Genome-Wide Identification and Characterization of NODULE-INCEPTION-Like Protein (NLP) Family Genes in Brassica napus
Abstract
:1. Introduction
2. Results
2.1. Identification of NLP Genes in B. napus
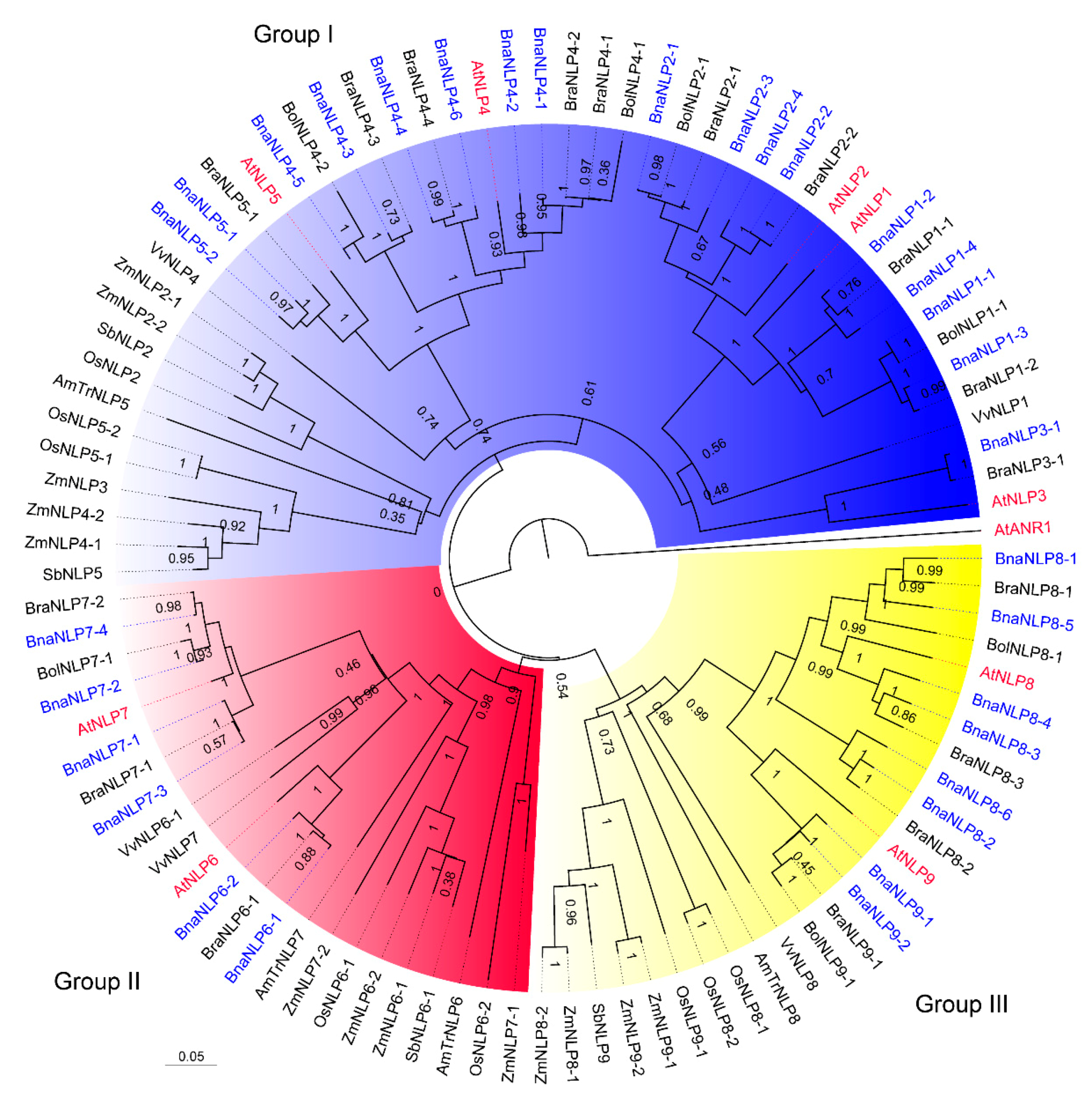
2.2. Phylogenetic Analysis of BnaNLP Proteins
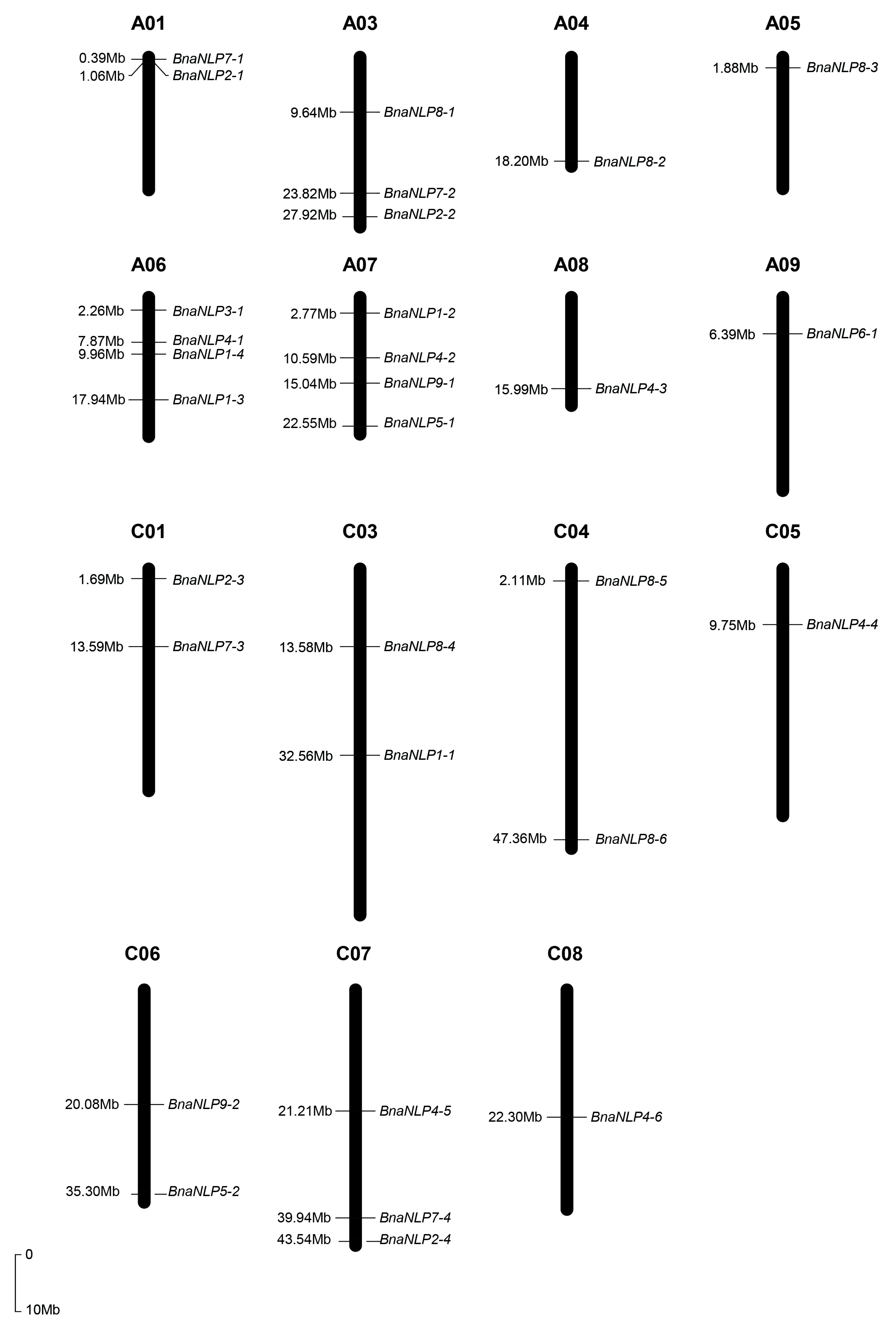
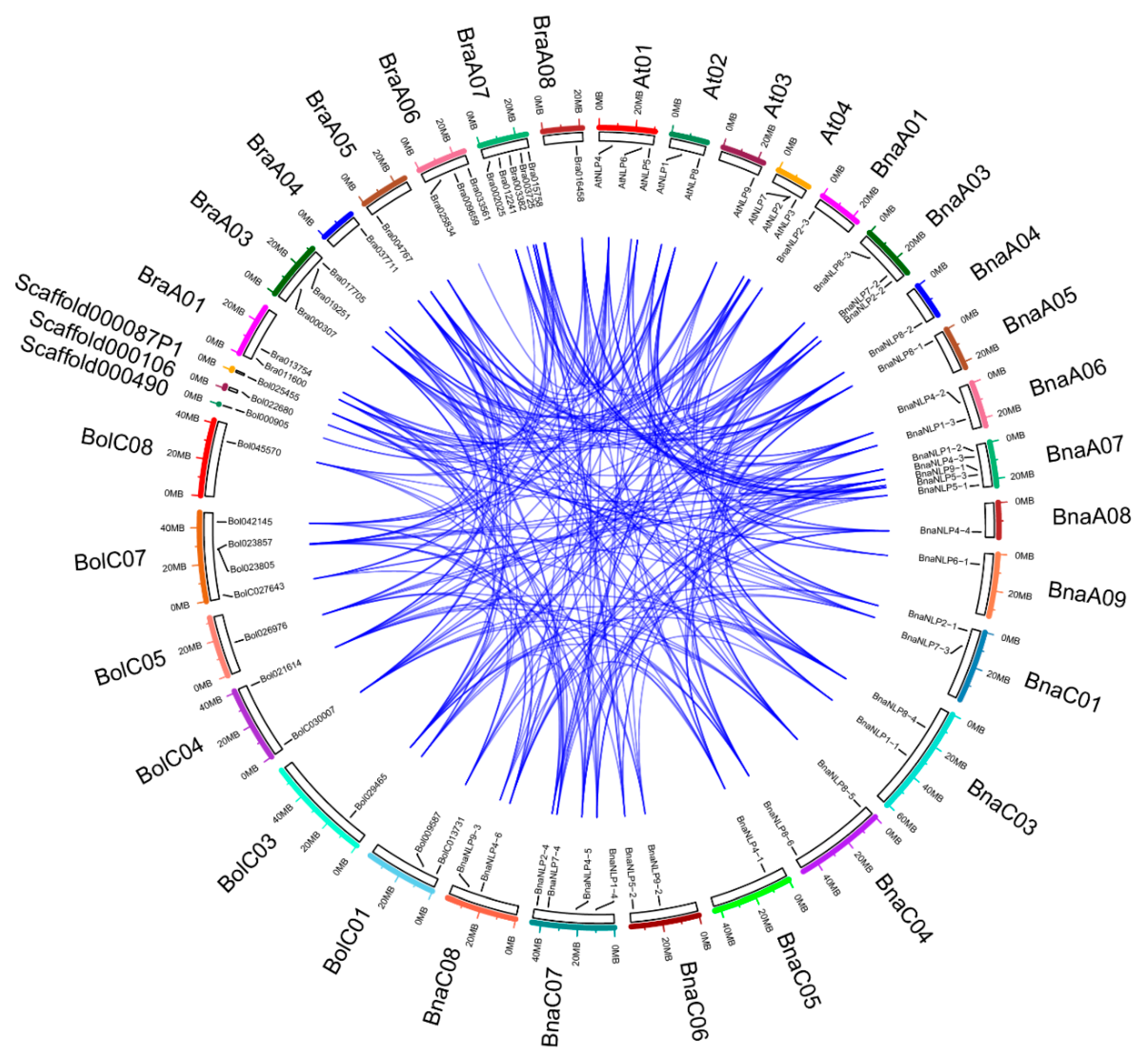
2.3. Chromosome Location and Ka/Ks Ratio Calculation
2.4. Gene Structure and Conserved Motif Analyses
2.5. Prediction of TF Binding Sites in the BnaNLP Promoters
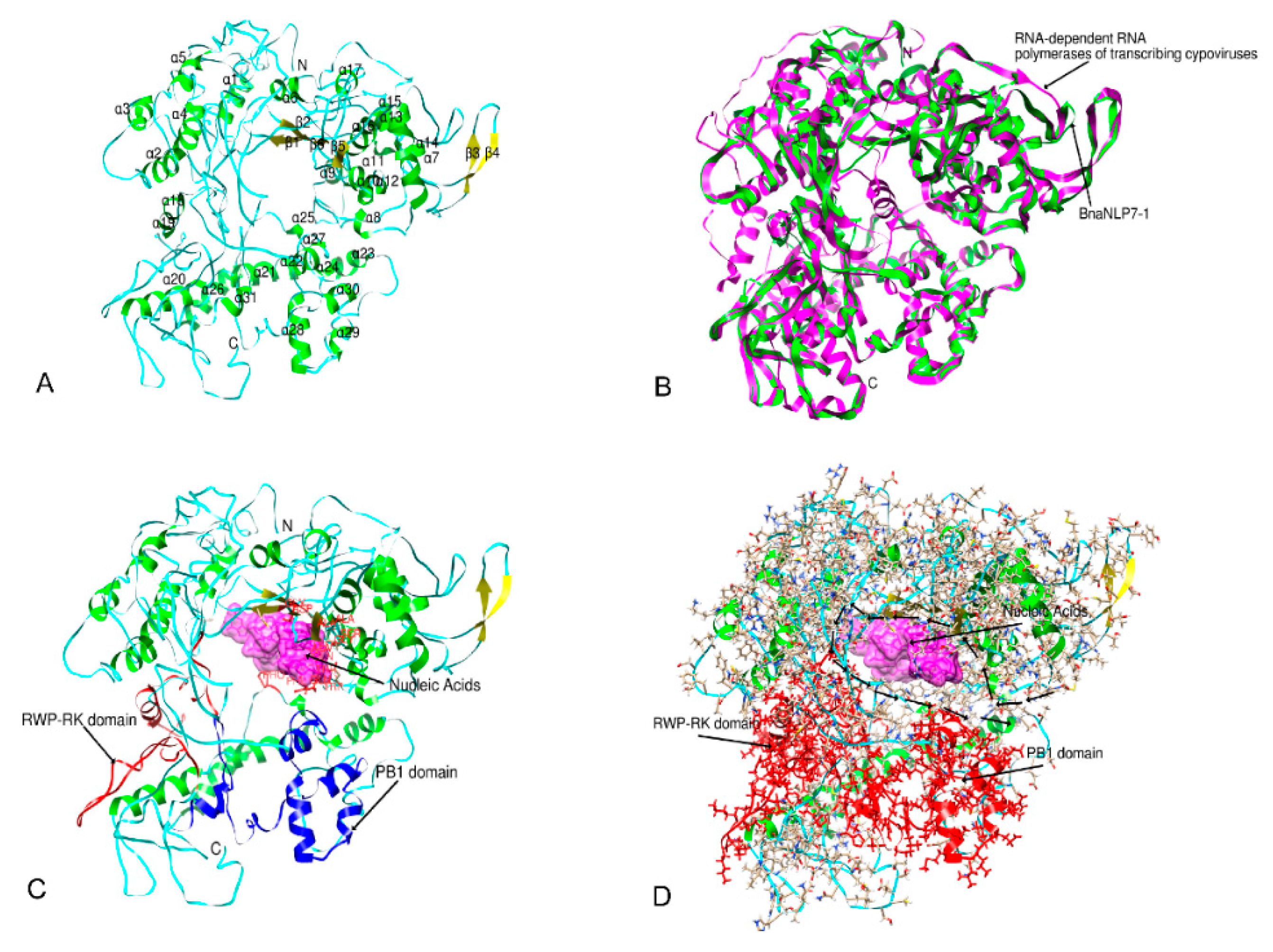
2.6. Three-Dimensional Structure Prediction
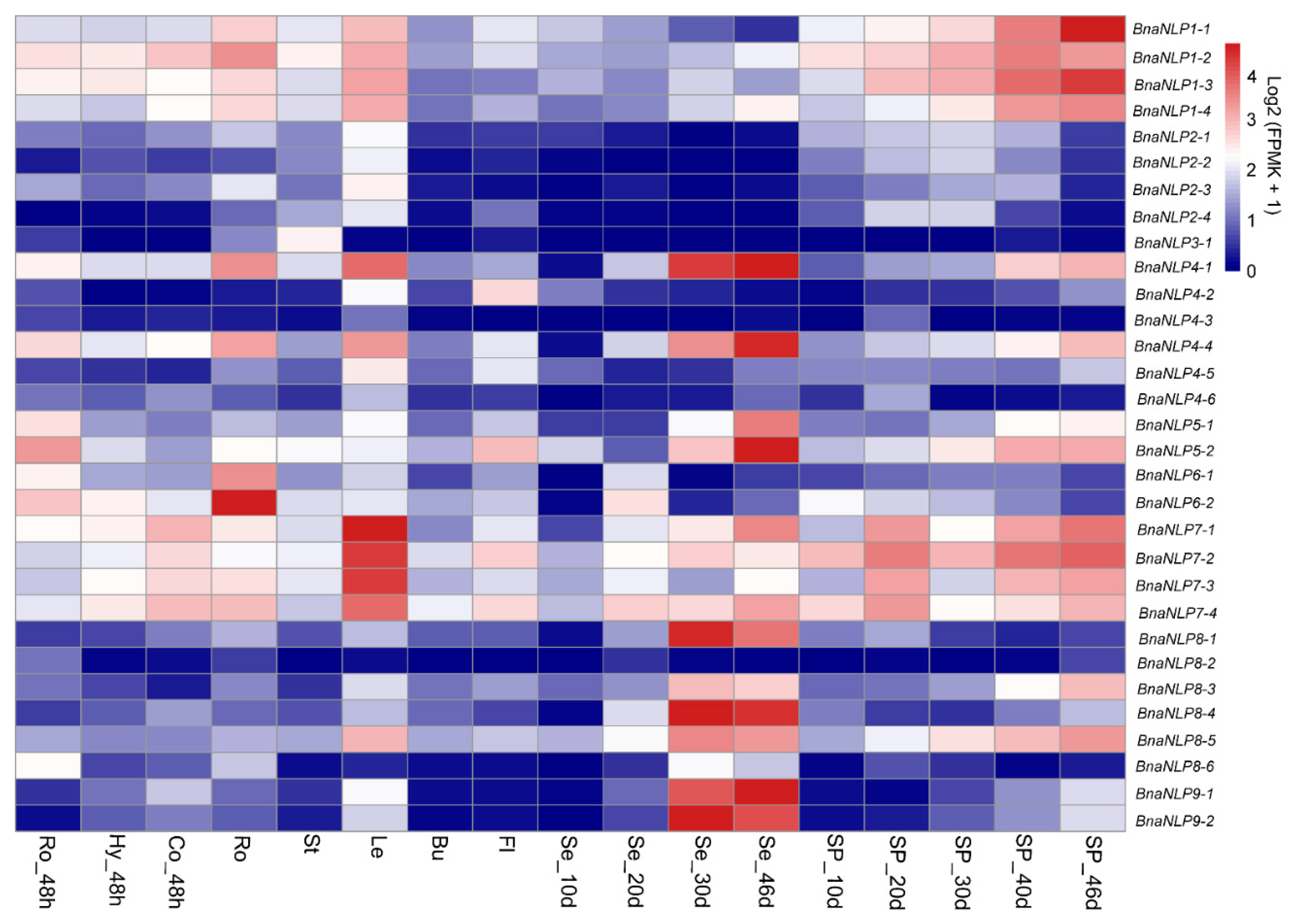
2.7. Organ-Specific Expression Patterns of BnaNLP Genes
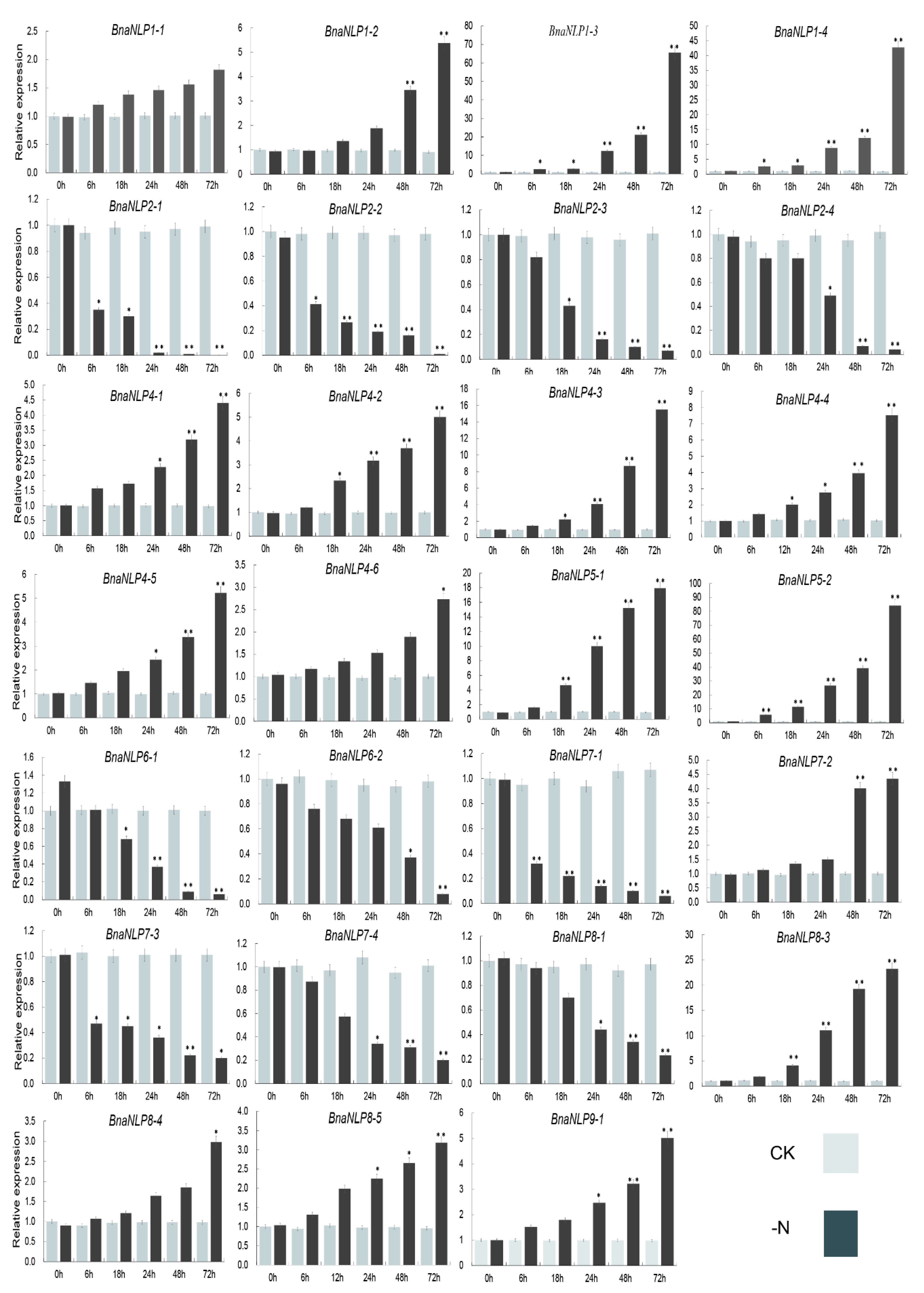
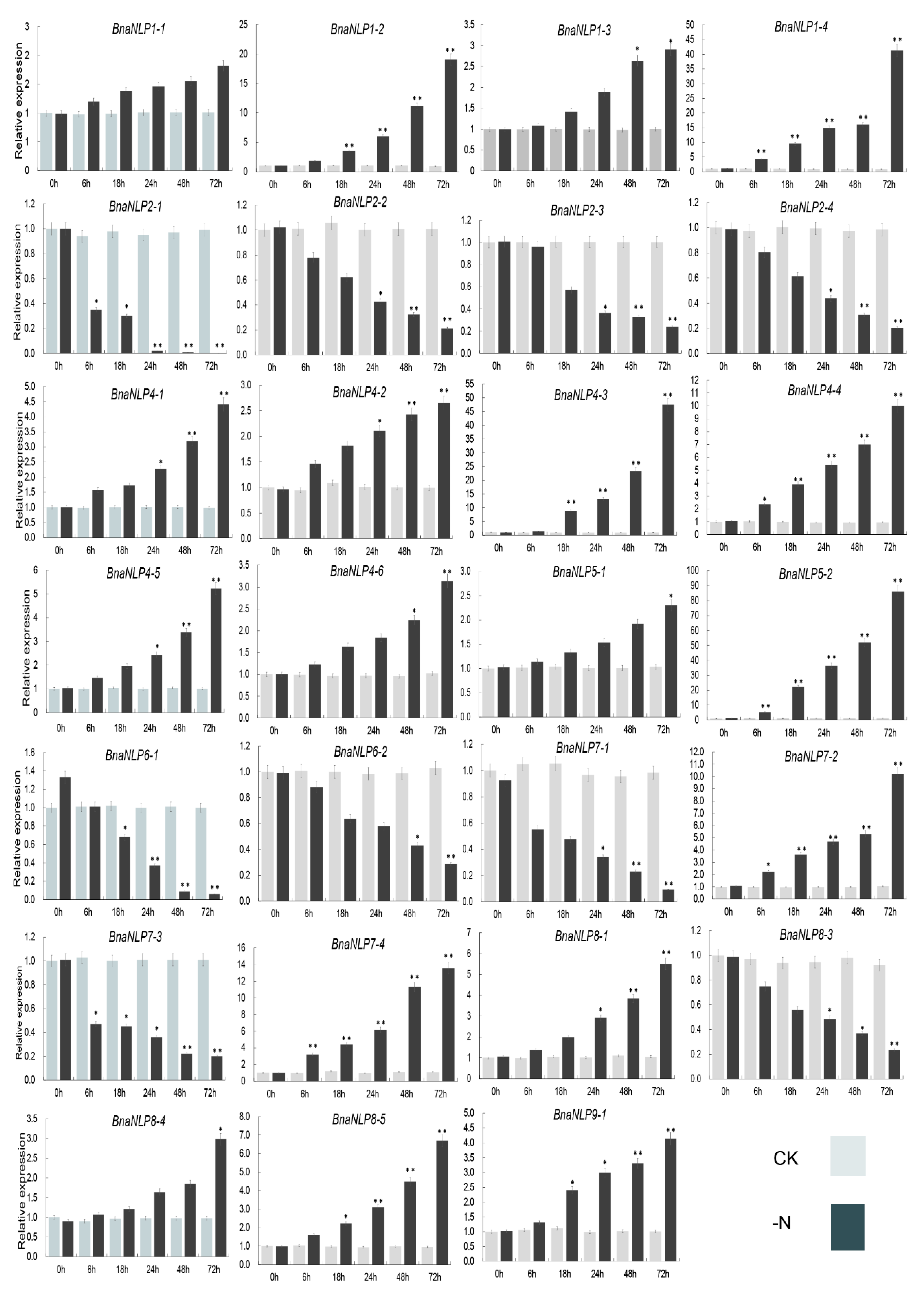
2.8. Expression Patterns of BnaNLP Genes under Nitrogen Deficiency
3. Discussion
3.1. Characterization of the NLP Gene Family in B. napus
3.2. Phylogenetic Relationship and Duplication Analysis of BnaNLPs
3.3. Expression Profiling and Response of Genes to Nitrogen Deficient Conditions
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification of NLP Family Genes in Plants
4.3. Phylogenetic Analysis of the NLP Family in B. napus
4.4. Chromosomal Locations and Protein Property Analysis
4.5. Motif Identification and TF Binding Site Analysis
4.6. Three-Dimensional Structure Prediction of BnaNLP7-1
4.7. RNA Isolation, RNA-Sequencing, and Quantitative Reverse-Transcription PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xing, X.J.; Wang, Y.; Tran, A.; Crawford, N. A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol. 2009, 151, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.J.; Burden, L.M.; Hurley, J.H. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J. 2000, 19, 5288–5299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.C.; Wang, Y.Y.; Tsay, Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009, 57, 264–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; von Wirén, N.; Yamaya, T.; Yamaguchi, J. Distinct expression and function of three ammonium transporter genes (OsAMT1;1-1;3) in rice. Plant Cell Physiol. 2003, 44, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.M.; Wang, R.L.; Zhu, T.; Rothstein, S.J. Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genom. 2007, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Mizokami, Y.; Miyata, K.; Tholen, D.; Watanabe, C.K.; Noguchi, K. Evidence for a nitrate-independent function of the nitrate sensor NRT1.1 in Arabidopsis thaliana. J. Plant Res. 2011, 124, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Castaings, L.; Antonio, C.; Delphine, P.; Virginie, G.; Yves, T.; Stéphanie, B.M.; Ludivine, T.; Jean-Pierre, R.; Françoise, D.; Emilio, F.; et al. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J. 2009, 57, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Schauser, L.; Wioletta, W.; Jens, S. Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J. Mol. Evol. 2005, 60, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A plant regulator controlling development of symbiotic root nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.Y.; Madsen, L.H.; Tsyganov, V.E.; Umehara, Y.; Voroshilova, V.A.; Batagov, A.O.; Sandal, N.; Mortensen, A.; Schauser, L.; Ellis, N.; et al. The Sym35 gene required for root nodule development in pea is an ortholog of Nin from Lotus japonicus. Plant Physiol. 2003, 131, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, W.; Konishi, M.; Yanagisawa, S. The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal. Behav. 2013, 8, e25975. [Google Scholar] [CrossRef] [PubMed]
- Chardin, C.; Thomas, G.; Francois, R.; Christian, M.; Anne, K. The plant RWP-RK transcription factors: Key regulators of nitrogen responses and of gametophyte development. J. Exp. Bot. 2014, 65, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H.; Sachiko, K.; Takashi, I. Structure and function of the PB1 domain, a protein interaction module conserved in animals, fungi, amoebas, and plants. Sci. STKE 2007, 401, re6. [Google Scholar] [CrossRef] [PubMed]
- Studholme, D.J.; Dixon, R. Domain architectures of σ54-dependent transcriptional activators. J. Bacteriol. 2003, 185, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.; Dixon, R. The role of bacterial enhancer binding proteins as specialized activators of s54-dependent transcription. Microbiol. Mol. Biol. Rev. 2012, 76, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Transcription factors involved in controlling the expression of nitrate reductase genes in higher plants. Plant Sci. 2014, 229, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.W.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.C.; et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Liu, Y.H.; Jiang, L.; Wang, Y.C.; Lv, Y.D.; Zhou, L.; Liang, S.Q.; Bao, H.B.; Zhao, H. Genome-wide analysis of maize NLP transcription factor family revealed the roles in nitrogen response. Plant Growth Regul. 2018, 84, 95–105. [Google Scholar] [CrossRef]
- Konishi, M.; Yanagisawa, S. The regulatory region controlling the nitrate-responsive expression of a nitrate reductase gene, NIA1, in Arabidopsis. Plant Cell Physiol. 2011, 52, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Yanagisawa, S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 2013, 4, 1617. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Zhang, D.W.; Zhu, F.; Wang, S.X.; Zhu, T.; Pu, X.J.; Zheng, T.; Feng, H.; Lin, H.H. Nitrate reductase-dependent nitric oxide production is required for regulation alternative oxidase pathway involved in the resistance to Cucumber mosaic virus infection in Arabidopsis. Plant Growth Regul. 2015, 77, 99–107. [Google Scholar] [CrossRef]
- Marchive, C.; Roudier, F.; Castaings, L.; Bréhaut, V.; Blondet, E.; Colot, V.; Meyer, C.; Krapp, A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013, 4, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, P.; Ripoll, J.J.; Wang, R.H.; Vuong, L.; Bailey-Steinitz, L.J.; Ye, D.N.; Crawford, N.M. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. USA 2017, 114, 2419–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.H.; Niu, Y.J.; Konishi, M.; Wu, Y.; Du, H.; Sun, H.; Li, L.; Boundsocq, M.; Mccormack, M.; Maekawa, S.; et al. Discovery of nitrate–CPK–NLP signalling in central nutrient–growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaharu, U.; Nagaharu, N. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Wu, X.Y.; Xu, Z.R.; Qu, C.P.; Li, W.; Sun, Q.; Liu, G.J. Genome-wide identification and characterization of NLP gene family in Populus trichocarpa. Bull. Bot. Res. 2014, 34, 37–43. [Google Scholar]
- Feng, C.; Wu, J.; Wang, X.W. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar]
- Anton, N.; Makova, K.D.; Li, W.H. The Ka/Ks ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar]
- Chow, C.N.; Zheng, H.Q.; Wu, N.Y.; Chien, C.H.; Huang, H.D.; Lee, T.Y.; Chiang-Hsieh, Y.F.; Hou, P.F.; Yang, T.Y.; Chang, W.C. Plant PAN 2.0: An update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 2015, 44, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, L. Cryo-EM shows the polymerase structures and a nonspooled genome within a dsRNA virus. Nucleic Acids Res. 2015, 349, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Roy, A.; Zhang, Y. BioLiP: A semi-manually curated database for biologically relevant ligand-protein interactions. Nucleic Acids Res. 2013, 41, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; McDonald, L.; Cygler, M.; Ekiel, I. Coiled-coil helix rotation selects repressing or activating state of transcriptional regulator DhaR. Structure 2014, 22, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Niemann, V.; Koch-Singenstreu, M.; Nilkens, S.; Götz, F.; Unden, G.; Stehle, T. The NreA protein functions as a nitrate receptor in the Staphylococcal nitrate regulation system. J. Mol. Biol. 2014, 426, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, T.; White, P.J.; Cong, R.H.; Lu, J. Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J. Exp. Bot. 2018, 69, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.C.; Sangigrahi, S.; Mandal, L.N. Effect of inoculation of Azospirillum lipoferum on nitrogen fixation in rhizosphere soil, their association with root, yield and nitrogen uptake by mustard (Brassica juncea). Plant Soil 1985, 87, 273–280. [Google Scholar] [CrossRef]
- Scheible, W.R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Okamoto, M.; Xing, X.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Rizi, V.; Franzaring, J.; Fangmeier, A.; Kunze, K. Divergent N Deficiency-Dependent Senescence and Transcriptome Response in Developmentally Old and Young Brassica napus Leaves. Front. Plant Sci. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Daniel, I.A. The Water-Culture Method For Growing Plants without Soil; California Agricultural Experiment Station, Circular; University of California: Berkeley, CA, USA, 1950; Volume 31, p. 347. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Yu, M.N.; Liu, M.; Zhang, R.; Sun, W.; Qian, M.C.; Duan, H.C.; Chang, W.; Ma, J.Q.; Qu, C.M.; et al. Genome-wide identification, evolutionary and expression analyses of the GALACTINOL SYNTHASE gene family in rapeseed and tobacco. Int. J. Mol. Sci. 2017, 18, 2768. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Stoffel, W. TMbase-A database of membrane spanning proteins segments. Biol. Chem. 1993, 374, 166. [Google Scholar]
- Henrik, N. Protein function prediction. Mol. Biol. 2017, 1611, 59–73. [Google Scholar]
- Blum, T.; Briesemeister, S.; Kohlbacher, O. MultiLoc2: Integrating phylogeny and gene ontology terms improves subcellular protein localization prediction. BMC Bioinform. 2009, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindvalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhang, Y. LOMETS: A local meta-threading-server for protein structure prediction. Nucleic Acids Res. 2007, 35, 3375–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified plat form for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Yang, J.; Zhang, Y. COFACTOR: An accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res. 2012, 40, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.; Fan, Y.; Ma, J.; Sun, W.; et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Xiao, Z.; Jian, H.; Peng, L.; Qu, C.; Fu, M.; He, B.; Tie, L.; Liang, Y.; Xu, X.; et al. A combination of genome-wide association and transcriptome analysis reveals candidate genes controlling harvest index-related traits in Brassica napus. Sci. Rep. 2016, 6, 36452. [Google Scholar] [CrossRef] [PubMed]

| Gene Name in B. napus | Gene ID in B. napus | Gene ID in A. thaliana | Chromosome | Position (bp) | Number of Introns | Protein Length (aa) | Protein Molecular Weight (kDa) | Protein Isoelectric Point (pI) | Protein Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| BnaNLP1-1 | BnaC03g47510D | AT2G17150 | C03 | 32,561,336~32,565,236 | 3 | 870 | 95.78 | 5.1 | Nucleus |
| BnaNLP1-2 | BnaA07g03130D | AT2G17150 | A07 | 2,770,119~2,774,330 | 3 | 895 | 99.32 | 4.98 | Nucleus |
| BnaNLP1-3 | BnaA06g25930D | AT2G17150 | A06 | 17,936,012~17,942,485 | 3 | 872 | 96.02 | 5.18 | Nucleus |
| BnaNLP1-4 | BnaC07g06170D | AT2G17150 | C07 | 9,960,829~9,965,297 | 3 | 896 | 99.33 | 4.98 | Nucleus |
| BnaNLP2-1 | BnaA01g02150D | AT4G35270 | A01 | 1,061,127~1,065,935 | 4 | 889 | 98.91 | 6.87 | Nucleus |
| BnaNLP2-2 | BnaA03g53210D | AT4G35270 | A03 | 27,917,856~27,921,412 | 3 | 897 | 100.07 | 6.67 | Nucleus |
| BnaNLP2-3 | BnaC01g03280D | AT4G35270 | C01 | 1,694,787~1,699,810 | 3 | 966 | 107.19 | 5.92 | Nucleus |
| BnaNLP2-4 | BnaC07g45500D | AT4G35270 | C07 | 43,543,401~43,546,699 | 3 | 901 | 100.71 | 7.04 | Chloroplast |
| BnaNLP3-1 | BnaA06g40940D | AT4G38340 | A06 | 2,255,358~2,256,385 | 3 | 681 | 76.37 | 7.31 | Nucleus |
| BnaNLP4-1 | BnaA06g14540D | AT1G20640 | A06 | 7,867,109~7,871,401 | 3 | 814 | 91.05 | 5.59 | Nucleus |
| BnaNLP4-2 | BnaA07g11340D | AT1G20640 | A07 | 10,591,730~10,594,794 | 3 | 775 | 86.88 | 5.36 | Nucleus |
| BnaNLP4-3 | BnaA08g21720D | AT1G20640 | A08 | 15,991,108~15,994,588 | 4 | 764 | 85.19 | 5.61 | Nucleus |
| BnaNLP4-4 | BnaC05g16000D | AT1G20640 | C05 | 9,745,808~9,749,062 | 3 | 703 | 77.85 | 5.84 | Nucleus |
| BnaNLP4-5 | BnaC07g15260D | AT1G20640 | C07 | 21,212,376~21,215,440 | 3 | 769 | 86.07 | 5.47 | Nucleus |
| BnaNLP4-6 | BnaC08g19370D | AT1G20640 | C08 | 22,293,545~22,296,521 | 4 | 761 | 85.02 | 5.75 | Nucleus |
| BnaNLP5-1 | BnaA07g32630D | AT1G76350 | A07 | 22,548,833~22,552,146 | 3 | 802 | 89.64 | 5.89 | Nucleus |
| BnaNLP5-2 | BnaC06g37080D | AT1G76350 | C06 | 35,298,227~35,301,829 | 3 | 802 | 89.61 | 5.94 | Nucleus |
| BnaNLP6-1 | BnaA09g12180D | AT1G64530 | A09 | 6,394,926~6,398,454 | 5 | 822 | 91.48 | 5.56 | Nucleus |
| BnaNLP6-2 | BnaCnng38990D | AT1G64530 | Unknown | 5 | 822 | 91.56 | 5.74 | Nucleus | |
| BnaNLP7-1 | BnaA01g35090D | AT4G24020 | A01 | 386,973~387,241 | 5 | 934 | 102.95 | 5.5 | Nucleus |
| BnaNLP7-2 | BnaA03g46410D | AT4G24020 | A03 | 23,822,691~23,826,304 | 5 | 934 | 102.68 | 5.6 | Nucleus |
| BnaNLP7-3 | BnaC01g15850D | AT4G24020 | C01 | 10,891,090~10,895,162 | 5 | 978 | 108.05 | 5.96 | Nucleus |
| BnaNLP7-4 | BnaC07g38670D | AT4G24020 | C07 | 39,940,917~39,945,105 | 5 | 937 | 103.06 | 5.61 | Nucleus |
| BnaNLP8-1 | BnaA03g20260D | AT2G43500 | A03 | 9,638,886~9,642,606 | 5 | 895 | 98.71 | 5.41 | Nucleus |
| BnaNLP8-2 | BnaA04g25110D | AT2G43500 | A04 | 18,201,925~18,205,255 | 5 | 798 | 88.35 | 5.94 | Nucleus |
| BnaNLP8-3 | BnaA05g03380D | AT2G43500 | A05 | 1,878,196~1,880,941 | 4 | 809 | 89.36 | 5.94 | Nucleus |
| BnaNLP8-4 | BnaC03g24230D | AT2G43500 | C03 | 13,588,155~13,591,741 | 5 | 897 | 99.00 | 5.42 | Nucleus |
| BnaNLP8-5 | BnaC04g02980D | AT2G43500 | C04 | 2,115,457~2,118,921 | 5 | 799 | 88.08 | 5.92 | Nucleus |
| BnaNLP8-6 | BnaC04g48980D | AT2G43500 | C04 | 47,362,522~47,365,605 | 6 | 811 | 90.07 | 5.94 | Nucleus |
| BnaNLP9-1 | BnaA07g18430D | AT3G59580 | A07 | 15,037,510~15,041,748 | 6 | 852 | 94.09 | 5.47 | Nucleus |
| BnaNLP9-2 | BnaC06g17440D | AT3G59580 | C06 | 20,075,721~20,079,938 | 6 | 851 | 94.15 | 5.58 | Nucleus |
| Group | Gene ID in A. thaliana | Gene ID in B. napus | Model | Ka | Ks | Ka/Ks | Average Ka/Ks |
|---|---|---|---|---|---|---|---|
| Group I | AT2G17150 | BnaNLP1-1 | NG | 0.095573 | 0.381817 | 0.250311 | 0.169549 |
| AT2G17150 | BnaNLP1-2 | NG | 0.084925 | 0.375565 | 0.226126 | ||
| AT2G17150 | BnaNLP1-3 | NG | 0.094742 | 0.382276 | 0.247837 | ||
| AT2G17150 | BnaNLP1-4 | NG | 0.089774 | 0.376577 | 0.238395 | ||
| AT4G35270 | BnaNLP2-1 | NG | 0.064136 | 0.464096 | 0.138196 | ||
| AT4G35270 | BnaNLP2-2 | NG | 0.068387 | 0.409203 | 0.167123 | ||
| AT4G35270 | BnaNLP2-3 | NG | 0.063939 | 0.470639 | 0.135856 | ||
| AT4G35270 | BnaNLP2-4 | NG | 0.070692 | 0.431699 | 0.163754 | ||
| AT4G38340 | BnaNLP3-1 | NG | 0.123420 | 0.365014 | 0.338126 | ||
| AT1G20640 | BnaNLP4-1 | NG | 0.044027 | 0.522956 | 0.084189 | ||
| AT1G20640 | BnaNLP4-2 | NG | 0.057461 | 0.430702 | 0.133413 | ||
| AT1G20640 | BnaNLP4-3 | NG | 0.063875 | 0.37724 | 0.169322 | ||
| AT1G20640 | BnaNLP4-4 | NG | 0.056956 | 0.602642 | 0.094510 | ||
| AT1G20640 | BnaNLP4-5 | NG | 0.056344 | 0.418456 | 0.134646 | ||
| AT1G20640 | BnaNLP4-6 | NG | 0.063963 | 0.361807 | 0.176786 | ||
| AT1G76350 | BnaNLP5-1 | NG | 0.055669 | 0.60493 | 0.092026 | ||
| AT1G76350 | BnaNLP5-2 | NG | 0.057236 | 0.62409 | 0.091711 | ||
| Group II | AT1G64530 | BnaNLP6-1 | NG | 0.069587 | 0.545178 | 0.127642 | 0.103387 |
| AT1G64530 | BnaNLP6-2 | NG | 0.070799 | 0.519949 | 0.136166 | ||
| AT4G24020 | BnaNLP7-1 | NG | 0.027387 | 0.347158 | 0.07889 | ||
| AT4G24020 | BnaNLP7-2 | NG | 0.034582 | 0.363912 | 0.095029 | ||
| AT4G24020 | BnaNLP7-3 | NG | 0.029575 | 0.345875 | 0.085507 | ||
| AT4G24020 | BnaNLP7-4 | NG | 0.035223 | 0.3628 | 0.097086 | ||
| Group III | AT2G43500 | BnaNLP8-1 | NG | 0.083688 | 0.393217 | 0.212829 | 0.264202 |
| AT2G43500 | BnaNLP8-2 | NG | 0.105273 | 0.419328 | 0.251052 | ||
| AT2G43500 | BnaNLP8-3 | NG | 0.096503 | 0.434656 | 0.222021 | ||
| AT2G43500 | BnaNLP8-4 | NG | 2.395310 | 4.04226 | 0.592566 | ||
| AT2G43500 | BnaNLP8-5 | NG | 0.093713 | 0.471195 | 0.198883 | ||
| AT2G43500 | BnaNLP8-6 | NG | 0.107375 | 0.405135 | 0.265035 | ||
| AT3G59580 | BnaNLP9-1 | NG | 0.084630 | 0.469167 | 0.180383 | ||
| AT3G59580 | BnaNLP9-2 | NG | 0.089617 | 0.469558 | 0.190854 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Chang, W.; Fan, Y.; Sun, W.; Qu, C.; Zhang, K.; Liu, L.; Xu, X.; Tang, Z.; Li, J.; et al. Genome-Wide Identification and Characterization of NODULE-INCEPTION-Like Protein (NLP) Family Genes in Brassica napus. Int. J. Mol. Sci. 2018, 19, 2270. https://doi.org/10.3390/ijms19082270
Liu M, Chang W, Fan Y, Sun W, Qu C, Zhang K, Liu L, Xu X, Tang Z, Li J, et al. Genome-Wide Identification and Characterization of NODULE-INCEPTION-Like Protein (NLP) Family Genes in Brassica napus. International Journal of Molecular Sciences. 2018; 19(8):2270. https://doi.org/10.3390/ijms19082270
Chicago/Turabian StyleLiu, Miao, Wei Chang, Yonghai Fan, Wei Sun, Cunmin Qu, Kai Zhang, Liezhao Liu, Xingfu Xu, Zhanglin Tang, Jiana Li, and et al. 2018. "Genome-Wide Identification and Characterization of NODULE-INCEPTION-Like Protein (NLP) Family Genes in Brassica napus" International Journal of Molecular Sciences 19, no. 8: 2270. https://doi.org/10.3390/ijms19082270






