AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin
Abstract
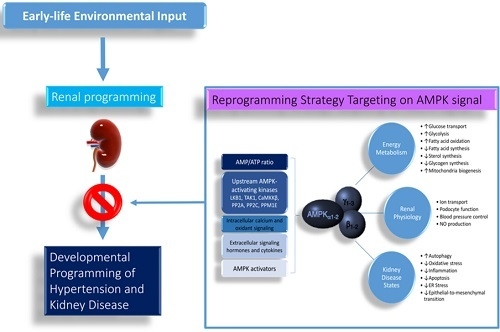
:1. Introduction
2. AMP-Activated Protein Kinase in the Renal System
2.1. The Structure and Function of AMP-Activated Protein Kinase
2.2. Regulation of AMP-Activated Protein Kinase AMP-Activated Protein Kinase in the Kidney
2.3. AMP-Activated Protein Kinase in Hypertension and Kidney Disease
3. Common Mechanisms Link AMP-Activated Protein Kinase to Renal Programming
3.1. Renin–Angiotensin System
3.2. Sodium Transporters
3.3. Nutrient-Sensing Signals
3.4. Oxidative Stress
4. Reprogramming Strategy Targeting AMP-Activated Protein Kinase Signaling
4.1. Metformin
4.2. Resveratrol and Other Polyphenols
4.3. Thiazolidinediones
4.4. Others
5. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| AICAR | 5-aminoimidazole-4carboxamide riboside |
| AMPK | AMP-activated protein kinase |
| AT1R | Angiotensin II type 1 receptor |
| CaMKKβ | Ca2+-/calmodulin-dependent protein kinase β |
| DOHaD | Developmental origins of health and disease |
| LKB1 | Liver kinase B1 |
| mTOR | Mammalian target of rapamycin |
| NCC | Na+/Cl− cotransporter |
| NHE3 | Type 3 sodium hydrogen exchanger |
| NKCC2 | Na-K-2Cl cotransporter |
| PGC-1α | Peroxisome proliferator-activated receptor γ coactivator-1α |
| PPAR | Peroxisome proliferator-activated receptor |
| PPM1E | Mg2+-/Mn2+-dependent protein phosphatase 1E |
| PP2A | Protein phosphatase 2A |
| PP2C | protein phosphatase 2C |
| RAS | Renin-angiotensin system |
| SD | Sprague–Dawley |
| SHR | Spontaneously hypertensive rat |
| SIRT | Silent information regulator transcript |
| TAK1 | TGFβ-activated kinase 1 |
| Ulk1 | Unc-51-like kinase 1 |
References
- Chong, E.; Yosypiv, I.V. Developmental programming of hypertension and kidney disease. Int. J. Nephrol. 2012, 2012, 15. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Si, L.Y. Protective effects of AMP-activated protein kinase in the cardiovascular system. J. Cell Mol. Med. 2010, 14, 2604–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajani, R.; Pastor-Soler, N.M.; Hallows, K.R. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.; Silvestre, R.; Cordeiro-da-Silva, A.; Estaquier, J.; Foretz, M.; Viollet, B. AMP-activated Protein Kinase as a Target For Pathogens: Friends Or Foes? Curr. Drug Targets 2016, 17, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.; Mount, P.; Hill, R.; Levidiotis, V.; Katsis, F.; Stapleton, D.; Kemp, B.E.; Power, D.A. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am. J. Physiol. Renal Physiol. 2005, 288, F578–F586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Caton, P.W.; Holness, M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010, 204, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 2007, 115, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y. PPARs Link Early Life Nutritional insults to later programmed hypertension and metabolic syndrome. Int. J. Mol. Sci. 2015, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Calvani, R.; Bernabei, R.; Leeuwenburgh, C.; Marzetti, E. Contribution of impaired mitochondrial autophagy to cardiac aging: Mechanisms and therapeutic opportunities. Circ. Res. 2012, 110, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Allouch, S.; Munusamy, S. AMP-activated Protein Kinase as a Drug Target in Chronic Kidney Disease. Curr. Drug Targets 2018, 19, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Carattino, M.D.; Edinger, R.S.; Grieser, H.J.; Wise, R.; Neumann, D.; Schlattner, U.; Johnson, J.P.; Kleyman, T.R.; Hallows, K.R. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J. Biol. Chem. 2005, 280, 17608–17616. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.A.; Gimenez, I.; Cook, N.; Jennings, I.; Katerelos, M.; Katsis, F.; Levidiotis, V.; Kemp, B.E.; Power, D.A. Regulation of the renal-specific Na+–K+–2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem. J. 2007, 405, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, I.; Dada, L.A.; Briva, A.; Trejo, H.E.; Welch, L.C.; Chen, J.; Toth, P.T.; Lecuona, E.; Witters, L.A.; Schumacker, P.T.; et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Invest. 2008, 118, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Hallows, K.R.; Alzamora, R.; Li, H.; Gong, F.; Smolak, C.; Neumann, D.; Pastor-Soler, N.M. AMP-activated protein kinase inhibits alkaline pH and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am. J. Physiol. Cell Physiol. 2009, 296, C672–C681. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.M.; Hallows, K.R. AMP-activated protein kinase regulation of kidney tubular transport. Curr. Opin. Nephrol. Hypertens. 2012, 21, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.J.; Teschke, S.R.; Reid, E.B.; Durham, K.K.; Kroetsch, J.T.; Rush, J.W. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J. Hypertens. 2012, 30, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Kuo, H.C.; Hsu, C.N.; Huang, L.T.; Tain, Y.L. Metformin reduces asymmetric dimethylarginine and prevents hypertension in spontaneously hypertensive rats. Transl. Res. 2014, 164, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Care, A.S.; Sung, M.M.; Panahi, S.; Gragasin, F.S.; Dyck, J.R.; Davidge, S.T.; Bourque, S.L. Perinatal Resveratrol Supplementation to Spontaneously Hypertensive Rat Dams Mitigates the Development of Hypertension in Adult Offspring. Hypertension 2016, 67, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.H.; Lee, W.C.; Leu, S.; Chan, J.Y.H. Prenatal Metformin Therapy Attenuates Hypertension of Developmental Origin in Male Adult Offspring Exposed to Maternal High-Fructose and Post-Weaning High-Fat Diets. Int. J. Mol. Sci. 2018, 19, 1066. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int. J. Mol. Sci. 2017, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, S.H.H.; Chan, J.Y.H. Biochemical basis for pharmacological intervention as a reprogramming strategy against hypertension and kidney disease of developmental origin. Biochem. Pharmacol. 2018, 153, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Yosypiv, I.V. Renin-angiotensin system in ureteric bud branching morphogenesis: Insights into the mechanisms. Pediatr. Nephrol. 2011, 26, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. 2014, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Siragy, H.M. The angiotensin II type 2 receptor and the kidney. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Dhande, I.; Samuel, P.; Hussain, T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin II type 2 receptor/angiotensin (1-7)/Mas receptor and exhibit greater high-fat diet-induced kidney injury. J. Renin Angiotensin Aldosterone Syst. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin. Sci. 1998, 94, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y.; Tain, Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.K.; Sui, Y.; Zhou, H.R.; Shen, J.; Tan, N.; Huang, Y.M.; Li, S.S.; Pan, Y.H.; Zhang, X.X.; Zhao, H.L. Cross-talk between AMP-activated protein kinase and renin-angiotensin system in uninephrectomised rats. J. Renin Angiotensin Aldosterone Syst. 2016, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlstedt, K.; Trouvain, C.; Boettger, T.; Shi, L.; Fisslthaler, B.; Fleming, I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ. Res. 2013, 112, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Leu, S.; Wu, K.; Chan, J. High salt exacerbates programmed hypertension in maternal fructose-fed male offspring. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.; Leu, S.; Chan, J.Y.H. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Mol. Nutr. Food Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Berry, B.J.; Wojtovich, A.P. Exercise and Mitochondrial Dynamics: Keeping in Shape with ROS and AMPK. Antioxidants 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zou, M.H. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 2012, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nüsken, E.; Dötsch, J.; Weber, L.T.; Nüsken, K.D. Developmental Programming of Renal Function and Re-Programming Approaches. Front. Pediatr. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Luo, T.; Luo, X.; Tang, Z. Resveratrol prevents Ang II-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.J.; Rush, J.W. Endothelium-dependent vasorelaxation to the AMPK activator AICAR is enhanced in aorta from hypertensive rats and is NO and EDCF dependent. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H64–H75. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Foretz, M.; Viollet, B. Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 2018, 153, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Salatto, C.T.; Miller, R.A.; Cameron, K.O.; Cokorinos, E.; Reyes, A.; Ward, J.; Calabrese, M.F.; Kurumbail, R.G.; Rajamohan, F.; Kalgutkar, A.S.; et al. Selective Activation of AMPK β1-Containing Isoforms Improves Kidney Function in a Rat Model of Diabetic Nephropathy. J. Pharmacol. Exp. Ther. 2017, 361, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Javkhedkar, A.A.; Banday, A.A. Antioxidant resveratrol restores renal sodium transport regulation in SHR. Physiol. Rep. 2015, 3, e12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Morton, J.S.; Dolinsky, V.W.; Dyck, J.R.; Davidge, S.T. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R418–R426. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xing, W.; He, J.; Fu, F.; Zhang, W.; Su, F.; Liu, F.; Ji, L.; Gao, F.; Su, H.; et al. Magnolol administration in normotensive young spontaneously hypertensive rats postpones the development of hypertension: Role of increased PPARγ, reduced TRB3 and resultant alleviative vascular insulin resistance. PLoS ONE 2015, 10, e0120366. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sun, H.; Zhang, H.; Zhang, Y. Anti-hypertensive and renoprotective effects of berberine in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2015, 37, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.M.; Peng, N.; Clark, J.T.; Novak, L.; Roysommuti, S.; Prasain, J.; Wyss, J.M. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology 2007, 148, 5396–5402. [Google Scholar] [CrossRef] [PubMed]
- Dovinová, I.; Barancik, M.; Majzunova, M.; Zorad, S.; Gajdosechová, L.; Gresová, L.; Cacanyiova, S.; Kristek, F.; Balis, P.; Chan, J.Y. Effects of PPARγ agonist pioglitazone on redox-sensitive cellular signaling in young spontaneously hypertensive rats. PPAR Res. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, R.; de Champlain, J.; Wilson, T.W. Beneficial and deleterious effects of rosiglitazone on hypertension development in spontaneously hypertensive rats. Am. J. Hypertens. 2004, 17, 749–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.W.; He, S.J.; Feng, X.; Cheng, J.; Luo, Y.T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- De Broe, M.E.; Kajbaf, F.; Lalau, J.D. Renoprotective Effects of Metformin. Nephron 2018, 138, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The effect of fructose on renal biology and disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, J.; Symons, J.D.; Wu, T.C.; Bruno, R.S.; Litwin, S.E.; Jalili, T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J. Nutr. 2007, 137, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Wesseling, S.; Sánchez, M.; Braam, B.; Joles, J.A. Perinatal Inhibition of NF-κB has long-term antihypertensive and renoprotective effects in fawn-hooded hypertensive rats. Am. J. Hypertens. 2016, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Vicente, A.; Hopfer, U.; Garvin, J.L. Developing Tools for Analysis of Renal Genomic Data: An Invitation to Participate. J. Am. Soc. Nephrol. 2017, 28, 3438–3440. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Braam, B.; Joles, J.A. Perinatal inhibition of NF-kappaB has long-term antihypertensive effects in spontaneously hypertensive rats. J. Hypertens. 2011, 29, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Ozaki, H.; Serita, Y.; Sato, S. Maternal fructose intake during pregnancy modulates hepatic and hypothalamic AMP-activated protein kinase signalling in a sex-specific manner in offspring. Clin. Exp. Pharmacol. Physiol. 2014, 41, 331–337. [Google Scholar] [CrossRef] [PubMed]

| Animal Models | Gender/Species | Age at Evaluation | Dose and Period of Treatment | Reprogramming Effects | Ref. |
|---|---|---|---|---|---|
| Metformin | |||||
| SHR 1 | Male SHR | 12 weeks | Metformin (500 mg/kg/day) between 4 to 12 weeks of age | Prevented hypertension | [25] |
| Maternal high-fructose plus post-weaning high-fat diet | Male SD 2 rats | 12 weeks | Metformin (500 mg/kg/day) for 3 weeks during pregnancy | Attenuated hypertension; | [28] |
| Resveratrol and other polyphenols | |||||
| SHR | Male SHR | 11 weeks | Resveratrol (50 mg/L) in drinking water between 3–11 weeks of age | Attenuated hypertension | [55] |
| SHR | Male and female SHR | 12 weeks | Resveratrol (4g/kg of diet) between gestational day 0.5 and postnatal day 21 | Attenuated hypertension | [26] |
| SHR | Male SHR | 13 weeks | Resveratrol (50 mg/L) in drinking water between 3–13 weeks of age | Attenuated hypertension | [56] |
| Prenatal hypoxia and postnatal high-fat diet | Male SD rats | 12 weeks | Resveratrol (4g/kg of diet) between 3–12 weeks of age | Prevented hypertension | [57] |
| Maternal plus post-weaning high-fructose diets | Male SD rats | 12 weeks | Resveratrol (50 mg/L) in drinking water from weaning to three months of age | Prevented hypertension | [46] |
| Maternal plus post-weaning high-fat diets | Male SD rats | 16 weeks | 0.5% resveratrol in drinking water between 2 and 4 months of age | Prevented hypertension | [27] |
| SHR | Male SHR | 7 weeks | Magnolol (100 mg/kg/day) between 4 to 7 weeks of age | Attenuated hypertension | [58] |
| SHR | Male SHR | 20 weeks | Berberine (100 mg/kg/day) between 3 to 20 weeks of age | Attenuated hypertension and kidney damage | [59] |
| High-salt stroke-prone SHR | Male stroke-prone SHR | 16 weeks | Genistein (0.06% wt/wt diet) between 7 to 16 weeks of age | Attenuated hypertension and kidney damage | [60] |
| Thiazolidinediones | |||||
| SHR | Male SHR | 7 weeks | Pioglitazone (10 mg/kg/day) between 5 to 7 weeks of age | Attenuated hypertension | [61] |
| SHR | Male SHR | 13 weeks | Rosiglitazone (150 mg/kg/day) between 5 to 13 weeks of age | Attenuated hypertension | [62] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. https://doi.org/10.3390/ijms19061744
Tain Y-L, Hsu C-N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. International Journal of Molecular Sciences. 2018; 19(6):1744. https://doi.org/10.3390/ijms19061744
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2018. "AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin" International Journal of Molecular Sciences 19, no. 6: 1744. https://doi.org/10.3390/ijms19061744
APA StyleTain, Y.-L., & Hsu, C.-N. (2018). AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. International Journal of Molecular Sciences, 19(6), 1744. https://doi.org/10.3390/ijms19061744