Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure
Abstract
:1. Introduction
2. Functional Closure
2.1. Vasoconstriction
2.1.1. Oxygen Pathways
2.1.2. Pathways Unrelated to Oxygen
2.2. Vasodilation
2.2.1. PGE2
2.2.2. Vasodilating Factors Unrelated to PGE
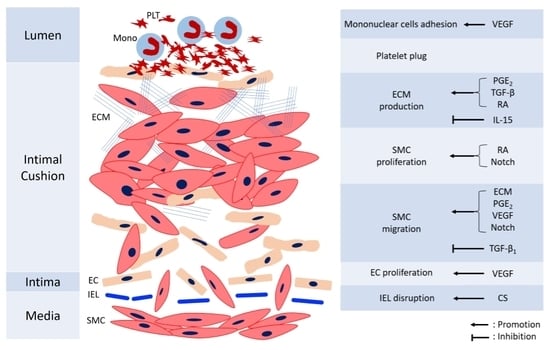
3. Anatomical Closure
3.1. Factors Regulating SMC Proliferation and Migration
3.1.1. PGE2
3.1.2. Retinoic Acid
3.1.3. TGF-β1
3.1.4. Notch Signaling
3.2. Extracellular Matrix (ECM)
3.2.1. Hyaluronan
3.2.2. Fibronectin
3.2.3. Chondroitin Sulfate
3.2.4. Elastin
3.3. Factors Affecting Endothelial Cells (ECs)
3.4. Blood Cells’ Interaction
4. Pharmacological Agents for Management of DA Patency
4.1. Agents for Closing the DA
4.2. Agents for Opening the DA
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001, 107, E1. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; McCoy, M.; Friedlich, P.; Bright, B.; Gottipati, V.; Seri, I.; Sekar, K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 2009, 123, e138–e144. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.A.; Rudolph, A.M. Control of the ductus arteriosus. Physiol. Rev. 1975, 55, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C. Persistent ductus arteriosus: Most probably a primary congenital malformation. Br. Heart J. 1977, 39, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; van Ertbruggen, I.; Moulaert, A.J.; Harinck, E. The ductus arteriosus in the preterm infant: Histologic and clinical observations. J. Pediatr. 1980, 96, 88–93. [Google Scholar] [CrossRef] [Green Version]
- De Reeder, E.; Girard, N.; Poelmann, R.; Van Munsteren, J.; Patterson, D.; Gittenberger-De Groot, A. Hyaluronic acid accumulation and endothelial cell detachment in intimal thickening of the vessel wall. The normal and genetically defective ductus arteriosus. Am. J. Pathol. 1988, 132, 574–585. [Google Scholar] [PubMed]
- Kovalčík, V. The response of the isolated ductus arteriosus to oxygen and anoxia. J. Physiol. 1963, 169, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.Y.; Brown, D.M. Effect of dexamethasone or fetal lung 15-hydroxy-prostaglandin dehydrogenase: Possible mechanism for the prevention of patent ductus arteriosus by maternal dexamethasone therapy. Prostaglandins Leuko. Med. 1987, 27, 237–245. [Google Scholar] [CrossRef]
- Thorburn, G.D. The placenta, PGE2 and parturition. Early Hum. Dev. 1992, 29, 63–73. [Google Scholar] [CrossRef]
- Bouayad, A.; Kajino, H.; Waleh, N.; Fouron, J.C.; Andelfinger, G.; Varma, D.R.; Skoll, A.; Vazquez, A.; Gobeil, F., Jr.; Clyman, R.I.; et al. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2342–H2349. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Glaser, A.; Wegmann, M.; Schranz, D.; Seyberth, H.; Nusing, R. Expression of prostanoid receptors in human ductus arteriosus. Br. J. Pharmacol. 2003, 138, 655–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, U.; Minamisawa, S.; Katayama, A.; Tang, T.; Suzuki, S.; Iwatsubo, K.; Iwasaki, S.; Kurotani, R.; Okumura, S.; Sato, M.; et al. Differential regulation of vascular tone and remodeling via stimulation of type 2 and type 6 adenylyl cyclases in the ductus arteriosus. Circ. Res. 2010, 106, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Tristani-Firouzi, M.; Reeve, H.L.; Tolarova, S.; Weir, E.K.; Archer, S.L. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J. Clin. Investig. 1996, 98, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Rebeyka, I.; Wu, X.; Nsair, A.; Thebaud, B.; Hashimoto, K.; Dyck, J.R.; Haromy, A.; Harry, G.; Barr, A.; et al. O2 sensing in the human ductus arteriosus: Regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ. Res. 2002, 91, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Kutty, S.; Toth, P.T.; Marsboom, G.; Hammel, J.M.; Chamberlain, C.; Ryan, J.J.; Zhang, H.J.; Sharp, W.W.; Morrow, E.; et al. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ. Res. 2013, 112, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, H.; Hashimoto, K.; Bonnet, S.N.; Haromy, A.; Harry, G.; Moudgil, R.; Nakanishi, T.; Rebeyka, I.; Thébaud, B.; Michelakis, E.D.; et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: A newly recognized mechanism for sustaining ductal constriction. Circulation 2007, 115, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Waleh, N.; Black, S.M.; Riemer, R.K.; Mauray, F.; Chen, Y.Q. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: The role of gestation, oxygen tension, and vasa vasorum. Pediatr. Res. 1998, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Seidner, S.R.; Chen, Y.Q.; Oprysko, P.R.; Mauray, F.; Mary, M.T.; Lin, E.; Koch, C.; Clyman, R.I. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr. Res. 2001, 50, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sodini, D.; Baragatti, B.; Barogi, S.; Laubach, V.E.; Coceani, F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br. J. Pharmacol. 2008, 153, 1631–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coceani, F.; Kelsey, L.; Seidlitz, E.; Korzekwa, K. Inhibition of the contraction of the ductus arteriosus to oxygen by 1-aminobenzotriazole, a mechanism-based inactivator of cytochrome P450. Br. J. Pharmacol. 1996, 117, 1586–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coceani, F.; Breen, C.A.; Leesm, J.G.; Falck, J.R.; Olley, P.M. Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ. Res. 1988, 62, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.; Momma, K.; Imamura, S.; Nakanishi, T. In vivo dilatation of the postnatal ductus arteriosus by atrial natriuretic peptide in the rat. Neonatology 2007, 92, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.R.; Jing, S.; Momma, K.; Nakanishi, T. The effect of vitamin A on contraction of the ductus arteriosus in fetal rat. Pediatr. Res. 2001, 49, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Minamisawa, S.; Adachi-Akahane, S.; Akaike, T.; Naguro, I.; Funakoshi, K.; Iwamoto, M.; Nakagome, M.; Uemura, N.; Hori, H.; et al. Multiple transcripts of Ca2+ channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1660–H1670. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Kelsey, L.; Seidlitz, E.; Marks, G.S.; McLaughlin, B.E.; Vreman, H.J.; Stevenson, D.K.; Rabinovitch, M.; Ackerley, C. Carbon monoxide formation in the ductus arteriosus in the lamb: Implications for the regulation of muscle tone. Br. J. Pharmacol. 1997, 120, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Kelsey, L.; Seidlitz, E. Carbon monoxide-induced relaxation of the ductus arteriosus in the lamb: Evidence against the prime role of guanylyl cyclase. Br. J. Pharmacol. 1996, 118, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Yokoyama, U.; Ishiwata, R.; Aoki, R.; Nagao, K.; Masukawa, D.; Umemura, M.; Fujita, T.; Iwasaki, S.; Nishimaki, S.; et al. Glutamate promotes contraction of the rat ductus arteriosus. Circ. J. 2016, 80, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Baragatti, B.; Ciofini, E.; Sodini, D.; Luin, S.; Scebba, F.; Coceani, F. Hydrogen sulfide in the mouse ductus arteriosus: A naturally occurring relaxant with potential EDHF function. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H927–H934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Yan, C.D.; Bian, J.S. Hydrogen sulfide: A novel signaling molecule in the vascular system. J. Cardiovasc. Pharmacol. 2011, 58, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Yokoyama, U.; Ichikawa, Y.; Taguri, M.; Kumagaya, S.; Ishiwata, R.; Yanai, C.; Fujita, S.; Umemura, M.; Fujita, T.; et al. Decreased serum osmolality promotes ductus arteriosus constriction. Cardiovasc. Res. 2014, 104, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateson, E.A.; Schulz, R.; Olley, P.M. Response of fetal rabbit ductus arteriosus to bradykinin: Role of nitric oxide, prostaglandins, and bradykinin receptors. Pediatr. Res. 1999, 45, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Mauray, F.; Roman, C.; Rudolph, A.M.; Heymann, M.A. Glucocorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. J. Pediatr. 1981, 98, 126–128. [Google Scholar] [CrossRef]
- Momma, K.; Takao, A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and betamethasone in fetal rats. Pediatr. Res. 1989, 25, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Hong, Z.G.; Xiong, P.Y.; Del Paggio, J.C.; Herr, J.E.; Johri, A.M.; Archer, S.L. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Arch. 2016, 468, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Armstrong, C.; Kelsey, L. Endothelin is a potent constrictor of the lamb ductus arteriosus. Can. J. Physiol. Pharmacol. 1989, 67, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Liu, Y.-A.; Seidlitz, E.; Kelsey, L.; Kuwaki, T.; Ackerley, C.; Yanagisawa, M. Endothelin A receptor is necessary for O2 constriction but not closure of ductus arteriosus. Am. J. Physiol. 1999, 277, H1521–H1531. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Kelsey, L.; Seidlitz, E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can. J. Physiol. Pharmacol. 1992, 70, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Freed, M.D.; Heymann, M.A.; Lewis, A.B.; Roehl, S.L.; Kensey, R.C. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation 1981, 64, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I. Mechanisms regulating the ductus arteriosus. Biol. Neonate 2006, 89, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; McGrath, J.C. Prostaglandin E2 and fetal oxygen tension synergistically inhibit response of isolated fetal rabbit ductus arteriosus to norepinephrine. J. Cardiovasc. Pharmacol. 1991, 17, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; McGrath, J.C. Characterisation of the effect of oxygen tension on response of fetal rabbit ductus arteriosus to vasodilators. Cardiovasc. Res. 1993, 27, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Walford, G.; Loscalzo, J. Nitric oxide in vascular biology. J. Thromb. Haemost. 2003, 1, 2112–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.H.; Yang, S.N.; Chen, H.L.; Tseng, H.I.; Dai, Z.K.; Wu, J.R. B-type natriuretic peptide predicts responses to indomethacin in premature neonates with patent ductus arteriosus. J. Pediatr. 2010, 157, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [PubMed]
- Hsu, J.H.; Liou, S.F.; Yang, S.N.; Wu, B.N.; Dai, Z.K.; Chen, I.J.; Yeh, J.L.; Wu, J.R. B-type natriuretic peptide inhibits angiotensin II-induced proliferation and migration of pulmonary arterial smooth muscle cells. Pediatr. Pulmonol. 2014, 49, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Van der Sterren, S.; Kleikers, P.; Zimmermann, L.J.; Villamor, E. Vasoactivity of the gasotransmitters hydrogen sulfide and carbon monoxide in the chicken ductus arteriosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1186–R1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Akaike, T.; Suzuki, S.; Jin, M.; Jiao, Q.; Watanabe, M.; Otsu, K.; Iwasaki, S.; et al. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J. Biol. Chem. 2008, 283, 28702–28709. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.E.; Waleh, N.S.; Mauray, F.; Breuss, J.; Pytela, R.; Kramer, R.H.; Clyman, R.I. Transforming growth factor beta 1 inhibits fetal lamb ductus arteriosus smooth muscle cell migration. Pediatr. Res. 1995, 37, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Yeh, J.L.; Liou, S.F.; Dai, Z.K.; Wu, B.N.; Hsu, J.H. Gamma-secretase inhibitor prevents proliferation and migration of ductus arteriosus smooth muscle cells through the Notch3-HES1/2/5 pathway. Int. J. Biol. Sci. 2016, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Ghatak, S.; Akaike, T.; Segi-Nishida, E.; Iwasaki, S.; Iwamoto, M.; Misra, S.; Tamura, K.; et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J. Clin. Investig. 2006, 116, 3026–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, C.A.; Bigras, J.L.; O’Blenes, S.B.; Zhou, B.; McIntyre, B.; Nakamura, N.; Kaneda, Y.; Rabinovitch, M. Gene transfer in utero biologically engineers a patent ductus arteriosus in lambs by arresting fibronectin-dependent neointimal formation. Nat. Med. 1999, 5, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Seidner, S.R.; Kajino, H.; Roman, C.; Koch, C.J.; Ferrara, N.; Waleh, N.; Mauray, F.; Chen, Y.Q.; Perkett, E.A.; et al. VEGF regulates remodeling during permanent anatomic closure of the ductus arteriosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R199–R206. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Xu, S.J.; Teng, J.Y.; Wu, W.; Ye, D.Y.; Wu, X.Z. Differential response of human fetal smooth muscle cells from arterial duct to retinoid acid. Acta Pharmacol. Sin. 2008, 29, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, U.; Sato, Y.; Akaike, T.; Ishida, S.; Sawada, J.; Nagao, T.; Quan, H.; Jin, M.; Iwamoto, M.; Yokota, S.; et al. Maternal vitamin A alters gene profiles and structural maturation of the rat ductus arteriosus. Physiol. Genom. 2007, 31, 139–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudreau, N.; Clausell, N.; Boyle, J.; Rabinovitch, M. Transforming growth factor-beta regulates increased ductus arteriosus endothelial glycosaminoglycan synthesis and a post-transcriptional mechanism controls increased smooth muscle fibronectin, features associated with intimal proliferation. Lab. Investig. 1992, 67, 350–359. [Google Scholar] [PubMed]
- Iwasaki, S.; Minamisawa, S.; Yokoyama, U.; Akaike, T.; Quan, H.; Nagashima, Y.; Nishimaki, S.; Ishikawa, Y.; Yokota, S. Interleukin-15 inhibits smooth muscle cell proliferation and hyaluronan production in rat ductus arteriosus. Pediatr. Res. 2007, 62, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Minamisawa, S.; Shioda, A.; Ishiwata, R.; Jin, M.H.; Masuda, M.; Asou, T.; Sugimoto, Y.; Aoki, H.; Nakamura, T.; et al. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation 2014, 129, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Minamisawa, S. Oxygenation decreases elastin secretion from rat ductus arteriosus smooth muscle cells. Pediatr. Int. 2015, 57, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Chan, C.Y.; Mauray, F.; Chen, Y.Q.; Cox, W.; Seidner, S.R.; Lord, E.M.; Weiss, H.; Waleh, N.; Evans, S.M.; et al. Permanent anatomic closure of the ductus arteriosus in newborn baboons: The roles of postnatal constriction, hypoxia, and gestation. Pediatr. Res. 1999, 45, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Waleh, N.; Seidner, S.; McCurnin, D.; Giavedoni, L.; Hodara, V.; Goelz, S.; Liu, B.M.; Roman, C.; Clyman, R.I. Anatomic closure of the premature patent ductus arteriosus: The role of CD14+/CD163+ mononuclear cells and VEGF in neointimal mound formation. Pediatr. Res. 2011, 70, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Mecham, R.P.; Keeley, F.; Rabinovitch, M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J. Clin. Investig. 1991, 88, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Engur, D.; Kaynak-Turkmen, M.; Deveci, M.; Yenisey, C. Platelets and platelet-derived growth factor in closure of the ductus arteriosus. Turk. J. Pediatr. 2015, 57, 242–247. [Google Scholar] [PubMed]
- Gittenberger-de Groot, A.C.; Strengers, J.L.; Mentink, M.; Poelmann, R.E.; Patterson, D.F. Histologic studies on normal and persistent ductus arteriosus in the dog. J. Am. Coll. Cardiol. 1985, 6, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.E.; Waleh, N.S.; Mauray, F.; Gold, L.; Perkett, E.A.; Clyman, R.I. Transforming growth factor-beta protein and messenger RNA expression is increased in the closing ductus arteriosus. Pediatr. Res. 1996, 39, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Gridley, T.; Liaw, L. Molecular pathways of notch signaling in vascular smooth muscle cells. Front. Physiol. 2012, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.T.; Jackson, A.R.; McHugh, K.M.; Lilly, B. Loss of Notch2 and Notch3 in vascular smooth muscle causes patent ductus arteriosus. Genesis 2015, 53, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Krebs, L.T.; Norton, C.R.; Gridley, T. Notch signal reception is required in vascular smooth muscle cells for ductus arteriosus closure. Genesis 2016, 54, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Minamisawa, S.; Ishikawa, Y. Regulation of vascular tone and remodeling of the ductus arteriosus. J. Smooth Muscle Res. 2010, 46, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinek, A.; Boyle, J.; Rabinovitch, M. Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate-induced “shedding” of the 67-kDa cell surface elastin binding protein. Exp. Cell Res. 1992, 203, 344–353. [Google Scholar] [CrossRef]
- Rabinovitch, M. Cell-extracellular matrix interactions in the ductus arteriosus and perinatal pulmonary circulation. Semin. Perinatol. 1996, 20, 531–541. [Google Scholar] [CrossRef]
- Clyman, R.I.; Goetzman, B.W.; Chen, Y.Q.; Mauray, F.; Kramer, R.H.; Pytela, R.; Schnapp, L.M. Changes in endothelial cell and smooth muscle cell integrin expression during closure of the ductus arteriosus: An immunohistochemical comparison of the fetal, preterm newborn, and full-term newborn rhesus monkey ductus. Pediatr. Res. 1996, 40, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Mauray, F.; Kramer, R.H. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp. Cell Res. 1992, 200, 272–284. [Google Scholar] [CrossRef]
- Waleh, N.; Seidner, S.; McCurnin, D.; Yoder, B.; Liu, B.M.; Roman, C.; Mauray, F.; Clyman, R.I. The role of monocyte-derived cells and inflammation in baboon ductus arteriosus remodeling. Pediatr. Res. 2005, 57, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Echtler, K.; Stark, K.; Lorenz, M.; Kerstan, S.; Walch, A.; Jennen, L.; Rudelius, M.; Seidl, S.; Kremmer, E.; Emambokus, N.R.; et al. Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat. Med. 2010, 16, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Poggi, C.; Fontanelli, G. Relationship between platelet count and volume and spontaneous and pharmacological closure of ductus arteriosus in preterm infants. Am. J. Perinatol. 2013, 30, 359–364. [Google Scholar] [PubMed]
- Mitra, S.; Chan, A.K.; Paes, B.A. The association of platelets with failed patent ductus arteriosus closure after a primary course of indomethacin or ibuprof1en: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2017, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Walia, R.; Shah, S.S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. 2015, CD003481. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Iwatsubo, K.; Umemura, M.; Fujita, T.; Ishikawa, Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 2013, 65, 1010–1052. [Google Scholar] [CrossRef] [PubMed]
- Hammerman, C.; Bin-Nun, A.; Markovitch, E.; Schimmel, M.S.; Kaplan, M.; Fink, D. Ductal closure with paracetamol: A surprising new approach to patent ductus arteriosus treatment. Pediatrics 2011, 128, e1618–e1621. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Warner, T.D.; Vojnovic, I.; Mitchell, J.A. Cellular mechanisms of acetaminophen: Role of cyclo-oxygenase. FASEB J. 2005, 19, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Wang, D.; Zhang, C.; Zhou, W.; Zhou, Q.; Wu, H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: A randomized controlled trial. PLoS ONE 2013, 8, e77888. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.M.; Niknafs, P.; Sabsevari, F.; Torabi, M.H.; Bijari, B.B.; Noroozi, E.; Mossavi, H. Comparison of Oral Acetaminophen Versus Ibuprofen in Premature Infants With Patent Ductus Arteriosus. Iran. J. Pediatr. 2016, 26, e3975. [Google Scholar] [CrossRef] [PubMed]
- Roofthooft, D.W.; van Beynum, I.M.; de Klerk, J.C.; van Dijk, M.; van den Anker, J.N.; Reiss, I.K.; Tibboel, D.; Simons, S.H. Limited effects of intravenous paracetamol on patent ductus arteriosus in very low birth weight infants with contraindications for ibuprofen or after ibuprofen failure. Eur. J. Pediatr. 2015, 174, 1433–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, Y.; Yokoyama, U.; Iwamoto, M.; Oshikawa, J.; Okumura, S.; Sato, M.; Yokota, S.; Masuda, M.; Asou, T.; Ishikawa, Y. Inhibition of phosphodiesterase type 3 dilates the rat ductus arteriosus without inducing intimal thickening. Circ. J. 2012, 76, 245624–245664. [Google Scholar] [CrossRef]
- Takizawa, T.; Oda, T.; Arishima, K.; Yamamoto, M.; Somiya, H.; Eguchi, Y.; Shiota, K. Inhibitory effect of enalapril on the constriction of the ductus arteriosus in newborn rats. J. Vet. Med. Sci. 1994, 56, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Horikoshi, E.; Shen, M.H.; Masaoka, T.; Takagi, H.; Yamamoto, M.; Kasai, K.; Arishima, K. Effects of TAK-044, a nonselective endothelin receptor antagonist, on the spontaneous and indomethacin- or methylene blue-induced constriction of the ductus arteriosus in rats. J. Vet. Med. Sci. 2000, 62, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.J.; Ziegler, J.W.; Ivy, D.D.; Halbower, A.C.; Kinsella, J.P.; Abman, S.H. Role of nitric oxide and cGMP system in regulation of ductus arteriosus tone in ovine fetus. Am. J. Physiol. 1996, 271, H2638–H2645. [Google Scholar] [CrossRef] [PubMed]
- Coceani, F.; Olley, P.M. The response of the ductus arteriosus to prostaglandins. Can. J. Physiol. Pharmacol. 1973, 51, 220–225. [Google Scholar] [CrossRef] [PubMed]


| Vasoconstrictors | References | Vasodilators | References |
|---|---|---|---|
| Oxygen sensing | Prostaglandin E2 | [10,11,12,13] | |
| Mitochondria | [14,15,16,17] | Nitric oxide | [18,19,20] |
| Cytochrome P450 | [21,22] | Natriuretic peptides | [23] |
| Retinoic acid | [24,25] | Carbon monoxide | [26,27] |
| Glutamate | [28] | Hydrogen sulfide | [29,30] |
| Hypoosmolality | [31] | ||
| Bradykinin | [32] | ||
| Corticosteroid | [33,34] |
| Cells | Mechanisms | Factors | Effects | Reference |
|---|---|---|---|---|
| SMCs | Migration | PGE2 | + | [48] |
| TGF-β1 | − | [49] | ||
| Notch | + | [50] | ||
| Fibronectin & Hyaluronan | + | [51,52] | ||
| VEGF | + | [53] | ||
| Proliferation | Retinoic acid | + | [54] | |
| Notch | + | [50] | ||
| ECM production | Hyaluronan | Retinoic acid | + | [55] |
| TGF-β | + | [56] | ||
| PGE2 | + | [51] | ||
| IL-15 | − | [57] | ||
| Fibronectin | Retinoic acid | + | [55] | |
| Chondroitin sulfate | TGF-β | + | [56] | |
| Elastin | PGE2 | − | [58] | |
| Oxygen | − | [59] | ||
| ECs | Proliferation | VEGF | + | [53,60,61] |
| IEL | Disruption | Chondroitin sulfate | + | [62] |
| Blood cells | Mononuclear cells adhesion | VEGF | + | [61] |
| Platelet plug | PDGF | + | [63] |
| Ductus Closure | References | Ductus Patency | References |
|---|---|---|---|
| Indomethacin * | [80] | Notch inhibitor | [50] |
| Ibuprofen * | [80] | Prostaglandin E1 * | [81] |
| Acetaminophen | [82,83,84,85,86] | Milrinone | [87] |
| Enalapril | [88] | ||
| Endothelin receptor antagonist | [89] | ||
| Nitric oxide | [90] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-C.; Yeh, J.-L.; Hsu, J.-H. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. Int. J. Mol. Sci. 2018, 19, 1861. https://doi.org/10.3390/ijms19071861
Hung Y-C, Yeh J-L, Hsu J-H. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. International Journal of Molecular Sciences. 2018; 19(7):1861. https://doi.org/10.3390/ijms19071861
Chicago/Turabian StyleHung, Yu-Chi, Jwu-Lai Yeh, and Jong-Hau Hsu. 2018. "Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure" International Journal of Molecular Sciences 19, no. 7: 1861. https://doi.org/10.3390/ijms19071861