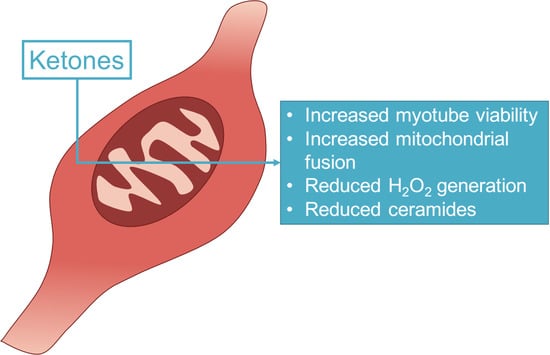
?-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle
Abstract
:1. Introduction
2. Results
2.1. β-Hydroxybutyrate Elicits Favorable Changes in Mitochondrial Respiration in Muscle Cells
2.2. β-Hydroxybutyrate Increases Cell Viability
2.3. β-Hydroxybutyrate Induces Mitochondrial Fusion
2.4. Myotube Ceramides Are Reduced with β-HB Treatment
2.5. Ketogenic Diet in Rats Alters Muscle Mitochondrial Physiology
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. Cell Viability Assays
4.4. Mitochondrial Morphology
4.5. Mitochondrial Respiration
4.6. ATP Analysis
4.7. H2O2 Emission
4.8. Lipids
4.9. Tissue Homogenization
4.10. Citrate Synthase Activity
4.11. Western Blotting
4.12. Statistical Methods
Author Contributions
Funding
Conflicts of Interest
References
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ijff, D.M.; Postulart, D.; Lambrechts, D.; Majoie, M.; de Kinderen, R.J.A.; Hendriksen, J.G.M.; Evers, S.; Aldenkamp, A.P. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: A randomized controlled trial. Epilepsy Behav 2016, 60, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Davis, L.M.; Sullivan, P.G.; Maalouf, M.; Simeone, T.A.; van Brederode, J.; Rho, J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007, 101, 1316–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 651–658. [Google Scholar] [CrossRef]
- Smith, M.E.; Tippetts, T.S.; Brassfield, E.S.; Tucker, B.J.; Ockey, A.; Swensen, A.C.; Anthonymuthu, T.S.; Washburn, T.D.; Kane, D.A.; Prince, J.T.; et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J. 2013, 456, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, C.M.; Day, L.J.; Gerschenson, M. Insulin resistance in the HIV-infected population: The potential role of mitochondrial dysfunction. Curr. Drug Targets Infect. Disord. 2005, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.H.; Neufer, P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 2012, 23, 142–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myette-Cote, E.; Neudorf, H.; Rafiei, H.; Clarke, K.; Little, J.P. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J. Physiol. 2018, 596, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- McClernon, F.J.; Yancy, W.S.; Eberstein, J.A.; Atkins, R.C.; Westman, E.C. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity 2007, 15, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S., Jr.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Foy, M.E.; Westman, E.C. A low-carbohydrate, ketogenic diet for type 2 diabetes mellitus. J. Gen. Intern. Med. 2004, 19, 110. [Google Scholar]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kaddai, V.; Ching, J.; Fridianto, K.T.; Sieli, R.J.; Sugii, S.; Summers, S.A. A Role for Ceramides, but Not Sphingomyelins, as Antagonists of Insulin Signaling and Mitochondrial Metabolism in C2C12 Myotubes. J. Biol. Chem. 2016, 291, 23978–23988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Buchhalter, J.R.; D’Alfonso, S.; Connolly, M.; Fung, E.; Michoulas, A.; Sinasac, D.; Singer, R.; Smith, J.; Singh, N.; Rho, J.M. The relationship between d-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open 2017, 2, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. 2017, 38 (Suppl. 1), 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ciavardelli, D.; Piras, F.; Consalvo, A.; Rossi, C.; Zucchelli, M.; Di Ilio, C.; Frazzini, V.; Caltagirone, C.; Spalletta, G.; Sensi, S.L. Medium-chain plasma acylcarnitines, ketone levels, cognition, and gray matter volumes in healthy elderly, mildly cognitively impaired, or Alzheimer’s disease subjects. Neurobiol. Aging 2016, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 4021–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milder, J.; Patel, M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012, 100, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahola-Erkkila, S.; Carroll, C.J.; Peltola-Mjosund, K.; Tulkki, V.; Mattila, I.; Seppanen-Laakso, T.; Oresic, M.; Tyynismaa, H.; Suomalainen, A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010, 19, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Kruger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Sharman, M.J.; Love, D.M.; Avery, N.G.; Gomez, A.L.; Scheett, T.P.; Kraemer, W.J. Body composition and hormonal responses to a carbohydrate-restricted diet. Metab. Clin. Exp. 2002, 51, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, T.; De Smet, S.; Ramaekers, M.; Van Thienen, R.; De Bock, K.; Clarke, K.; Hespel, P. Intake of a Ketone Ester Drink during Recovery from Exercise Promotes mTORC1 Signaling but Not Glycogen Resynthesis in Human Muscle. Front. Physiol. 2017, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Wu, G. The effect of ketone bodies on nitrogen metabolism in skeletal muscle. Comp. Biochem. Physiol. B 1991, 100, 209–216. [Google Scholar] [CrossRef]
- Zou, X.; Meng, J.; Li, L.; Han, W.; Li, C.; Zhong, R.; Miao, X.; Cai, J.; Zhang, Y.; Zhu, D. Acetoacetate Accelerates Muscle Regeneration and Ameliorates Muscular Dystrophy in Mice. J. Biol. Chem. 2016, 291, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Guenther, G.G.; Peralta, E.R.; Rosales, K.R.; Wong, S.Y.; Siskind, L.J.; Edinger, A.L. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 17402–17407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metab. Clin. Exp. 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Kephart, W.C.; Mumford, P.W.; Mao, X.; Romero, M.A.; Hyatt, H.W.; Zhang, Y.; Mobley, C.B.; Quindry, J.C.; Young, K.C.; Beck, D.T.; et al. The 1-Week and 8-Month Effects of a Ketogenic Diet or Ketone Salt Supplementation on Multi-Organ Markers of Oxidative Stress and Mitochondrial Function in Rats. Nutrients 2017, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Reynaud, O.; Chabot, F.; Mercier, J.; Andrich, D.E.; St-Pierre, D.H.; Gouspillou, G. The impact of a short-term high-fat diet on mitochondrial respiration, reactive oxygen species production, and dynamics in oxidative and glycolytic skeletal muscles of young rats. Physiol. Rep. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Lathen, D.R.; Dallon, B.W.; Tessem, J.S.; Bikman, B.T. β-Hydroxybutyrate improves β-cell mitochondrial function and survival. J. Insul. Resist. 2017, 2, 1. [Google Scholar]
- Dallon, B.W.; Parker, B.A.; Hodson, A.E.; Tippetts, T.S.; Harrison, M.E.; Appiah, M.M.A.; Witt, J.E.; Gibbs, J.L.; Gray, H.M.; Sant, T.M.; et al. Insulin selectively reduces mitochondrial uncoupling in brown adipose tissue in mice. Biochem. J. 2018, 475, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Tippetts, T.S.; Winden, D.R.; Swensen, A.C.; Nelson, M.B.; Thatcher, M.O.; Saito, R.R.; Condie, T.B.; Simmons, K.J.; Judd, A.M.; Reynolds, P.R.; et al. Cigarette smoke increases cardiomyocyte ceramide accumulation and inhibits mitochondrial respiration. BMC Cardiovasc. Disord. 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Tippetts, T.; Anderson, M.; Holub, Z.; Saito, R.; Swensen, A.; Prince, J.; Bikman, B.T. Insulin Increases Skeletal Muscle Ceramide Biosynthesis. Diabetes 2014, 63, A443. [Google Scholar]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.J.; Thatcher, M.O.; Carr, S.T.; Gibbs, J.L.; Trumbull, A.M.; Harrison, M.E.; Winden, D.R.; Pearson, M.J.; Tippetts, T.S.; Holland, W.L.; et al. High-Mobility Group Box 1 Disrupts Metabolic Function with Cigarette Smoke Exposure in a Ceramide-Dependent Manner. Int. J. Mol. Sci. 2017, 18, 1099. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.B.; Madsen, S.R.; Hallowell, D.M.; Goring, D.M.; Moore, T.M.; Hardman, S.E.; Heninger, M.R.; Atwood, D.R.; Thomson, D.M. Mitochondrial and performance adaptations to exercise training in mice lacking skeletal muscle LKB1. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1018–E1029. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. ?-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2247. https://doi.org/10.3390/ijms19082247
Parker BA, Walton CM, Carr ST, Andrus JL, Cheung ECK, Duplisea MJ, Wilson EK, Draney C, Lathen DR, Kenner KB, et al. ?-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. International Journal of Molecular Sciences. 2018; 19(8):2247. https://doi.org/10.3390/ijms19082247
Chicago/Turabian StyleParker, Brian A., Chase M. Walton, Sheryl T. Carr, Jacob L. Andrus, Eric C. K. Cheung, Michael J. Duplisea, Esther K. Wilson, Carrie Draney, Daniel R. Lathen, Kyle B. Kenner, and et al. 2018. "?-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle" International Journal of Molecular Sciences 19, no. 8: 2247. https://doi.org/10.3390/ijms19082247
APA StyleParker, B. A., Walton, C. M., Carr, S. T., Andrus, J. L., Cheung, E. C. K., Duplisea, M. J., Wilson, E. K., Draney, C., Lathen, D. R., Kenner, K. B., Thomson, D. M., Tessem, J. S., & Bikman, B. T. (2018). ?-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. International Journal of Molecular Sciences, 19(8), 2247. https://doi.org/10.3390/ijms19082247