ohyama, H.; Mineshiba, J.; Chou, H.H.; Takashiba, S.; Murayama, Y. Comparison of in vitro proliferative capacity of human periodontal ligament cells in juvenile and aged donors. Oral Dis. 1997, 3, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S.; Tonna, E.A. H3-proline study of aging periodontal ligament matrix formation: Comparison between matrices adjacent to either cemental or bone surfaces. J. Periodontal Res. 1977, 12, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Goseki, T.; Yamaguchi, M.; Iwasawa, T.; Takiguchi, H.; Abiko, Y. In vitro cellular aging stimulates interleukin-1 beta production in stretched human periodontal-ligament-derived cells. J. Dent. Res. 1997, 76, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Wu, RX.; Bi, CS.; Yu, Y.; Zhang, LL.; Chen, FM. Age-related decline in the matrix contents and functional properties of human periodontal ligament stem cell sheets. Acta Biomater. 2015, 22, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, U. Effect of age on the periodontium. J. Clin. Periodontol. 1984, 11, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Konstantonis, D.; Papadopoulou, A.; Makou, M.; Eliades, T.; Basdra, E.K.; Kletsas, D. The role of cellular senescence on the cyclic stretching-mediated activation of MAPK and ALP expression and activity in human periodontal ligament fibroblasts. Exp. Gerontol. 2014, 57, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Konstantonis, D.; Papadopoulou, A.; Makou, M.; Eliades, T.; Basdra, E.K.; Kletsas, D. Senescent human periodontal ligament fibroblasts after replicative exhaustion or ionizing radiation have a decreased capacity towards osteoblastic differentiation. Biogerontology 2013, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res. 2011, 51, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Noh, K.; Jue, S.S.; Lee, S.Y.; Kim, E.C. Melatonin promotes hepatic differentiation of human dental pulp stem cells: Clinical implications for the prevention of liver fibrosis. J. Pineal Res. 2015, 58, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Aging and oxygen toxicity: Relation to changes in melatonin. Age 1997, 20, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig, Á.; Rancan, L.; Paredes, S.D.; Carrasco, A.; Escames, G.; Vara, E.; Tresguerres, J.A. Melatonin decreases the expression of inflammation and apoptosis markers in the lung of a senescence-accelerated mice model. Exp. Gerontol. 2016, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, J.A.; Cuesta, S.; Kireev, R.A.; Garcia, C.; Acuña-Castroviejo, D.; Vara, E. Beneficial effect of melatonin treatment on age-related insulin resistance and on the development of type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2013, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernández, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Gutierrez-Cuesta, J.; Pallas, M.; Camins, A.; et al. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in senescence-accelerated mice. J. Pineal Res. 2008, 45, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Kim, A.J.; Kim, H.J.; Jee, H.J.; Kim, M.; Yoo, Y.H.; Yun, J. Melatonin suppressesdoxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J. Pineal Res. 2012, 53, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Cho, Y.C.; Sung, I.Y.; Kim, I.R.; Park, B.S.; Kim, Y.D. Melatonin promotes osteoblast differentiation and mineralization of MC3T3-E1 cells under hypoxic conditions through activation of PKD/p38 pathways. J. Pineal Res. 2014, 57, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kang, J.W.; Lee, E.M.; Kim, J.S.; Rhee, Y.H.; Kim, M.; Jeong, S.J.; Park, Y.G.; Kim, S.H. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J. Pineal Res. 2011, 51, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Liu, T.; Gong, Y.; Chen, S.; Pan, G.; Cui, W.; Luo, Z.P.; Pei, M.; Yang, H.; et al. Melatonin reverses H2O2-induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J. Pineal Res. 2015, 59, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Amaral Cda, S.; Vettore, M.V.; Leão, A. The relationship of alcohol dependence and alcohol consumption with periodontitis: A systematic review. J. Dent. 2009, 37, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Haley, R.L.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereasestradiol attenuates the effects of ethanol in osteoblasts. J. Bone Miner. Res. 2009, 24, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Toko, H.; Hariharan, N.; Konstandin, M.H.; Ormachea, L.; McGregor, M.; Gude, N.A.; Sundararaman, B.; Joyo, E.; Joyo, A.Y.; Collins, B.; et al. Differential regulation of cellular senescence and differentiation by prolylisomerase Pin1 in cardiac progenitor cells. J. Biol. Chem. 2014, 289, 5348–5356. [Google Scholar] [CrossRef]
- Cetinus, E.; Kilinc, M.; Uzel, M.; Inanc, F.; Kurutas, E.B.; Bilgic, E.; Karaoguz, A. Does long-term ischemia affect the oxidant status during fracture healing? Arch. Orthop. Trauma Surg. 2005, 125, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Yeler, H.; Tahtabas, F.; Candan, F. Investigation of oxidative stress during fracture healing in the rats. Cell Biochem. Funct. 2005, 23, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Nakade, O.; Takada, Y.; Kaku, T.; Lau, K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 2002, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.; Uslu, S.; Vatansever, S.H.; Aktug, H.; Yurtseven, M.E.; Uysal, A. Evaluation of the relationship between inducible nitric oxide synthase (iNOS) activity and effects of melatonin in experimental osteoporosis in the rat. Surg. Radiol. Anat. 2006, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Histing, T.; Anton, C.; Scheuer, C.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Pohlemann, T.; Menger, M.D. Melatonin impairs fracture healing by suppressing RANKL-mediated bone remodeling. J. Surg. Res. 2012, 173, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Uslu, S.; Uysal, A.; Oktem, G.; Yurtseven, M.; Tanyalçin, T.; Başdemir, G. Constructive effect of exogenous melatonin against osteoporosis after ovariectomy in rats. Anal. Quant. Cytol. Histol. 2007, 29, 317–325. [Google Scholar] [PubMed]
- Satué, M.; Ramis, J.M.; del Mar Arriero, M.; Monjo, M. A new role for 5-methoxytryptophol on bone cells function in vitro. J. Cell. Biochem. 2015, 116, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Arana, C.; Gómez-Moreno, G.; Escames, G.; López, A.; Ferrera, M.J.; Reiter, R.J.; Acuña-Castroviejo, D. Local application of melatonin into alveolar sockets of beagle dogs reduces tooth removal–induced oxidative stress. J. Periodontol. 2007, 78, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Ramis, J.M.; Monjo, M. Anti-fibrotic and anti- inflammatory properties of melatonin on human gingival fibroblasts in vitro. Biochem. Pharmacol. 2013, 86, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Yonemura, T.; Kaya, H. Increased oxidative product formation by peripheral blood polymorphonuclear leukocytes in human periodontal diseases. J. Periodontal Res. 1993, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, C.; Zucchelli, G.; Bernardi, F.; Scheda, M.; Valentini, A.F.; Calandriello, M. Enhanced superoxide production with no change of the antioxidant activity in gingival fluid of patients with chronic adult periodontitis. Free Radic. Res. Commun. 1991, 15, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S. Alcohol consumption a risk factor for periodontal disease. Evid. Based Dent. 2011, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshima, T.; Enoki, N.; Kobayashi, I.; Sakai, T.; Nagata, K.; Wada, H.; Fujiwara, H.; Ookuma, Y.; Sakai, H. Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. Int. J. Mol. Med. 2012, 30, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, L.; Gouveia, A.; Almeida, H. Copper ability to induce premature senescence in human fibroblasts. Age (Dordr.) 2012, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Osuna, C.; García-Pergañeda, A.; Carrillo-Vico, A.; Guerrero, J.M. The pineal secretory product melatonin reduces hydrogen peroxide-induced DNA damage in U-937 cells. J. Pineal Res. 1999, 26, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Rodella, L.F.; Nardo, L.; Giugno, L.; Cocchi, M.A.; Borsani, E.; Reiter, R.J.; Rezzani, R. A comparison of melatonin and α-lipoic acid in the induction of antioxidant defences in L6 rat skeletal muscle cells. Age (Dordr.) 2015, 37, 9824. [Google Scholar] [CrossRef] [PubMed]
- Satomura, K.; Tobiume, S.; Tokuyama, R.; Yamasaki, Y.; Kudoh, K.; Maeda, E.; Nagayama, M. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J. Pineal Res. 2007, 42, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, Y.M.; Kim, H.S.; Lee, K.Y. Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci. Rep. 2017, 7, 5716. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Liou, Y.C.; Zhou, X.Z. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002, 12, 164–172. [Google Scholar] [CrossRef]
- Lu, K.P.; Zhou, X.Z. The prolylisomerasePIN1: A pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.J.; Shin, M.R.; Kang, S.K.; Kim, J.Y.; Lee, S.C.; Kim, E.C. HIF-2 inhibition supresses inflammatory responses and osteoclastic differentiation in human periodontal ligament cells. J. Cell. Biochem. 2015, 116, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Kwak, H.B.; Lee, B.K.; Oh, J.; Yeon, J.T.; Choi, S.W.; Cho, H.J.; Lee, M.S.; Kim, J.J.; Bae, J.M.; Kim, S.H.; et al. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone 2010, 46, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Natarajan, V.; Demidenko, Z.N.; Burdelya, L.G.; Gudkov, A.V.; Blagosklonny, M.V. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc. Natl. Acad. Sci. USA 2012, 109, 13314–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef] [PubMed]
- Sarlak, G.; Jenwitheesuk, A.; Chetsawang, B.; Govitrapong, P. Effects of melatonin on nervous system aging: Neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013, 123, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.K.; Wang, Y.J.; Wang, Y.; Wang, S.; Tan, P.; Huang, W.; Liu, Y.S. The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can. J. Cardiol. 2014, 30, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Xie, Z.; Case, N.; Thompson, W.R.; Uzer, G.; Styner, M.; Rubin, J. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 2014, 29, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kim, J.K.; Mortensen, R.; Mutyaba, L.P.; Hankenson, K.D.; Krebsbach, P.H. Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem Cells 2013, 31, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Kudo, Y.; Iizuka, S.; Ogawa, I.; Abiko, Y.; Miyauchi, M.; Takata, T. Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem. Biophys. Res. Commun. 2006, 349, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Tahara, H.; Kitagawa, S.; Oka, H.; Kudo, Y.; Sato, S.; Ogawa, I.; Miyaichi, M.; Takata, T. Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo. Bone 2006, 39, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Lee, D.W.; Yun, H.M.; Cha, H.J.; Bae, C.H.; Cho, E.S.; Kim, E.C. Expression of thymosin beta-4 in human periodontal ligament cells and mouse periodontal tissue and its role in osteoblastic/cementoblastic differentiation. Differentiation 2015, 90, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Auh, Q.S.; Kang, S.K.; Kim, H.J.; Lee, J.W.; Noh, K.; Jang, J.H.; Kim, E.C. Combined effects of dentin sialoprotein and bone morphogenetic protein-2 on differentiation in human cementoblasts. Cell Tissue Res. 2014, 357, 119–132. [Google Scholar] [CrossRef] [PubMed]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, W.-J.; Park, J.S.; Kang, S.-K.; Kwon, I.-K.; Kim, E.-C. Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts. Int. J. Mol. Sci. 2018, 19, 1742. https://doi.org/10.3390/ijms19061742
Bae W-J, Park JS, Kang S-K, Kwon I-K, Kim E-C. Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts. International Journal of Molecular Sciences. 2018; 19(6):1742. https://doi.org/10.3390/ijms19061742
Chicago/Turabian StyleBae, Won-Jung, Jae Suh Park, Soo-Kyung Kang, Il-Keun Kwon, and Eun-Cheol Kim. 2018. "Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts" International Journal of Molecular Sciences 19, no. 6: 1742. https://doi.org/10.3390/ijms19061742