Peribiliary Glands as the Cellular Origin of Biliary Tract Cancer
Abstract
:1. Introduction
2. Chronic Inflammation Is Key in the Epidemiology and Risk Factors of CCA
3. Mutation Spectrum of CCA
4. Cellular Origins of iCAA
5. PBGs Are Possible Stem/Progenitor Niches for BECs
6. A Mouse Model Suggests PBGs Are the Cellular Origin of CCA
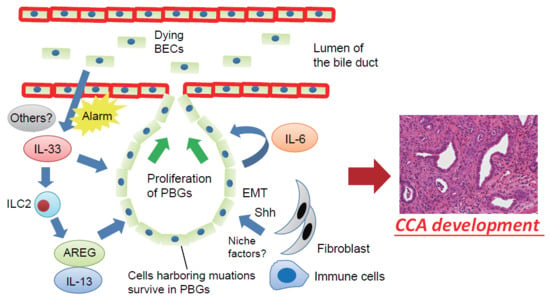
7. Interleukin (IL)-33 Promotes Proliferation of PBGs and Development of CCAs
8. PBGs Are the Potential Cellular Origin of Intraductal Papillary Neoplasms of the Biliary Duct
9. Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Miyata, T.; Uchida, T. Latest advances in the pathological understanding of cholangiocarcinomas. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Gores, G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017, 67, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Sia, D.; Bardeesy, N.; Mazzaferro, V.; Llovet, J.M. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2016, 22, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Tang, D.G. Cell-of-origin of cancer versus cancer stem cells: Assays and interpretations. Cancer Res. 2015, 75, 4003–4011. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Fox, J.G.; Wang, T.C. The origins of gastric cancer from gastric stem cells: Lessons from mouse models. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, S.; Suzuki, A. Intrahepatic cholangiocarcinoma can arise from notch-mediated conversion of hepatocytes. J. Clin. Investig. 2012, 122, 3914–3918. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Malato, Y.; Calvisi, D.F.; Naqvi, S.; Razumilava, N.; Ribback, S.; Gores, G.J.; Dombrowski, F.; Evert, M.; Chen, X.; et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Investig. 2012, 122, 2911–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Suzuki, N.; Hirata, Y.; Hikiba, Y.; Hayakawa, Y.; Kinoshita, H.; Ihara, S.; Uchino, K.; Nishikawa, Y.; Ijichi, H.; et al. Biliary epithelial injury-induced regenerative response by IL-33 promotes cholangiocarcinogenesis from peribiliary glands. Proc. Natl. Acad. Sci. USA 2017, 114, E3806–E3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the european network for the study of cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010, 101, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergquist, A.; Ekbom, A.; Olsson, R.; Kornfeldt, D.; Loof, L.; Danielsson, A.; Hultcrantz, R.; Lindgren, S.; Prytz, H.; Sandberg-Gertzen, H.; et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J. Hepatol. 2002, 36, 321–327. [Google Scholar] [CrossRef]
- Burak, K.; Angulo, P.; Pasha, T.M.; Egan, K.; Petz, J.; Lindor, K.D. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2004, 99, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Morris-Stiff, G.; Bhati, C.; Olliff, S.; Hubscher, S.; Gunson, B.; Mayer, D.; Mirza, D.; Buckels, J.; Bramhall, S.R. Cholangiocarcinoma complicating primary sclerosing cholangitis: A 24-year experience. Dig. Surg. 2008, 25, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Nakagawa, H.; Maeda, S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J. Gastroenterol. 2012, 18, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kamata, H.; Luo, J.L.; Leffert, H.; Karin, M. Ikkbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005, 121, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Umemura, A.; Taniguchi, K.; Font-Burgada, J.; Dhar, D.; Ogata, H.; Zhong, Z.; Valasek, M.A.; Seki, E.; Hidalgo, J.; et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Arai, Y.; Totoki, Y. Molecular genomic landscapes of hepatobiliary cancer. Cancer Sci. 2018, 109, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Chan-On, W.; Nairismagi, M.L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.; Nguyen, H.H.; et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- De Jong, I.E.M.; van Leeuwen, O.B.; Lisman, T.; Gouw, A.S.H.; Porte, R.J. Repopulating the biliary tree from the peribiliary glands. Biochim. Biophys. Acta 2018, 1864, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Roskams, T.; Desmet, V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat. Rec. 2008, 291, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Samak, G. Bile duct epithelial tight junctions and barrier function. Tissue Barriers 2013, 1, e25718. [Google Scholar] [CrossRef] [PubMed]
- De Assuncao, T.M.; Jalan-Sakrikar, N.; Huebert, R.C. Regenerative medicine and the biliary tree. Semin. Liver Dis. 2017, 37, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kordes, C.; Haussinger, D. Hepatic stem cell niches. J. Clin. Investig. 2013, 123, 1874–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanpain, C. Tracing the cellular origin of cancer. Nat. Cell Biol. 2013, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yanger, K.; Zong, Y.; Maggs, L.R.; Shapira, S.N.; Maddipati, R.; Aiello, N.M.; Thung, S.N.; Wells, R.G.; Greenbaum, L.E.; Stanger, B.Z. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013, 27, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarlow, B.D.; Pelz, C.; Naugler, W.E.; Wakefield, L.; Wilson, E.M.; Finegold, M.J.; Grompe, M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 2014, 15, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Tanimizu, N.; Nishikawa, Y.; Ichinohe, N.; Akiyama, H.; Mitaka, T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J. Biol. Chem. 2014, 289, 7589–7598. [Google Scholar] [CrossRef] [PubMed]
- Aishima, S.; Fujita, N.; Mano, Y.; Iguchi, T.; Taketomi, A.; Maehara, Y.; Oda, Y.; Tsuneyoshi, M. P62+ hyaline inclusions in intrahepatic cholangiocarcinoma associated with viral hepatitis or alcoholic liver disease. Am. J. Clin. Pathol. 2010, 134, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Terakado, Y.; Nakagawa, H.; Hikiba, Y.; Fujii, T.; Matsubara, D.; Noguchi, R.; Zhu, C.; Yamamoto, K.; Kudo, Y.; et al. A novel mouse model of intrahepatic cholangiocarcinoma induced by liver-specific Kras activation and Pten deletion. Sci. Rep. 2016, 6, 23899. [Google Scholar] [CrossRef] [PubMed]
- Guest, R.V.; Boulter, L.; Kendall, T.J.; Minnis-Lyons, S.E.; Walker, R.; Wigmore, S.J.; Sansom, O.J.; Forbes, S.J. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014, 74, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Lange, A.W.; Lin, S.C.; Kaestner, K.H.; Lowy, A.M.; Kim, I.; Whitsett, J.A.; Wells, J.M. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 2009, 17, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.A.; Kagami, H.; Shi, L.; Holland, A.M.; Elsasser, H.P.; Hammer, R.E.; MacDonald, R.J. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev. Biol. 2005, 286, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Raynaud, P.; Cordi, S.; Zong, Y.; Tronche, F.; Stanger, B.Z.; Jacquemin, P.; Pierreux, C.E.; Clotman, F.; Lemaigre, F.P. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 2009, 136, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Carpino, G.; Reid, L.; Gaudio, E.; Alvaro, D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J. Gastrointest. Oncol. 2012, 4, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.; Carpino, G.; Cui, C.B.; Gatto, M.; Rossi, M.; Berloco, P.B.; Cantafora, A.; Wauthier, E.; Furth, M.E.; et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011, 54, 2159–2172. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, F.; Shivakumar, P.; Pfister, J.; Walters, S.; Sabla, G.; Bezerra, J.A. Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology 2013, 58, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.E.; op den Dries, S.; Koster, M.H.; Lisman, T.; Gouw, A.S.; Porte, R.J. Regeneration of human extrahepatic biliary epithelium: The peribiliary glands as progenitor cell compartment. Liver Int. 2012, 32, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Cardinale, V.; Renzi, A.; Hov, J.R.; Berloco, P.B.; Rossi, M.; Karlsen, T.H.; Alvaro, D.; Gaudio, E. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J. Hepatol. 2015, 63, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.R.; Pairojkul, C.; Royce, S.G.; Clouston, A.; Bhathal, P.S. Liver fluke-associated and sporadic cholangiocarcinoma: An immunohistochemical study of bile duct, peribiliary gland and tumour cell phenotypes. J. Clin. Pathol. 2006, 59, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Nakanuma, Y. Pathologic observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum. Pathol. 1992, 23, 483–490. [Google Scholar] [CrossRef]
- Op den Dries, S.; Westerkamp, A.C.; Karimian, N.; Gouw, A.S.; Bruinsma, B.G.; Markmann, J.F.; Lisman, T.; Yeh, H.; Uygun, K.; Martins, P.N.; et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 2014, 60, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Puca, R.; Cardinale, V.; Renzi, A.; Scafetta, G.; Nevi, L.; Rossi, M.; Berloco, P.B.; Ginanni Corradini, S.; Reid, L.M.; et al. Peribiliary glands as a niche of extrapancreatic precursors yielding insulin-producing cells in experimental and human diabetes. Stem Cells 2016, 34, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a003129. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Mitsuhashi, T.; Hatanaka, Y.; Miyamoto, M.; Oba, K.; Tsuchikawa, T.; Suzuki, Y.; Hatanaka, K.C.; Hirano, S.; Matsuno, Y. Prognostic significance of epithelial-mesenchymal transition-related markers in extrahepatic cholangiocarcinoma: Comprehensive immunohistochemical study using a tissue microarray. Br. J. Cancer 2014, 111, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hikiba, Y.; Hirata, Y.; Font-Burgada, J.; Sakamoto, K.; Hayakawa, Y.; Taniguchi, K.; Umemura, A.; Kinoshita, H.; Sakitani, K.; et al. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 1090–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.H.; Krauland, L.; Singh, V.; Zou, B.; Devaraj, P.; Stolz, D.B.; Franks, J.; Monga, S.P.; Sasatomi, E.; Behari, J. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology 2010, 52, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Herr, K.J.; Tsang, Y.H.; Ong, J.W.; Li, Q.; Yap, L.L.; Yu, W.; Yin, H.; Bogorad, R.L.; Dahlman, J.E.; Chan, Y.G.; et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration. Sci. Rep. 2014, 4, 6835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hengel, J.; Van den Broeke, C.; Pieters, T.; Libbrecht, L.; Hofmann, I.; van Roy, F. Inactivation of p120 catenin in mice disturbs intrahepatic bile duct development and aggravates liver carcinogenesis. Eur. J. Cell Biol. 2016, 95, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, K.A.; Robertson, H.; Marshall, H.L.; Pekalski, M.; Zhao, L.; Booth, T.A.; Jones, D.E.; Burt, A.D.; Kirby, J.A. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab. Investig. 2008, 88, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, M.J.; Racedo, S.; Mikels-Vigdal, A.; Marshall, D.; Bowlus, C.; Lackner, C.; Madl, T.; Karlsen, T.H.; Hov, J.R.; Lyman, S.K.; et al. Lysyl oxidase-like protein 2 (LOXL2) modules barrier function in cholangiocytes in cholestasis. J. Hepatol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Nakanuma, Y. Pathological observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers. II. A possible source of cholangiocarcinoma. Hepatology 1990, 12, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Sasaki, M.; Igarashi, S.; Sato, Y.; Nakanuma, Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer 2013, 119, 1669–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razumilava, N.; Gradilone, S.A.; Smoot, R.L.; Mertens, J.C.; Bronk, S.F.; Sirica, A.E.; Gores, G.J. Non-canonical hedgehog signaling contributes to chemotaxis in cholangiocarcinoma. J. Hepatol. 2014, 60, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nakanuma, Y.; Kozaka, K.; Sato, Y.; Ikeda, H. Spread of hilar cholangiocarcinomas via peribiliary gland network: A hither-to-unrecognized route of periductal infiltration. Int. J. Clin. Exp. Pathol. 2013, 6, 318–322. [Google Scholar] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Razumilava, N.; Gores, G.J.; Walters, S.; Mizuochi, T.; Mourya, R.; Bessho, K.; Wang, Y.H.; Glaser, S.S.; Shivakumar, P.; et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J. Clin. Investig. 2014, 124, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Luster, A.D. T cell homing to epithelial barriers in allergic disease. Nat. Med. 2012, 18, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Li, X.Y.; Cheng, X.D.; Shen, L.P.; Fang, F.; Zhang, B.; Hua, H.; Yan, C.; Tang, R.X.; Zheng, K.Y. Expression and potential roles of IL-33/ST2 in the immune regulation during clonorchis sinensis infection. Parasitol. Res. 2016, 115, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Sawada, R.; Ku, Y.; Akita, M.; Otani, K.; Fujikura, K.; Itoh, T.; Ajiki, T.; Fukumoto, T.; Kakeji, Y.; Zen, Y. IL-33 overexpression reflects less aggressive tumour features in large-duct type cholangiocarcinomas. Histopathology 2018. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Rizvi, S.; Razumilava, N.; Bronk, S.F.; Davila, J.I.; Champion, M.D.; Borad, M.J.; Bezerra, J.A.; Chen, X.; Gores, G.J. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 2015, 61, 1627–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Dhar, D.; Nakagawa, H.; Font-Burgada, J.; Ogata, H.; Jiang, Y.; Shalapour, S.; Seki, E.; Yost, S.E.; Jepsen, K.; et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell 2013, 155, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Maeda, S.; Yoshida, H.; Tateishi, R.; Masuzaki, R.; Ohki, T.; Hayakawa, Y.; Kinoshita, H.; Yamakado, M.; Kato, N.; et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis c patients: An analysis based on gender differences. Int. J. Cancer 2009, 125, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Goydos, J.S.; Brumfield, A.M.; Frezza, E.; Booth, A.; Lotze, M.T.; Carty, S.E. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: Validation of utility as a clinical marker. Ann. Surg. 1998, 227, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.K.; Cho, Y.D.; Moon, J.H.; Jang, J.Y.; Kim, Y.S.; Kim, Y.S.; Lee, M.S.; Lee, J.S.; Shim, C.S. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am. J. Gastroenterol. 2007, 102, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Pradere, J.P.; Antoine, D.J.; Friedman, R.A.; Schwabe, R.F. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J. Clin. Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Huda, N.; Chen, X.; Antoine, D.J.; Li, Y.; Dai, G.; Kohler, U.A.; Zong, W.X.; Waguri, S.; Werner, S.; et al. HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J. Clin. Investig. 2018, 128. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.G.; Lee, H.; Katabi, N.; DeMatteo, R.P.; Fong, Y.; D’Angelica, M.I.; Allen, P.J.; Klimstra, D.S.; Jarnagin, W.R. Intraductal papillary neoplasm of the bile duct: A biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012, 56, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zen, Y.; Fujii, T.; Itatsu, K.; Nakamura, K.; Minato, H.; Kasashima, S.; Kurumaya, H.; Katayanagi, K.; Kawashima, A.; Masuda, S.; et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006, 44, 1333–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, Y.; Zen, Y.; Hirano, S.; Tanaka, E.; Takahashi, O.; Yonemori, A.; Doumen, H.; Kawakami, H.; Itoh, T.; Nakanuma, Y.; et al. Intraductal oncocytic papillary neoplasm of the bile duct: The first case of peribiliary gland origin. J. Hepato Biliary Pancreat. Surg. 2009, 16, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Nakanuma, Y.; Ohara, M.; Iwao, T.; Kimura, N.; Ishidate, T.; Kijima, H. Intraductal papillary neoplasm arising from peribiliary glands connecting with the inferior branch of the bile duct of the anterior segment of the liver. Pathol. Int. 2011, 61, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Uesaka, K.; Nakanuma, Y. Cystic and papillary neoplasm at the hepatic hilum possibly originating in the peribiliary glands. Case Rep. Pathol. 2016, 2016, 9130754. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Yamamoto, Y.; Ito, T.; Okamura, Y.; Sugiura, T.; Uesaka, K.; Nakanuma, Y. Cystic micropapillary neoplasm of peribiliary glands with concomitant perihilar cholangiocarcinoma. World J. Gastroenterol. 2016, 22, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.; Carpino, G.; Reid, L.M.; Gaudio, E.; Alvaro, D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 2012, 55, 2041–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.F.; Carithers, R.L., Jr. Aasld practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005, 41, 1407–1432. [Google Scholar] [CrossRef] [PubMed]
- Skaro, A.I.; Jay, C.L.; Baker, T.B.; Wang, E.; Pasricha, S.; Lyuksemburg, V.; Martin, J.A.; Feinglass, J.M.; Preczewski, L.B.; Abecassis, M.M. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: The untold story. Surgery 2009, 146, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck, R.L., III; de Brito, M.C.; Berntsen, N.L.; Gomez-Vazquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Lourdusamy, V.; Konjeti, R.; Vargo, J.J.; Parsi, M.A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 81, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]


| Types of CCA | Methods for CCA Induction | Methods for Lineage Tracing | Cellular Origin |
|---|---|---|---|
| Intrahepatic CCA | Chronic thioacetamide (TAA) administration | Rosa26-LSL-LacZ mice crossed with AlbuminCreERT mice or K19CreERT mice | Hepatocytes |
| Hepatocyte-specific activation of Akt and Notch pathways by hydrodynamic tail vein injection | Injection of adenoassociated virus serotype 8 vector expressing Cre from transthyretin promoter into Rosa26-LSL-EYFP mice | Hepatocytes | |
| Duct cell-specific Kras activation and PTEN deletion | K19CreERT mice crossed with LSL-KrasG12D mice and Ptenflox/flox mice | Cholangiocytes | |
| Duct cell-specific p53 deletion in combination with chronic TAA administration | K19CreERT mice crossed with p53flox/flox mice and Rosa26-LSL-EYFP mice | Cholangiocytes | |
| Extrahepatic CCA | Duct cell-specific activation of Kras and deletion of TGFβR2 and E-cadherin | K19CreERT mice crossed with LSL-KrasG12D, Tgfbr2flox/flox, CDH1flox/flox, and Rosa26-LSL-LacZ mice | Peribiliary glands (PBGs) |
| Types of Stem/Progenitor Cell Markers | Stem/Progenitor Cell Markers Expressed in PBGs |
|---|---|
| Pluripotency genes | Oct4, Nanog |
| Stem cell surface markers | CD133, CXCR4, CD44 |
| Markers of endodermal stem cells | Pdx1, Sox9, Sox17, Foxa2 |
| Markers of hepatic stem cells | EpCAM, NCAM |
| Markers of intestinal stem cells | Lgr5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, H.; Hayata, Y.; Yamada, T.; Kawamura, S.; Suzuki, N.; Koike, K. Peribiliary Glands as the Cellular Origin of Biliary Tract Cancer. Int. J. Mol. Sci. 2018, 19, 1745. https://doi.org/10.3390/ijms19061745
Nakagawa H, Hayata Y, Yamada T, Kawamura S, Suzuki N, Koike K. Peribiliary Glands as the Cellular Origin of Biliary Tract Cancer. International Journal of Molecular Sciences. 2018; 19(6):1745. https://doi.org/10.3390/ijms19061745
Chicago/Turabian StyleNakagawa, Hayato, Yuki Hayata, Tomoharu Yamada, Satoshi Kawamura, Nobumi Suzuki, and Kazuhiko Koike. 2018. "Peribiliary Glands as the Cellular Origin of Biliary Tract Cancer" International Journal of Molecular Sciences 19, no. 6: 1745. https://doi.org/10.3390/ijms19061745