Protective Effects of Rhodiola Crenulata Extract on Hypoxia-Induced Endothelial Damage via Regulation of AMPK and ERK Pathways
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of RCE
2.2. RCE Protects against Hypoxia-Induced Endothelial Cell Death and Restores NO Production
2.3. RCE Protects Endothelial Cells from Oxidative Stress
2.4. RCE Restores Hypoxia-Impaired NO Production via AMPK-AKT-eNOS Signaling Pathway
2.5. RCE Protects Endothelial Cells from Hypoxia-Induced Apoptosis
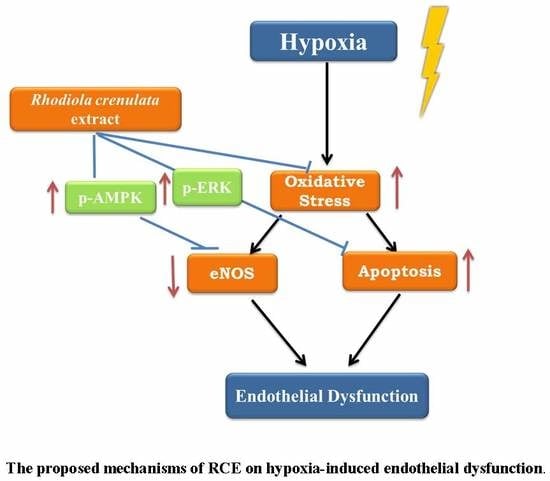
3. Discussion
4. Materials and Methods
4.1. Preparations and HPLC Analysis of RCE
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Measurement of Nitrite
4.5. Malondialdehyde Analysis
4.6. Measurement of Intracellular ROS
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cummins, E.P.; Crean, D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017, 19, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2002, 23, 168–175. [Google Scholar] [CrossRef]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bartsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [PubMed]
- Hatoum, O.A.; Miura, H.; Binion, D.G. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1791–H1796. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012, 4, 1391–1403. [Google Scholar] [CrossRef]
- Lee, S.Y.; Li, M.H.; Shi, L.S.; Chu, H.; Ho, C.W.; Chang, T.C. Rhodiola crenulata Extract Alleviates Hypoxic Pulmonary Edema in Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 718739. [Google Scholar] [PubMed]
- Lee, R.; Channon, K.M.; Antoniades, C. Therapeutic strategies targeting endothelial function in humans: Clinical implications. Curr. Vasc. Pharmacol. 2012, 10, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Ohkita, M.; Kiso, Y.; Matsumura, Y. Pharmacology in Health Foods: Improvement of Vascular Endothelial Function by French Maritime Pine Bark Extract (Flavangenol). J. Pharmacol. Sci. 2011, 115, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Härtel, F.; Holl, M.; Arshad, M.; Aslam, M.; Gündüz, D.; Weyand, M.; Micoogullari, M.; Abdallah, Y.; Piper, H.; Noll, T. Transient hypoxia induces ERK-dependent anti-apoptotic cell survival in endothelial cells. Am. J. Physiol. Cell Physiol. 2010, 298, C1501–C1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Zhou, L.; Wu, X.; Liu, C.; Fan, Y.; Li, Q. Hypoxic preconditioning protects cardiomyocytes against hypoxia/reoxygenation injury through AMPK/eNOS/PGC-1α signaling pathway. Int. J. Clin. Exp. Pathol. 2014, 7, 7378–7388. [Google Scholar] [PubMed]
- Lin, K.T.; Chang, T.C.; Lai, F.Y.; Lin, C.S.; Chao, H.L.; Lee, S.Y. Rhodiola crenulata Attenuates gamma-Ray Induced Cellular Injury via Modulation of Oxidative Stress in Human Skin Cells. Am. J. Chin. Med. 2018, 46, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shi, L.S.; Chu, H.; Li, M.H.; Ho, C.W.; Lai, F.Y.; Huang, C.Y.; Chang, T.C. Rhodiola crenulata and Its Bioactive Components, Salidroside and Tyrosol, Reverse the Hypoxia-Induced Reduction of Plasma-Membrane-Associated Na,K-ATPase Expression via Inhibition of ROS-AMPK-PKCξ Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 284150. [Google Scholar]
- Hsu, S.W.; Chang, T.C.; Wu, Y.K.; Lin, K.T.; Shi, L.S.; Lee, S.Y. Rhodiola crenulata extract counteracts the effect of hypobaric hypoxia in rat heart via redirection of the nitric oxide and arginase 1 pathway. BMC Complement. Altern. Med. 2017, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Yen, I.C.; Tsai, W.C.; Ahmetaj-Shala, B.; Chang, T.C.; Tsai, C.S.; Lee, S.Y. Rhodiola crenulata Attenuates High Glucose Induced Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells. Am. J. Chin. Med. 2017, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.; Mahaseth, H.; Welch, T.; Vilback, A.; Sonbol, K.; Kalambur, V.; Bowlin, P.; Bischof, J.; Hebbel, R.; Vercellotti, G. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2715–H2725. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Franca, C.N.; Wenzel, D.; Fleischmann, B.; Nickenig, G.; Werner, N.; Skowasch, D. Hypoxia-induced endothelial dysfunction in apolipoprotein E-deficient mice; effects of infliximab and l-glutathione. Atherosclerosis 2014, 236, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, D.; Chen, B. Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath 2012, 16, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Isaak, C.K.; Zhou, Y.; Petkau, J.C.; Karmin, O.; Liu, Y.; Siow, Y.L. Salidroside and Tyrosol from Rhodiola protect H9c2 cell from ischemia/reperfusion-induced apoptosis. Life Sci. 2012, 91, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Qian, E.W.; Ge, D.T.; Kong, S.K. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J. Ethnopharmacol. 2011, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Zhao, X.; Lin, X.; Tan, C.; Cao, G.; Wang, Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnink, S.; van Haelst, P.; van Boven, A.; Stroes, E.; Tio, R.; Plokker, T.; Smit, A.; Veeger, N.; Crijns, H.; van Gilst, W. Endothelial dysfunction in patients with coronary artery disease: A comparison of three frequently reported tests. J. Investig. Med. 2002, 50, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, D.; Rao, V.; Tumiati, L.C.; Xu, N.; Sheshgiri, R.; Miriuka, S.; Delgado, D.H.; Ross, H.J. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation 2006, 114 (Suppl. 1), I319–I326. [Google Scholar] [CrossRef] [PubMed]
- El Solh, A.A.; Akinnusi, M.E.; Baddoura, F.H.; Mankowski, C.R. Endothelial cell apoptosis in obstructive sleep apnea: A link to endothelial dysfunction. Am. J. Respir. Crit. Care Med. 2007, 175, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.B.; Gao, M.; Xu, W.R.; Yang, X.Y.; Zhu, X.M.; Du, G.H. Protective effects of salidroside on endothelial cell apoptosis induced by cobalt chloride. Biol. Pharm. Bull. 2009, 32, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Sievenpiper, J.L.; Wong, J.; Xu, Z.; Beljan-Zdravkovic, U.; Arnason, J.T.; Assinewe, V.; Stavro, M.P.; Jenkins, A.L.; Leiter, L.A.; et al. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am. J. Clin. Nutr. 2001, 73, 753–758. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bi, X.; Lu, X.; Zhao, M.; Yu, X.; Sun, L.; Xu, M.; Wier, W.; Zang, W. Reduction of Mitochondria-Endoplasmic Reticulum Interactions by Acetylcholine Protects Human Umbilical Vein Endothelial Cells from Hypoxia/Reoxygenation Injury. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Nakata, S.; Koike, Y.; Miyata, S.; Kitaichi, K.; Nishizawa, T.; Nagata, K.; Yasuma, F.; Murohara, T.; Yokota, M. Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens. Res. 2007, 30, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Sata, M.; Kakoki, M.; Nagata, D.; Nishimatsu, H.; Suzuki, E.; Aoyagi, T.; Sugiura, S.; Kojima, H.; Nagano, T.; Kangawa, K.; et al. Adrenomedullin and nitric oxide inhibit human endothelial cell apoptosis via a cyclic GMP-independent mechanism. Hypertension 2000, 36, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Tamilarasan, K.P.; Rajkumar, A.S.; Geetha Priya, S.; Rajaram, M.; Saleem, N.K.; Majumder, S.; Jaffar Ali, B.M.; Illavazagan, G.; Chatterjee, S. Nitric oxide/cGMP protects endothelial cells from hypoxia-mediated leakiness. Eur. J. Cell Biol. 2008, 87, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.; Manuel, P.; Oliveira, A.; Gil, I.; Nascimento, E.; Sanchez, A. The impact of obstructive sleep apnea in diabetes mellitus. Sleep Med. 2013, 14, e290. [Google Scholar] [CrossRef]
- Leung, S.B.; Zhang, H.; Lau, C.W.; Huang, Y.; Lin, Z. Salidroside improves homocysteine-induced endothelial dysfunction by reducing oxidative stress. Evid. Based Complement. Altern. Med. 2013, 2013, 679635. [Google Scholar] [CrossRef] [PubMed]
- Nagata, D.; Mogi, M.; Walsh, K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 2003, 278, 31000–31006. [Google Scholar] [CrossRef] [PubMed]
- Reihill, J.A.; Ewart, M.A.; Hardie, D.G.; Salt, I.P. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem. Biophys. Res. Commun. 2007, 354, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chen, P.Y.; Lin, J.C.; Kirkby, N.S.; Ou, C.H.; Chang, T.C. Melaleuca alternifolia Induces Heme Oxygenase-1 Expression in Murine RAW264.7 Cells through Activation of the Nrf2-ARE Pathway. Am. J. Chin. Med. 2017, 45, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Tsai, W.-C.; Lin, J.-C.; Ahmetaj-Shala, B.; Huang, S.-F.; Chang, W.-L.; Chang, T.-C. Astragaloside II promotes intestinal epithelial repair by enhancing L-arginine uptake and activating the mTOR pathway. Sci. Rep. 2017, 7, 12302. [Google Scholar] [CrossRef] [PubMed] [Green Version]









 : hypoxic stimulation.
: hypoxic stimulation.
 : hypoxic stimulation.
: hypoxic stimulation.
| Type | Antigen | Manufacturer | Dilution |
|---|---|---|---|
| Primary antibody | eNOS | Santa Cruz, CA | 1:1000 |
| Primary antibody | p-AKT (Thr308 and Ser473) | Santa Cruz, CA | 1:1000 |
| Primary antibody | AKT | Santa Cruz, CA | 1:1000 |
| Primary antibody | β-actin | Chemicon, Temecula, CA | 1:1000 |
| Primary antibody | p-eNOS (Ser1177) | Cell Signaling Tech. | 1:1000 |
| Primary antibody | AMPK | Cell Signaling Tech. | 1:1000 |
| Primary antibody | p-AMPK (T172) | Cell Signaling Tech. | 1:1000 |
| Primary antibody | ACC | Cell Signaling Tech. | 1:1000 |
| Primary antibody | p-ACC | Cell Signaling Tech. | 1:1000 |
| Primary antibody | PARP | Cell Signaling Tech. | 1:1000 |
| Primary antibody | Caspase 3 | Cell Signaling Tech. | 1:1000 |
| Primary antibody | Caspase 8 | Cell Signaling Tech. | 1:1000 |
| Primary antibody | ERK | Cell Signaling Tech. | 1:1000 |
| Primary antibody | p-ERK | Cell Signaling Tech. | 1:1000 |
| Secondary antibody | Anti-goat IgG-HRP | Santa Cruz, CA | 1:10,000 |
| Secondary antibody | Anti-rabbit IgG-HRP | GeneTex | 1:10,000 |
| Secondary antibody | Anti-mouse IgG-HRP | Jackson | 1:10,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-K.; Yen, I.-C.; Tsai, W.-C.; Chang, T.-C.; Lee, S.-Y. Protective Effects of Rhodiola Crenulata Extract on Hypoxia-Induced Endothelial Damage via Regulation of AMPK and ERK Pathways. Int. J. Mol. Sci. 2018, 19, 2286. https://doi.org/10.3390/ijms19082286
Chang P-K, Yen I-C, Tsai W-C, Chang T-C, Lee S-Y. Protective Effects of Rhodiola Crenulata Extract on Hypoxia-Induced Endothelial Damage via Regulation of AMPK and ERK Pathways. International Journal of Molecular Sciences. 2018; 19(8):2286. https://doi.org/10.3390/ijms19082286
Chicago/Turabian StyleChang, Pi-Kai, I-Chuan Yen, Wei-Cheng Tsai, Tsu-Chung Chang, and Shih-Yu Lee. 2018. "Protective Effects of Rhodiola Crenulata Extract on Hypoxia-Induced Endothelial Damage via Regulation of AMPK and ERK Pathways" International Journal of Molecular Sciences 19, no. 8: 2286. https://doi.org/10.3390/ijms19082286