Repair of Damaged Articular Cartilage: Current Approaches and Future Directions
Abstract
:1. Introduction
2. Clinically Used Approaches
2.1. Intra-Articular Injections of Various Compounds
2.1.1. Corticosteroid Injections
2.1.2. Hyaluronic Acid (Hyaluronan) Injections
2.1.3. Injections of Autologous Platelet-Rich Plasma
2.2. Surgical Approaches: Microfracture and Chondroplasty Surgery
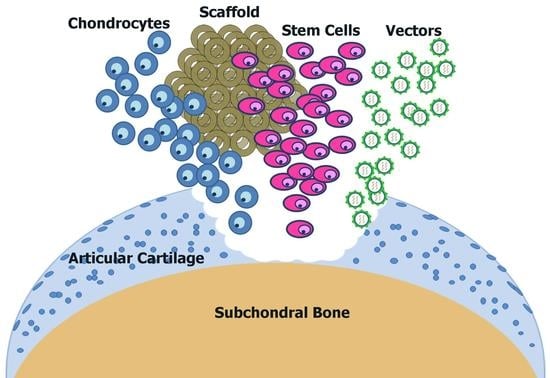
2.3. Regenerative Medicine and Cell-Based Approaches
3. Regeneration of Cartilage with Stem Cells
3.1. Mesenchymal Stem Cells
3.2. Embryonic Stem Cells
3.3. Induced Pluripotent Stem Cells
3.4. Chondrogenic Stem/Progenitor Cells from the Superficial Zone
4. Tissue-Engineered Constructs
4.1. Scaffolds
4.2. Production of Scaffolds
4.3. Three-Dimensional Bio-Printing
5. Approaches Mimicking the Natural Environment of Articular Cartilage
5.1. Lubrication
5.2. Mechanical Stimuli
5.3. Hypoxia
6. Regenerative Approaches for Treatment of Osteoarthritis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.; Choi, J.; Hwang, N.S. Regulation of lubricin for functional cartilage tissue regeneration: A review. Biomater. Res. 2018, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Tee, C.A.; Lai, Z.; Lim, C.T.; Han, J.; Lee, E.H. Characterization and application of size-sorted zonal chondrocytes for articular cartilage regeneration. Biomaterials 2018, 165, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017, 31, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Grol, M.W.; Lee, B.H. Gene therapy for repair and regeneration of bone and cartilage. Curr. Opin. Pharmacol. 2018, 40, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. rAAV-mediated combined gene transfer and overexpression of TGF-β and SOX9 remodels human osteoarthritic articular cartilage. J. Orthop. Res. 2016, 34, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Veronesi, F.; Carina, V.; Costa, V.; Raimondi, L.; De Luca, A.; Alessandro, R.; Fini, M.; Giavaresi, G. Gene therapy for chondral and osteochondral regeneration: Is the future now? Cell. Mol. Life Sci. 2018, 75, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Cobo-Molinos, J.; Antich, C.; López-Ruiz, E. Osteoarthritis: Trauma vs Disease. Adv. Exp. Med. Biol. 2018, 1059, 63–83. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis: Care and Management|Guidance and Guidelines|NICE. Available online: https://www.nice.org.uk/guidance/cg177 (accessed on 20 July 2018).
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sports Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, CD005328. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.W. Intra-articular hyaluronan therapy. Curr. Opin. Rheumatol. 2000, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.E.; Maurer, S.G.; Di Cesare, P.E. Viscosupplementation for osteoarthritis. Am. J. Orthop. 2000, 29, 80–88; discussion 88–89. [Google Scholar] [PubMed]
- Estades-Rubio, F.J.; Reyes-Martín, A.; Morales-Marcos, V.; García-Piriz, M.; García-Vera, J.J.; Perán, M.; Marchal, J.A.; Montañez-Heredia, E. Knee Viscosupplementation: Cost-Effectiveness Analysis between Stabilized Hyaluronic Acid in a Single Injection versus Five Injections of Standard Hyaluronic Acid. Int. J. Mol. Sci. 2017, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.H.; LaValley, M.; McAlindon, T.; Felson, D.T. Intra-articular Hyaluronic Acid in Treatment of Knee Osteoarthritis. JAMA 2003, 290, 3115. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.; Donnelly, P.; Brown, G.A.; Cummins, D.S. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J. Bone Joint Surg. Am. 2015, 97, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.S.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Reddi, A.H. Platelet-Rich Plasma Modulates Actions on Articular Cartilage Lubrication and Regeneration. Tissue Eng. Part B. Rev. 2016, 22, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Kundra, R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017, 2, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhart, J.M.; Gambaryan, S.; Watson, S.P.; Jurk, K.; Walter, U.; Sickmann, A.; Heemskerk, J.W.M.; Zahedi, R.P. What Can Proteomics Tell Us About Platelets? Circ. Res. 2014, 114, 1204–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: Insights into integrin signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef] [PubMed]
- Montañez-Heredia, E.; Irízar, S.; Huertas, P.J.; Otero, E.; Del Valle, M.; Prat, I.; Díaz-Gallardo, M.S.; Perán, M.; Marchal, J.A.; Hernandez-Lamas, M.D.C. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int. J. Mol. Sci. 2016, 17, 1064. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Schonholtz, G.J. Arthroscopic debridement of the knee joint. Orthop. Clin. North Am. 1989, 20, 257–263. [Google Scholar] [PubMed]
- Jacobi, M.; Villa, V.; Magnussen, R.A.; Neyret, P. MACI—A new era? Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Lamplot, J.D.; Schafer, K.A.; Matava, M.J. Treatment of Failed Articular Cartilage Reconstructive Procedures of the Knee A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118761871. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Kohn, D. Indication for and performance of articular cartilage drilling using the Pridie method. Orthopade 1999, 28, 4–10. [Google Scholar] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Kumar, A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. KNEE Surg. Sports Traumatol. Arthrosc. 2014, 22, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical Efficacy of the Microfracture Technique for Articular Cartilage Repair in the Knee. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Torrie, A.M.; Kesler, W.W.; Elkin, J.; Gallo, R.A. Osteochondral allograft. Curr. Rev. Musculoskelet. Med. 2015, 8, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangody, L.; Kish, G.; Kárpáti, Z.; Udvarhelyi, I.; Szigeti, I.; Bély, M. Mosaicplasty for the treatment of articular cartilage defects: Application in clinical practice. Orthopedics 1998, 21, 751–756. [Google Scholar] [PubMed]
- Nakagawa, Y.; Mukai, S.; Yabumoto, H.; Tarumi, E.; Nakamura, T. Serial Changes of the Cartilage in Recipient Sites and Their Mirror Sites on Second-Look Imaging After Mosaicplasty. Am. J. Sports Med. 2016, 44, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Füles, P. Autologous Osteochondral Mosaicplasty For The Treatment Of Full-thickness Defects Of Weight-bearing Joints: Ten Years Of Experimental And Clinical Experience. J. Bone Joint Surg. Am. 2003, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gracitelli, G.C.; Meric, G.; Briggs, D.T.; Pulido, P.A.; McCauley, J.C.; Belloti, J.C.; Bugbee, W.D. Fresh Osteochondral Allografts in the Knee. Am. J. Sports Med. 2015, 43, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.S.; Roberts, S. A histological comparison of the repair tissue formed when using either Chondrogide® or periosteum during autologous chondrocyte implantation. Osteoarthr. Cartil. 2013, 21, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Goyal, A.; Keyhani, S.; Lee, E.H.; Hui, J.H.P. Evidence-Based Status of Second- and Third-Generation Autologous Chondrocyte Implantation Over First Generation: A Systematic Review of Level I and II Studies. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Steinwachs, M.; Erggelet, C.; Krause, S.J.; Ossendorf, C.; Maier, D.; Ghanem, N.; Uhl, M.; Haag, M. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthr. Cartil. 2007, 15, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Devitt, B.M.; Bell, S.W.; Webster, K.E.; Feller, J.A.; Whitehead, T.S. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee 2017, 24, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Derrett, S.; Stokes, E.A.; James, M.; Bartlett, W.; Bentley, G. Cost and health status analysis after autologous chondrocyte implantation and mosaicplasty: A retrospective comparison. Int. J. Technol. Assess. Health Care 2005, 21, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, G.; Drogset, J.O.; Engebretsen, L.; Grøntvedt, T.; Ludvigsen, T.C.; Løken, S.; Solheim, E.; Strand, T.; Johansen, O. A Randomized Multicenter Trial Comparing Autologous Chondrocyte Implantation with Microfracture. J. Bone Jt. Surg. 2016, 98, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Zanasi, S.; Brittberg, M.; Marcacci, M. Basic science, clinical repair and reconstruction of articular cartilage defects: Current status and prospects. In Immunohistochemical and Biochemical Analysis of Cartilage Repair Tissue Biopsies; Timeo Editore: Rastignano, Italy, 2006; pp. 705–710. [Google Scholar]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Hoffman, T.; Wu, A.; Kohn, J. An Innovative Laboratory Procedure to Expand Chondrocytes with Reduced Dedifferentiation. Cartilage 2017, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Ma, B.; Liang, Y.; Chen, J.; Zhu, W.; Li, M.; Wang, D. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am. J. Transl. Res. 2015, 7, 194–208. [Google Scholar] [PubMed]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, E.W.; Jahr, H.; Koevoet, J.L.M.; van Leeuwen, J.P.T. M.; Weinans, H.; Verhaar, J.A.N.; van Osch, G.J.V.M. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004, 23, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.G.A.; Saris, D.B.F.; Geuze, R.E.; Helm, Y.J.M. Van Der; Rijen, M.H.P. Van; Verbout, A.J.; Dhert, W.J.A.; Creemers, L.B. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue Eng. 2006, 12, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.J.M.J.; Emans, P.J.J.; Coolsen, M.M.E.M.E.; Voss, L.; Surtel, D.A.M.A.M.; Cremers, A.; van Rhijn, L.W.W.; Welting, T.J.M.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Effects of passage number and post-expansion aggregate culture on tissue engineered, self-assembled neocartilage. Acta Biomater. 2016, 43, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Leijten, J.C.H.; Wu, L.; Kip, M.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr. Cartil. 2013, 21, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Rakic, R.; Bourdon, B.; Hervieu, M.; Branly, T.; Legendre, F.; Saulnier, N.; Audigié, F.; Maddens, S.; Demoor, M.; Galera, P. RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation. Int. J. Mol. Sci. 2017, 18, 1842. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; de Souza, P.; Castrejon, H.V.; John, T.; Merker, H.-J.; Scheid, A.; Shakibaei, M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002, 308, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Mandl, E.W.; Van Der Veen, S.W.; Verhaar, J.A.N.; Van Osch, G.J.V.M. Multiplication of Human Chondrocytes with Low Seeding Densities Accelerates Cell Yield without Losing Redifferentiation Capacity. Tissue Eng. 2004, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, X.; Zhou, J.; Wei, L. The age-related changes in cartilage and osteoarthritis. Biomed. Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Mabuchi, Y.; Yoshida, M.; Suto, E.G.; Suzuki, N.; Muneta, T.; Sekiya, I.; Akazawa, C. Purified Human Synovium Mesenchymal Stem Cells as a Good Resource for Cartilage Regeneration. PLoS ONE 2015, 10, e0129096. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, G.; Xu, X. Immunomodulatory functions of mesenchymal stem cells and possible mechanisms. Histol. Histopathol. 2016, 31, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Steck, E.; Fischer, J.; Lorenz, H.; Gotterbarm, T.; Jung, M.; Richter, W. Mesenchymal stem cell differentiation in an experimental cartilage defect: Restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009, 18, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Jukes, J.M.; van Blitterswijk, C.A.; de Boer, J. Skeletal tissue engineering using embryonic stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 165–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latchoumane, C.-F.V.; Jackson, L.; Sendi, M.S.E.; Tehrani, K.F.; Mortensen, L.J.; Stice, S.L.; Ghovanloo, M.; Karumbaiah, L. Chronic Electrical Stimulation Promotes the Excitability and Plasticity of ESC-derived Neurons following Glutamate-induced Inhibition In vitro. Sci. Rep. 2018, 8, 10957. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 2017, 6, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Vats, A.; Bielby, R.C.; Tolley, N.; Dickinson, S.C.; Boccaccini, A.R.; Hollander, A.P.; Bishop, A.E.; Polak, J.M. Chondrogenic differentiation of human embryonic stem cells: The effect of the micro-environment. Tissue Eng. 2006, 12, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Puttonen, K.A.; Lindeberg, H.; Ruponen, M.; Hovatta, O.; Koistinaho, J.; Lammi, M.J. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int. J. Biochem. Cell Biol. 2013, 45, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Li, W.-J. Chondrogenesis of Embryonic Stem Cell-Derived Mesenchymal Stem Cells Induced by TGFβ1 and BMP7 Through Increased TGFβ Receptor Expression and Endogenous TGFβ1 Production. J. Cell. Biochem. 2017, 118, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Craft, A.M.; Rockel, J.S.; Nartiss, Y.; Kandel, R.A.; Alman, B.A.; Keller, G.M. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Tsumaki, N.; Okada, M.; Yamashita, A. iPS cell technologies and cartilage regeneration. Bone 2015, 70, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R. iPS Cells—The Triumphs and Tribulations. Dent. J. 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Diecke, S.; Lenkov, O.D.; Chapelin, F.; Donig, J.; Tong, X.; Derugin, N.; Chan, R.C.F.; Gaur, A.; Yang, F.; et al. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev. 2015, 11, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yamashita, A.; Ozono, K.; Tsumaki, N. Limited Immunogenicity of Human Induced Pluripotent Stem Cell-Derived Cartilages. Tissue Eng. Part A 2016, 22, 1367–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.-Y.; Im, G.-I. Chondrogenic and Osteogenic Induction from iPS Cells. Methods Mol. Biol. 2016, 1357, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Craft, A.M.; Ahmed, N.; Rockel, J.S.; Baht, G.S.; Alman, B.A.; Kandel, R.A.; Grigoriadis, A.E.; Keller, G.M. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 2013, 140, 2597–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, N.; Miura, M.; Nakao, K.; Kondo, E.; Fujii, T.; Taura, D.; Kanamoto, N.; Sone, M.; Yasoda, A.; Arai, H.; et al. Human Induced Pluripotent Stem Cells Differentiated into Chondrogenic Lineage via Generation of Mesenchymal Progenitor Cells. Stem Cells Dev. 2013, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Liu, S.; Woltjen, K.; Thomas, B.; Meng, G.; Hotta, A.; Takahashi, K.; Ellis, J.; Yamanaka, S.; Rancourt, D.E. Cartilage tissue engineering identifies abnormal human induced pluripotent stem cells. Sci. Rep. 2013, 3, 1978. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Gourraud, P.-A.; Gilson, L.; Girard, M.; Peschanski, M. The role of human leukocyte antigen matching in the development of multiethnic"haplobank" of induced pluripotent stem cell lines. Stem Cells 2012, 30, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagin, A.S.; Medvedeva, E.V. Regenerative medicine: Cartilage stem cells identified, but can they heal? Nat. Rev. Rheumatol. 2017, 13, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S.; Um, H.-B.; Dyment, N.A.; Cottingham, N.; Usami, Y.; Enomoto-Iwamoto, M.; Kronenberg, M.S.; Maye, P.; Rowe, D.W.; Koyama, E.; et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017, 426, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Markway, B.D.; Weekes, K.J.; McCarthy, H.E.; Johnstone, B. Physioxia Promotes the Articular Chondrocyte-Like Phenotype in Human Chondroprogenitor-Derived Self-Organized Tissue. Tissue Eng. Part A 2018, 24, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Fickert, S.; Schattenberg, T.; Niks, M.; Weiss, C.; Thier, S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: A case series. Arch. Orthop. Trauma Surg. 2014, 134, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J.A. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng. Part C Methods 2012, 18, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selmi, T.A.S.; Verdonk, P.; Chambat, P.; Dubrana, F.; Potel, J.-F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel. J. Bone Jt. Surg. Br. 2008, 90-B, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Whyte, G.P. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am. J. Sports Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.A.; Melchels, F.P.W.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Feng, K.; Liu, X.; Ma, P.X. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials 2009, 30, 5061–5067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Cooper, J.A.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; da Silva Ramos, T.A.; Damanik, F.; Quang Le, B.; Wieringa, P.; Bennink, M.; van Blitterswijk, C.; de Boer, J.; Moroni, L. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci. Rep. 2015, 5, 14804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonomoto, K.; Yamaoka, K.; Kaneko, H.; Yamagata, K.; Sakata, K.; Zhang, X.; Kondo, M.; Zenke, Y.; Sabanai, K.; Nakayamada, S.; et al. Spontaneous Differentiation of Human Mesenchymal Stem Cells on Poly-Lactic-Co-Glycolic Acid Nano-Fiber Scaffold. PLoS ONE 2016, 11, e0153231. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Soleimani, M.; Chamheidari, G.A.; Seyedjafari, E.; Dodel, M.; Atashi, A.; Gheisari, Y. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J. Biomed. Mater. Res. A 2011, 99, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Tseng, C.-S.; Hsu, S.-H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R. Smart biomaterials for tissue engineering of cartilage. Injury 2008, 39, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.R.; Parry, J.; Dadsetan, M.; Bravo, D.; Riester, S.M.; van Wijnen, A.J.; Yaszemski, M.J.; Kakar, S. Chondrocyte Attachment, Proliferation, and Differentiation on Three-Dimensional Polycaprolactone Fumarate Scaffolds. Tissue Eng. Part A 2017, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Recha-Sancho, L.; Moutos, F.T.; Abellà, J.; Guilak, F.; Semino, C.E. Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold. Materials 2016, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Schagemann, J.; Behrens, P.; Paech, A.; Riepenhof, H.; Kienast, B.; Mittelstädt, H.; Gille, J. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch. Orthop. Trauma Surg. 2018, 138, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; de Girolamo, L. Sustained five-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. Bone Jt. J. 2015, 97-B, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Efe, T.; Theisen, C.; Fuchs-Winkelmann, S.; Stein, T.; Getgood, A.; Rominger, M.B.; Paletta, J.R.J.; Schofer, M.D. Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, A.; Ciatti, R.; Pascarella, F.; Latte, C.; Di Salvatore, M.G.; Liguori, L.; Iannella, G. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. KNEE Surg. Sports Traumatol. Arthrosc. 2010, 18, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Basad, E.; Ishaque, B.; Bachmann, G.; Stürz, H.; Steinmeyer, J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: A 2-year randomised study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cherubino, P.; Grassi, F.A.; Bulgheroni, P.; Ronga, M. Autologous chondrocyte implantation using a bilayer collagen membrane: A preliminary report. J. Orthop. Surg. 2003, 11, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Welsch, G.H.; Mamisch, T.C.; Zak, L.; Blanke, M.; Olk, A.; Marlovits, S.; Trattnig, S. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: Preliminary results. Am. J. Sports Med. 2010, 38, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Berruto, M.; Benazzo, F.; Zanon, G.; Della Villa, S.; Marcacci, M. Articular cartilage treatment in high-level male soccer players: A prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am. J. Sports Med. 2011, 39, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Zerbinati, F.; Gildone, A.; Faccini, R. Autologous chondrocyte implantation: A comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop. Belg. 2007, 73, 207–218. [Google Scholar] [PubMed]
- Visna, P.; Pasa, L.; Cizmár, I.; Hart, R.; Hoch, J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques—A randomized controlled study. Acta Chir. Belg. 2004, 104, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Guo, Q.; Huang, J.; Zhang, L.; Yao, J.; Yang, F.; Wang, S.; Xu, W.; Wang, A.; et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 2008, 29, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Lima, E.G.; Mauck, R.L.; Takai, E.; Taki, E.; LeRoux, M.A.; Lu, H.H.; Stark, R.G.; Guo, X.E.; Ateshian, G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J. Biomech. 2003, 36, 1853–1864. [Google Scholar] [CrossRef]
- Janjanin, S.; Li, W.-J.; Morgan, M.T.; Shanti, R.M.; Tuan, R.S. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J. Surg. Res. 2008, 149, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.; Liu, J.M.-J.; Chua, C.-K.; Chou, S.-M.; Shyu, V.B.-H.; Chen, J.-P. Cartilage Tissue Engineering with Silk Fibroin Scaffolds Fabricated by Indirect Additive Manufacturing Technology. Materials 2014, 7, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Giammona, G. Photocrosslinkable polyaspartamide/polylactide copolymer and its porous scaffolds for chondrocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Paraffin spheres as porogen to fabricate poly(l-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.Y.; Andriotis, O.G.; Li, S.; Basnett, P.; Su, B.; Roy, I.; Tare, R.S.; Sengers, B.G.; Stolz, M. Nanofibrous poly(3-hydroxybutyrate)/poly(3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J. Biomater. Appl. 2016, 31, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Chiang, H.; Kuo, T.-F.; Lee, H.-S.; Jiang, C.-C.; Tuan, R.S. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: A pilot study. J. Tissue Eng. Regen. Med. 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.K.; Yarin, A.L.; Megaridis, C.M.; Cho, M. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: Engineering the superficial zone of articular cartilage. Tissue Eng. Part A 2009, 15, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Yang, T.; Sun, Y.; You, Q.; Li, J.; Wang, Z.; Han, B. Composite poly(l-lactic-acid)/silk fibroin scaffold prepared by electrospinning promotes chondrogenesis for cartilage tissue engineering. J. Biomater. Appl. 2016, 30, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Levorson, E.J.; Raman Sreerekha, P.; Chennazhi, K.P.; Kasper, F.K.; Nair, S.V.; Mikos, A.G. Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed. Mater. 2013, 8, 014103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; Tran, K.; Chang, W.; Shelke, N.B.; Kumbar, S.G.; Yu, X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: Cartilage regeneration. J. Biomed. Nanotechnol. 2014, 10, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, H.; Zhang, L.; Xu, H.; Zhao, X. Self-assembly-peptide hydrogels as tissue-engineering scaffolds for three-dimensional culture of chondrocytes in vitro. Macromol. Biosci. 2010, 10, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gao, G.; Yonezawa, T.; Dai, G. Human Cartilage Tissue Fabrication Using Three-dimensional Inkjet Printing Technology. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41-56. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chik, T.-K.; Ngan, A.H.W.; Chan, S.C.H.; Shum, D.K.Y.; Chan, B.P. Correlation between Compositional and Mechanical Properties of Human Mesenchymal Stem Cell-Collagen Microspheres During Chondrogenic Differentiation. Tissue Eng. Part A 2011, 17, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, S.; Kumar, A.; Admane, P.; Bandyopadhyay, A.; Ghosh, S. Elucidating role of silk-gelatin bioink to recapitulate articular cartilage differentiation in 3D bioprinted constructs. Bioprinting 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Zhu, N.; Schreyer, D.J.; Chen, X. Modeling Process-Induced Cell Damage in the Biodispensing Process. Tissue Eng. Part C Methods 2010, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Jiang, Y.; Cai, Y.; Xu, G.; Tong, T.; Zhang, W.; Wang, L.; Ji, J.; Shi, P.; et al. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater. 2013, 9, 7236–7247. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Eames, B.F.; Chen, X. Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.P.; Abu-Lail, N.I.; Coles, J.M.; Guilak, F.; Jay, G.D.; Zauscher, S. Friction Force Microscopy of Lubricin and Hyaluronic Acid between Hydrophobic and Hydrophilic Surfaces. Soft Matter 2009, 5, 3438–3445. [Google Scholar] [CrossRef] [PubMed]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamchedu, N.P.; Tofte, J.N.; Waller, K.A.; Zhang, L.X.; Patel, T.K.; Jay, G.D. Superficial zone cellularity is deficient in mice lacking lubricin: A stereoscopic analysis. Arthritis Res. Ther. 2016, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.; Zhang, L.; Jay, G. Friction-Induced Mitochondrial Dysregulation Contributes to Joint Deterioration in Prg4 Knockout Mice. Int. J. Mol. Sci. 2017, 18, 1252. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.; Xu, X.; Bible, M.D.; Calve, S.; Neu, C.P.; Panitch, A. Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface. Biomaterials 2015, 73, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.R.C.; Flannery, C.R. Bioregulation of lubricin expression by growth factors and cytokines. Eur. Cell. Mater. 2007, 13, 40–45; discussion 45. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Reddi, A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007, 56, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, X.; Li, T.; Zhang, Q. Kartogenin, transforming growth factor-β1 and bone morphogenetic protein-7 coordinately enhance lubricin accumulation in bone-derived mesenchymal stem cells. Cell Biol. Int. 2015, 39, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Andrades, J.A.; Motaung, S.C.; Jiménez-Palomo, P.; Claros, S.; López-Puerta, J.M.; Becerra, J.; Schmid, T.M.; Reddi, A.H. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-β1 and bone morphogenetic protein-7. Arthritis Res. Ther. 2012, 14, R72. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, T.; Sakata, R.; Reddi, A.H. Induction of chondrogenesis and expression of superficial zone protein in synovial explants with TGF-β1 and BMP-7. Tissue Eng. Part A 2013, 19, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Nakagawa, T.; Reddi, A.H. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: Analysis of superficial zone protein/lubricin expression. Tissue Eng. Part A 2010, 16, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Lee, S.Y.; Reddi, A.H. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor Î21. Arthritis Rheum. 2009, 60, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.C.; Vunjak-Novakovic, G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res. Ther. 2016, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Mechanotransduction and cartilage integrity. Ann. N. Y. Acad. Sci. 2011, 1240, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, K.; Doran, P.M. Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnol. Bioeng. 2012, 109, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M.; Walker, J.M. Cartilage Tissue Engineering; Doran, P.M., Ed.; Humana Press: Melbourne, VIC, Australia, 2015; ISBN 9781493929375. [Google Scholar]
- Elder, B.D.; Athanasiou, K.A. Hydrostatic Pressure in Articular Cartilage Tissue Engineering: From Chondrocytes to Tissue Regeneration. Tissue Eng. Part B Rev. 2009, 15, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, H.; Kozhemyakina, E.; Hung, H.-H.; Grodzinsky, A.J.; Lassar, A.B. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014, 28, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Cui, Z.; Urban, J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheum. 2004, 50, 3915–3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia Promotes the Differentiated Human Articular Chondrocyte Phenotype through SOX9-dependent and -independent Pathways. J. Biol. Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Martens, D.E.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Cartilage tissue engineering: Controversy in the effect of oxygen. Crit. Rev. Biotechnol. 2003, 23, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Schrobback, K.; Malda, J.; Crawford, R.W.; Upton, Z.; Leavesley, D.I.; Klein, T.J. Effects of oxygen on zonal marker expression in human articular chondrocytes. Tissue Eng. Part A 2012, 18, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, T.; Kishimoto, K.N.; Okuno, H.; Itoi, E. Oxygen tension affects lubricin expression in chondrocytes. Tissue Eng. Part A 2014, 20, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Polak, J.M. Control of human articular chondrocyte differentiation by reduced oxygen tension. J. Cell. Physiol. 2004, 199, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune modulation to improve tissue engineering outcomes for cartilage repair in the osteoarthritic joint. Tissue Eng. Part B. Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Su, W.-R.; Shi, S.-H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.V.; Abratte, C.M.; Schimenti, J.C.; Felippe, M.J.B.; Cassano, J.M.; Southard, T.L.; Cross, J.A.; Fortier, L.A. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen. Med. 2014, 9, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzo, R.M.; Gibson, J.; Xu, R.-H.; Lee, F.Y.; Drissi, H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J. Cell. Biochem. 2013, 114, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.J.; Copeland, R.O.; Yi, Y.; Choi, K.-B.; Meschter, C.; Hwang, S.; Lim, C.-L.; Yip, V.; Hyun, J.-P.; Lee, H.-Y.; et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 2010, 12, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.-W.; Cho, J.J.; Elmallah, R.K.; Cherian, J.J.; Kim, T.W.; Lee, M.-C.; Mont, M.A. A Multicenter, Single-Blind, Phase IIa Clinical Trial to Evaluate the Efficacy and Safety of a Cell-Mediated Gene Therapy in Degenerative Knee Arthritis Patients. Hum. Gene Ther. Clin. Dev. 2015, 26, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Parvizi, J.; Bramlet, D.; Lee, K.H.; Romness, D.W.; Mont, M.A. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr. Cartil. 2015, 23, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Asahara, H. Current Status and Strategy of microRNA Research for Cartilage Development and Osteoarthritis Pathogenesis. J. Bone Metab. 2016, 23, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsen, T.A.; de Souza, G.A.; Ødegaard, B.; Engebretsen, L.; Brinchmann, J.E. microRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-induced Osteoarthritis. Mol. Ther. Nucleic Acids 2016, 5, e373. [Google Scholar] [CrossRef] [PubMed]
- Si, H.-B.; Zeng, Y.; Liu, S.-Y.; Zhou, Z.-K.; Chen, Y.-N.; Cheng, J.-Q.; Lu, Y.-R.; Shen, B. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthr. Cartil. 2017, 25, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Wang, C.; Yu, H.; Yu, X.; Yu, H. MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1. Inflammation 2016, 39, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Gracitelli, G.C.; Moraes, V.Y.; Franciozi, C.E.; Luzo, M.V.; Belloti, J.C. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst. Rev. 2016, 9, CD010675. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. https://doi.org/10.3390/ijms19082366
Medvedeva EV, Grebenik EA, Gornostaeva SN, Telpuhov VI, Lychagin AV, Timashev PS, Chagin AS. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. International Journal of Molecular Sciences. 2018; 19(8):2366. https://doi.org/10.3390/ijms19082366
Chicago/Turabian StyleMedvedeva, Ekaterina V., Ekaterina A. Grebenik, Svetlana N. Gornostaeva, Vladimir I. Telpuhov, Aleksey V. Lychagin, Peter S. Timashev, and Andrei S. Chagin. 2018. "Repair of Damaged Articular Cartilage: Current Approaches and Future Directions" International Journal of Molecular Sciences 19, no. 8: 2366. https://doi.org/10.3390/ijms19082366