Abstract
Plants have evolved a variety of dispersal units whereby the embryo is enclosed by various dead protective layers derived from maternal organs of the reproductive system including seed coats (integuments), pericarps (ovary wall, e.g., indehiscent dry fruits) as well as floral bracts (e.g., glumes) in grasses. Commonly, dead organs enclosing embryos (DOEEs) are assumed to provide a physical shield for embryo protection and means for dispersal in the ecosystem. In this review article, we highlight recent studies showing that DOEEs of various species across families also have the capability for long-term storage of various substances including active proteins (hydrolases and ROS detoxifying enzymes), nutrients and metabolites that have the potential to support the embryo during storage in the soil and assist in germination and seedling establishment. We discuss a possible role for DOEEs as natural coatings capable of “engineering” the seed microenvironment for the benefit of the embryo, the seedling and the growing plant.
1. Introduction
The seed is the fundamental unit of dispersal in higher plants and is at the focal point of consumers, farmers, seed companies and seed banks. However, plants possess a variety of dispersal units in which the embryo is covered by several layers that provide physical protection and means for dispersal [1,2]. Dry fruits consist of two groups: dehiscent, in which the fruit is splitting open at maturity to allow for seed dispersal, and indehiscent, whereby the fruit is not opened at maturity and constitutes the dispersal unit (Figure 1). Consequently, seeds, the dispersal unit of dry dehiscent fruits, have a single protective layer (the seed coat) enclosing the embryo while in fruits the embryo is covered by two protective layers, the seed coat and the fruit coat (the pericarp and its accessories). In various Poaceae species, the basic dispersal unit constitutes of a unique type of dry fruit in which the seed coat and the pericarp are fused together to form the caryopsis. Two additional types of dispersal units are common in Poaceae species: a floret, in which the caryopsis is covered by the lemma and palea, and a spikelet, whereby the floret is further covered by the glumes (Figure 1).
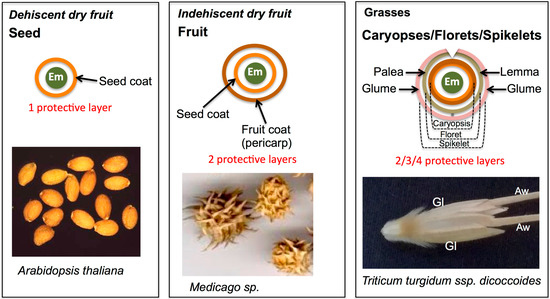
Figure 1.
Dispersal units of dry fruits and grasses. Dry fruits can be either dehiscent, in which the fruit is opened at maturity to release the dispersal units, the seeds (e.g., Arabidopsis thaliana), or indehiscent, whereby the fruit is not opened at maturity and represents the dispersal unit (e.g., Medicago species). In seed and fruit dispersal units, the embryo is covered by one and two protective layers, respectively. In grasses, the basic dispersal unit constitutes a unique type of dry fruit in which the seed coat and the pericarp are fused together to form the caryopsis. A floret is a type of dispersal unit, whereby the caryopsis is covered by the lemma and palea. In a spikelet, a floret or florets are further enclosed by glumes (e.g., Triticum turgidum ssp. dicoccoides). Note, in caryopsis, floret and spikelet, the embryo is enclosed by two, three and four protective layers, respectively. Em, embryo; Gl, glume; Aw, awn (a long appendage at the lemma).
All protective layers enclosing the embryo, namely, seed coats, pericarps, lemmas, paleas and glumes, are maternally-derived and undergo programmed cell death (PCD) at maturity. Although the term dispersal unit highlights an entity that is specialized for dispersal, several studies have shown that the dispersal unit serves multiple functions including protection from predation, seed positioning in the soil, moisture adsorption, seed anchoring, light filtering, and regulation of seed respiration [3]. The mechanisms of seed protection from predation include morphological characteristics such as hairiness, thickness, and hardness of seed coat as well as chemical protection, in which the dispersal unit contains secondary metabolites that control predation [4,5]. Sometimes the covering layers of the dispersal unit contribute to dormancy and/or inhibit germination due to permeability barriers preventing water uptake or gaseous exchange, the presence of germination inhibitory substances [6,7,8,9,10] or due to mechanical barriers preventing embryo expansion [11]. The composition and level of substances within the dead organs enclosing embryo (DOEEs) may be affected by environmental cues. For instance, the pericarps of pea (Pisum sativum) possess nutritional and antioxidant compounds that were further enhanced by plant growth promoting microbes [12]. Several reports demonstrated the positive effect of the intact dispersal units on seed longevity and seedling establishment when compared to naked seeds [13,14,15,16].
It is commonly believed that during PCD most macromolecules such as DNA, RNA and proteins are degraded and their constituents remobilized into other plant parts [17,18,19]. Contrary to this view, recent reports have demonstrated the capacity of DOEEs to store and maintain the integrity of hundreds of proteins; some can persist in active forms for decades, and are released to the immediate surrounding of the dispersal unit upon hydration [16,20,21].
In this review, we highlight recent studies showing that DOEEs of various species across families have the capability for long-term storage of various substances including active proteins (hydrolases and Reactive Oxygen Species (ROS) detoxification enzymes), nutrients and metabolites that have the potential to support the embryo during storage in the soil and assist in germination and seedling establishment. We discuss the potential function of DOEEs as natural coatings, acting as engineers of the seed microenvironment to increase survival rate of the seed.
2. DOEEs Release Hundreds of Proteins upon Hydration
Proteome analyses of DOEEs including seed coats, pericarps and glumes highlighted their function as a long-term storage for hundreds of proteins that are released upon hydration. Many of the identified proteins possess catalytic activity including hydrolytic activity and oxireductase activity; some of the stored proteins, such as nucleases, remain active after long periods (10–50 years) of storage within DOEEs [16,20,21]. Proteins released from DOEEs include several plant defensin-like (DEFL) molecules, also known as low molecular weight cysteine-rich (LCR) proteins, which are implicated in defense response to fungus [22,23,24]. These proteins, primarily found in seeds, are also present in leaves and flowers and often up-regulated following pathogenic attack or in response to environmental stress such as drought [25]. They can confer enhanced resistance to pathogen when overexpressed in transgenic plants [26,27]. Other proteins identified in the proteome data that could act against pathogens include chitinases, endochitinases, endonucleases and glucanases. Chitinases degrade chitin, a polysaccharide found in a variety of organisms including insects and fungi. Chitinase and glucanase genes are often over-expressed in plants and are implicated in combating fungal pathogens [28,29,30]. The proteome data also revealed S1 type endonucleases, which are released from DOEEs upon hydration [16,20,21]. Endonucleases, in general, are involved in multiple cellular processes including DNA synthesis and DNA repair [31] and in PCD [32,33,34]. The capacity of endonucleases to target unpaired regions within superhelical DNA to introduce nicks and double strand DNA breaks may implicate them as defense factors against plasmid-containing soil pathogens such as the Clavibacter michiganensis subsp. michiganensis, a Solanaceae species-pathogenic actinomycete that contains two plasmids, which are important for pathogenesis [35]. In addition, transgenic tobacco plants expressing a bovine pancreatic RNase, an extracellular ribonuclease, showed an increase resistance to plant RNA viruses, namely, Cucumber mosaic virus and Tobacco mosaic virus [36,37]. Notably, treatment of leaves with S-like RNase NE injected into the extracellular space suppresses growth of Phytophthora parasitica [38,39], an oomycete soil borne pathogen with a wide range of host plants. Also, proteome analysis identified Pathogenesis Related 4 (PR4 RNase) homolog ribonucleases released from dead seed coats of white mustard (Sinapis alba) and from dead glumes of wild emmer wheat [16,20]. The Wheatwin1 PR4 RNase activity was shown to be required for repressing fungal cells activity [40]. The presence of plant defense-related proteins within the dead, non-living organs of wheat and oat dispersal unit (DU) was reported. Accordingly, wheat bran was found to contain multiple plant defense-related proteins including oxalate oxidase (OXO), peroxidase (POX), and polyphenol oxidase (PPO) whose activities were highest in the outer layer [41]. In addition, when oat DU was incubated with Fusarium avenaceum strain F.a.1, polyphenol oxidase (PPO) was induced in the whole DU as well as in the dissected parts, namely the dead, non-living hulls (lemma and palea) and the caryopses [42]. The authors suggested that the induction of PPO in the non-living hulls, surprisingly, could have resulted from latent forms of PPO that are activated following challenge with F.a.1 [42].
The Proteome data also revealed two groups of proteins released upon hydration from DOEEs that might play an important role in seed persistence in the soil and seed germination, namely, ROS detoxifying enzymes (e.g., superoxide dismutases and peroxidases) and cell wall modification enzymes (e.g., pectinesterases and polygalacturonases) [16,21], which are discussed below.
3. ROS Detoxifying Enzymes
ROS have an important role in seed biology [43,44]. They are produced during seed maturation and desiccation, seed storage in the soil and seed germination. ROS could lead to oxidative stress that can harm macromolecules such as proteins and DNA, and consequently to seed weakening. Thus, ROS “detoxifying” enzymes such as superoxide dismutases (SODs), catalases and peroxidases are of prime importance in maintaining appropriate balance of ROS and seed viability. Although ROS have long been thought as toxic molecules, many reported studies highlighted ROS also as signaling molecules acting in releasing seed dormancy, germination as well as providing defense against soil pathogens [43,44,45].
The term ROS “detoxifying” enzymes may be ambiguous, inasmuch as many of the enzymes actually convert one ROS into another often even more potent species. Accordingly, radical derivatives of oxygen such as superoxide (O2−), resulting from reduction of oxygen, is a short-lived molecule that serves as a precursor for superoxide dismutase leading to the formation of other ROS including hydrogen peroxide (H2O2) and hydroxyl radical (OH·). Catalase catalyzes the conversion of H2O2 into water and oxygen. Alternatively, H2O2 may react with glutathione peroxidase to catalyze the formation of water and the conversion of reduced glutathione (GSH) into glutathione disulfide (GSSG). The presence of transition metal allows hydroxyl radical production from H2O2 by the Fenton reaction. Accordingly, Fe2+ is oxidized by H2O2 to Fe3+ to form a hydroxyl radical (HO•) and a hydroxide ion (OH−) [46].
During early development, many seeds are green and engaged in photosynthesis and thus the production of superoxide and singlet oxygen (1O2), another type of ROS, is inevitable. Singlet oxygen is a highly reactive oxygen species that mostly reacts with organic molecules having double bonds [47]. Damages imposed by singlet oxygen can reduce photosynthetic efficiency and even cause cell death. Various antioxidant compounds within the chloroplasts such as carotenoids, tocopherols and plastoquinones quench singlet oxygen and protect against its toxic effects [48]. Nevertheless, the cell responses to the presence of singlet oxygen are largely dependent on its levels. Accordingly, extreme production of singlet oxygen might lead to unavoidable death known as “accidental cell death” and moderate levels may induce programmed cell death, while low levels of singlet oxygen may signal for acclimation [48].
An important source for ROS is the mitochondrial respiratory system in which electron leakage from the transport chain can generate superoxide that can be dismutated into H2O2 [49]. Hence, the amount of ROS generated in the seed is proportional to the mitochondrial respiratory activity being high at early stages of embryogenesis but strongly reduced as seed mature and become quiescent. During imbibition and germination, the respiratory activity is significantly enhanced leading to production of ROS. Accordingly, superoxide and H2O2 are produced at high levels in embryonic axes of soybean during imbibition, which was associated with increase in activity of ROS enzymes including SODs, catalase, peroxidase, glutathione and ascorbate peroxidases [50]. ROS are released from the seed coat and the embryo of radish seeds upon imbibition in the dark. A correlation was found between inhibition of germination caused by far-red light and inhibition of ROS release; Gibberellic acid (GA) restores full germination under far-red light and the release of ROS from the seeds [51]. Similarly, in barley (Hordeum vulgare) aleurone cells, H2O2 production was induced by GA but suppressed by ABA [52]. Presently, it is not clear whether induction of ROS production during imbibition is a developmentally regulated process that facilitates germination or toxic by-products induced upon resumption of respiration activity during germination. The findings that ROS production is often associated with increase expression of genes encoding for ROS “detoxifying” enzymes suggest that ROS are toxic compounds whose levels must be decreased. Thus, the overrepresentation of ROS detoxifying/metabolizing enzymes in the dead glumes of wild emmer wheat [16,21] highlighted their importance in seed persistence in the soil, germination and seedling establishment. We hypothesize that these enzymes are released upon hydration to the immediate surrounding of the germinating seed to fulfill multiple functions. Accordingly, ROS detoxifying enzymes make sure that the seed microenvironment is free of hazardous radicals or allow for the production of specific radicals, which are important for germination, or are necessary for protection against potential soil pathogens. In this respect, the presence of multiple SOD enzymes in DOEEs might ensure reduction in superoxide level and generation of H2O2 that facilitates seed germination [53]. Consequently, H2O2 may be reduced to hydroxyl radical, which in turn might affect seed longevity and seed germination [54,55].
4. Cell Wall Modification Enzymes
The overrepresentation of pectinesterases (PMEs/PEs) and polygalacturonases (PGs) in DOEEs [21] suggests a role in modifying cell walls to allow for the radicle to protrude outside the seed coverings. Both PMEs and PGs are pectinases involved in multiple developmental processes in plants via loosening and softening pectin—the major constituent of plant cell walls. PMEs and PGs were reported to affect the mechanical stability of cell walls during fruit ripening. They are involved in cell wall loosening of the endosperm and the testa, which is necessary for radicle protrusion, cell wall extension during pollen germination and pollen tube growth, abscission and stem elongation [56,57].
PMEs are responsible for de-methyl esterfication of the most abundant pectin in cell walls, homoglacturonans (HG), which in turn affects their elasticity, permeability and porosity and consequently plant growth and development [58,59]. The removal of methyl groups from HG by PMEs can differently affect cell wall properties leading either to cell wall softening or stiffening. Notably, the activity of PMEs is subjected to regulation by pectin methylesterase inhibitors (PMEIs), a group of small proteins that physically interact with PMEs and affect their activity [60]. PMEs were isolated from germinating seeds of a variety of annual and perennial plant species including cowpea (Vigna sinensis) and yellow cedar (Chamaecyparis nootkatensis) and are assumed to play an important role in loosening cell walls to allow for radicle emergence [61,62]. Changes in expression pattern of PME enconding genes as well as in PME enzymatic activities observed during seed germination of Lepidium sativum (garden cress) are associated with testa rupture; exogenous application of PMEs to garden cress seeds promoted permeability and rupture of the testa [63]. Transgenic Arabidopsis plants overexpressing PME inhibitor (PMEI5) displayed, as expected, reduction in PME activities and increased cell wall methylesterification, which was accompanied by a significantly faster rate of germination [64]. Closer inspection of published data [64] revealed that most methylesterification, as deduced by JIM7 immunolabeling, was found in embronic cells, with no clear effect on methylesterification of the testa. It appears that the action of PMEs is complex and that demethylesterification may be required, in a temporal manner, to coordinate between the growing radicle and the rupture of the endosperm and the testa.
Polygalacturonases (PGs) are enzymes that hydrolyze the α-1,4 glycosidic bonds between galacturonic acid residues resulting in pectin depolymerization and softening of cell walls. A calcium-dependent exo-PG activity was detected in tomato seed protein extracts, which is related to the product of the LeXPG1 gene. Indeed, the LeXPG1 mRNA was increased during imbibition, and further enhanced in seeds upon completion of germination, suggesting that PG is involved in weakening of the endosperm cell walls for radicle protrusion [65]. Similarly, PG was reported to be involved in lateral root development in Allium porrum via loosening of cortical cells ahead of the growing tip of the root [66].
Taken together, PMEs and PGs, which are accumulated in DOEEs provide a complementary, maternally source for cell wall modifying enzymes to ensure proper seed germination by loosening seed coverings at the time of germination, to allow for radicle protrusion.
5. DOEEs as a Rich Storage for Nutrients and Growth Factors
The study of the nutritional value of seed coats, pericarps and glumes revealed that high levels of nutrients such as potassium, phosphorus and sulfur are stored within these dead organs and are likely to serve as an immediate nutritional supply for germinating seeds. Germination assays of wild emmer wheat showed the beneficial effect of the intact dispersal unit (DU) compared to naked caryopsis. Although germination from the DU was delayed by 4–5 days, post germination growth and development were enhanced in seedlings derived from the intact DU. Particularly, DU-seedlings have significantly higher number and higher length of lateral roots than seedlings derived from naked caryopsis [16], which are attributed to the effect of lateral root-promoting substances such as auxin [67,68,69] and to nutritional elements [70,71,72,73], respectively. Similar effects were reported for germination of the Winterfat (Krascheninnikovia lanata) dispersal unit, which consists of hairy bracts enveloping utricle (fruit) and the seed. Accordingly, the removal of the hairy bracts significantly reduced seedling establishment and vigor [13]. Notably, initial analysis of phytohormones in glumes derived from wild emmer wheat revealed the presence of abscisic acid (ABA) and auxin (IAA); interestingly, jasmonic acid (JA) and salicylic acid (SA) were most abundant in the glumes [74]. The significant of these phytohormones in seedling growth and development as well as in defense priming against biotic and abiotic stresses still need to be explored.
Potassium is one of the major essential nutrients for plant growth and development. The high levels of potassium identified in DOEEs might reflect the accumulation of large quantities of this element in plants, which constitutes 2–10% of plant dry weight [75,76]. Potassium has important regulatory roles, and is required for plant growth processes including enzyme activation, photosynthesis and protein synthesis; it also participates in plant response to biotic and abiotic stresses [77].
6. Control of Microbial Growth by DOEEs
Seeds are commonly germinated in the soil, whereby radicles (embryonic roots) are protruding into an unknown, potentially hazardous environment, which challenge their survival. Even before germination, during storage in the soil, seeds are subjected to biotic and abiotic stress conditions (microbial attack and humidity fluctuations), which might affect their longevity and persistence; however, seeds in the soil maintain viability for many years [78]. The mechanisms underlying long-term viability of seeds in the soil were addressed mainly with respect to chemical defense (secondary metabolites). Accordingly, examination of seeds of over 80 plant species from the British flora revealed that many possess and release upon hydration potent antimicrobial and antifeedant compounds including hydroxyphenols and hydrogen cyanide [79]. Seeds of various plant species secrete, following hydration, various proteins that function against fungal pathogens and Gram-positive bacteria [26,80,81], but their origin, maternal (e.g., dead seed coat) or zygotic (i.e., embryo) is not well known. Many seeds have pigmented testa resulting from production of phenolic compounds (e.g., tannins), which are often associated with defense activity against pathogens [82,83,84]. In addition, proteome analysis of soybean (Glycine max L.) live seed coats derived from developing seeds showed multiple proteins including an abundant class I chitinase [85] that plays a role in plant defense against pathogens [86,87].
Recent data showed that dead seed coats contain microbial growth promoting or suppressing activities. Analysis of seeds derived from two cruciferous species, namely, Sinapis alba and Anastatica hierochuntica, showed that they differ in their bacterial growth controlling activities. Substances secreted from A. hierochuntica embryonic tissues or seed coats displayed very strong antibacterial activity toward Gram-positive and Gram-negative bacterial strains Staphylococcus aureus and Escherichia coli, respectively, which was comparable to the inhibitory effect of 100 μg/L ampicillin [20]. Substances secreted from A. hierochntica seeds also displayed strong inhibitory effect on spore germination of Fusarium oxysporum f.sp. melonis [20]. On the other hand, substances released from S. alba seeds had no antibacterial activity but rather slight bacterial growth promoting activity. These differences may be attributed to the different habitats from which seeds were collected. Thus, S. alba seeds were collected from plants growing along the margins of agricultural fields in the northern Negev and supplemented with all intensive agricultural treatments including irrigation, fertilization and chemicals against pests and bacterial and fungal pathogens. Seeds of A. hierochuntica, on the other hand, were collected from natural habitat in the Negev desert and mother plants were subjected to all kinds of stresses prevailing in the desert ecosystem. Thus, while substances secreted from A. hierochuntica might combat potential soil pathogen, those secreted from S. alba seeds might promote growth of beneficial soil borne microbes to support plant growth and development. Plant growth-promoting bacteria can influence root development and enhance plant growth by various means including increasing nutrient availability (e.g., nitrogen (N), iron (Fe) and phosphorus (P)) and production of indolic compounds (e.g., IAA) as well as by protecting plants from diseases, at least partly by suppressing deleterious soil borne pathogens [88,89,90]. Thus, accumulation of microbe growth controlling substances in DOEEs might act directly or indirectly to control soil borne pathogens and to promote plant growth and development.
7. Concluding Remarks
DOEEs, including seed coats, pericarps and floral bracts, in grasses were evolved not just for providing a physical shield for embryo protection or means for seed dispersal and germination but also as storage organs for multiple active proteins and probably metabolites and other substances for the purpose of germination, nourishment as well as protection of germinating seeds from soil pathogens (Figure 2). Thus, our data suggest that DOEEs should be viewed as “natural coatings” capable of “engineering the microenvironment” to allow for seed persistence in the soil, germination and seedling establishment. These findings open a new realm of research in seed biology and raised several questions for future study:
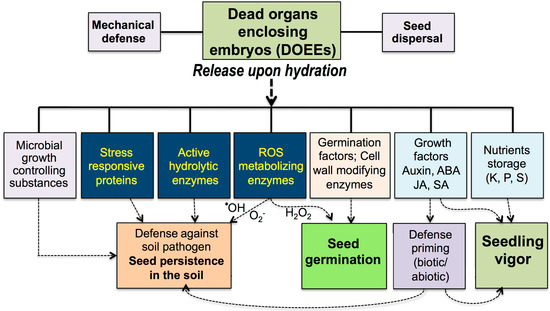
Figure 2.
Dead organs enclosing embryos (DOEEs): More than a physical shield for embryo protection and means for seed dispersal, DOEEs function as a rich, long-term storage for multiple beneficial substances that are released upon hydration to the immediate surroundings of the dispersal unit (DU) including seeds, indehiscent dry fruits, florets and spikelets. These substances comprise active proteins (hydrolases, ROS metabolizing enzymes, etc.), metabolites (e.g., phytohormones) and nutrients that are released from DOEEs (e.g., seed coat, pericarp, and glumes) and have the potential to facilitate germination, confer defense against soil pathogen, trigger defense priming in germinating seeds toward biotic and abiotic stresses and supply nutrients and growth factors that contribute to seedling establishment and vigor.
- Do substances released from DOEEs (e.g., JA, SA) have the capability of inducing plant defense priming against biotic and abiotic stresses?
- Can we use substances released from DOEEs as a substitute for the hazardous chemical coating of seeds?
- Does storage of seeds in gene banks with their associated dead organs better preserve and maintain seed viability?
- How do mother plant growth conditions affect the composition of substances stored in DOEEs and consequently seed longevity, germination and seedling establishment?
- Can we modify the composition of proteins and of other substances within DOEEs to build up a superior natural coating?
Taken together, the way we commonly refer to death in plants is challenged by the findings emerging from the study of DOEEs. The findings that the “dead can nurture” add another dimension, not recognized previously, to understanding seed biology and ecology, and might have important implications for seed economy and for ex-situ conservation of seeds in gene banks for future usage.
Funding
The research was supported by the Harbour Foundation (B.R.) and by the Margolin Foundation to G. Grafi.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Eriksson, O. Evolution of Seed Size and Biotic Seed Dispersal in Angiosperms: Paleoecological and Neoecological Evidence. Int. J. Plant Sci. 2008, 169, 863–870. [Google Scholar] [CrossRef]
- Booth, D.T. Plant diaspore functions. J. Seed Technol. 1990, 14, 61–73. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Zangerl, A.R.; Nitao, J.K. Constraints on Chemical Coevolution: Wild Parsnips and the Parsnip Webworm. Evolution 1986, 40, 1215. [Google Scholar] [CrossRef] [PubMed]
- Sroelov, R. On germination inhibitors. IV. Germination inhibitors of Sinapis alba and other seeds when enclosed in their fruit. Palestine J. Bot. 1940, 2, 33–45. [Google Scholar]
- Miyamoto, T.; Tolbert, N.E.; Everson, E.H. Germination inhibitors related to dormancy in wheat seeds. Plant Physiol. 1961, 36, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wurzburger, J.; Leshem, Y. Physiological action of the germination inhibitor in the husk of Aegilops kotschyi Boiss. New Phytol. 1969, 68, 337–341. [Google Scholar] [CrossRef]
- Gatford, K.T.; Eastwood, R.F.; Halloran, G.M. Germination inhibitors in bracts surrounding the grain of Triticum tauschii. Funct. Plant Biol. 2002, 29, 881–890. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Li, Q.; Liu, Y.; Hou, L.; Li, G. Influence of Pericarp, Cotyledon and Inhibitory Substances on Sharp Tooth Oak (Quercus aliena var. acuteserrata) Germination. PLoS ONE 2012, 7, e47682. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Chaudhary, A.; Singh, S.; Singh, H.B. Modulation of nutritional and antioxidant potential of seeds and pericarp of pea pods treated with microbial consortium. Food Res. Int. 2014, 64, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.T.; Schuman, G.E. Seedbed Ecology of Winterfat: Fruits versus Threshed Seeds. J. Range Manag. 1983, 36, 387. [Google Scholar] [CrossRef]
- Ohadi, S.; Mashhadi, H.R.; Tavakol-Afshari, R. Effects of Storage and Burial on Germination Responses of Encapsulated and Naked Seeds of Turnipweed (Rapistrum rugosum) to Light. Weed Sci. 2011, 59, 483–488. [Google Scholar] [CrossRef]
- Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of indehiscent pericarp in formation of soil seed bank in five cold desert Brassicaceae species. Plant Ecol. 2017, 218, 1187–1200. [Google Scholar] [CrossRef]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2002, 1, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.H.; Yang, J.J.; Liu, Y.D.; Shen, F.F. Protein degradation and nitrogen remobilization during leaf senescence. J. Plant Biol. 2008, 51, 11–19. [Google Scholar] [CrossRef]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Raviv, B.; Grafi, G. Dead Pericarps of Dry Fruits Function as Long-Term Storage for Active Hydrolytic Enzymes and Other Substances That Affect Germination and Microbial Growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.; Cammue, B.; Osborn, R.W. Plant Defensins: Novel Antimicrobial Peptides as Components of the Host Defense System. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Gomes, V.M. Plant Defensins and Defensin-Like Peptides—Biological Activities and Biotechnological Applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef]
- De Coninck, B.; Cammue, B.P.; Thevissen, K. Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biol. Rev. 2013, 26, 109–120. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.G.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Leuven, F.V.; Vanderleyden, J.; et al. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell 1995, 7, 573. [Google Scholar] [CrossRef] [PubMed]
- Coca, M.; Bortolotti, C.; Rufat, M.; Peñas, G.; Eritja, R.; Tharreau, D.; Pozo, A.M.D.; Messeguer, J.; Segundo, B.S. Transgenic rice plants expressing the antifungal AFP protein from aspergillus giganteus show enhanced resistance to the rice blast fungus Magnaporthe grisea. Plant Mol. Biol. 2004, 54, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, K.; Gaur, R.; Gupta, V. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Genetic engineering of crop plants for fungal resistance: Role of antifungal genes. Biotechnol. Lett. 2012, 34, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, L.; Bambara, R.A. Flap endonuclease 1. Annu. Rev. Biochem. 2013, 82, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Ito, J.; Aoyagi, S.; Fukuda, H. Endonucleases. Plant Mol. Biol. 2000, 44, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Granot, G.; Morgenstern, Y.; Khan, A.; Rapp, Y.G.; Pesok, A.; Nevo, E.; Grafi, G. Internucleosomal DNA fragmentation in wild emmer wheat is catalyzed by S1-type endonucleases translocated to the nucleus upon induction of cell death. BBA Gene Regul. Mech. 2015, 1849, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Givaty-Rapp, Y.; Yadav, N.S.; Khan, A.; Grafi, G. S1-Type Endonuclease 2 in Dedifferentiating Arabidopsis Protoplasts: Translocation to the Nucleus in Senescing Protoplasts Is Associated with De-Glycosylation. PLoS ONE 2017, 12, e0170067. [Google Scholar] [CrossRef] [PubMed]
- Gartemann, K.-H.; Kirchner, O.; Engemann, J.; Gräfen, I.; Eichenlaub, R.; Burger, A. Clavibacter michiganensis subsp. michiganensis: First steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 2003, 106, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.A.; Sapotsky, M.V.; Komarova, M.L.; Scherban, A.B.; Shumny, V.K.; Polyakova, A.M.; Lapshina, L.A.; Kochetov, A.V.; Malinovsky, V.I. Protection of transgenic tobacco plants expressing bovine pancreatic ribonuclease against tobacco mosaic virus. Plant Cell Rep. 2007, 26, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Trifonova, E.A.; Kochetov, A.V.; Kanayama, Y. Expression of an extracellular ribonuclease gene increases resistance to Cucumber mosaic virus in tobacco. BMC Plant Biol. 2016, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Galiana, E.; Bonnet, P.; Conrod, S.; Keller, H.; Panabieres, F.; Ponchet, M.; Poupet, A.; Ricci, P. RNase Activity Prevents the Growth of a Fungal Pathogen in Tobacco Leaves and Increases upon Induction of Systemic Acquired Resistance with Elicitin. Plant Physiol. 1997, 115, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Hugot, K.; Ponchet, M.; Marais, A.; Ricci, P.; Galiana, E. A tobacco S-like RNase inhibits hyphal elongation of plant pathogens. Mol. Plant Microbe Interact. 2002, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, A.; Kriegel, A.M.; Bradner, J.R.; Atwell, B.J.; Roberts, T.H.; Willows, R.D. Strategic distribution of protective proteins within bran layers of wheat protects the nutrient-rich endosperm. Plant Physiol. 2010, 152, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, E.P.; Okubara, P.A.; Anderson, J.V.; Morris, C.F. Polyphenol oxidase as a biochemical seed defense mechanism. Front. Plant Sci. 2014, 5, 689. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; Mcainsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2014, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Havaux, M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Puntarulo, S.; Galleano, M.; Sanchez, R.A.; Boveris, A. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochim. Biophys. Acta 1991, 1074, 277–283. [Google Scholar] [CrossRef]
- Schopfer, P.; Plachy, C.; Frahry, G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.H.; Yuasa, T.; Iwaya-Inoue, M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012, 158, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Baek, K.H.; Lee, H.S.; Kwak, S.S.; Bang, J.W.; Kwon, S.Y. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Gupta, R. Alkaline pectinases: A review. Biocatal. Agric. Biotechnol. 2015, 4, 279–285. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Paynel, F.; Leroux, C.; Surcouf, O.; Schaumann, A.; Pelloux, J.; Driouich, A.; Mollet, J.C.; Lerouge, P.; Lehner, A.; Mareck, A. Kiwi fruit PMEI inhibits PME activity, modulates root elongation and induces pollen tube burst in Arabidopsis thaliana. Plant Growth Regul. 2014, 74, 285–297. [Google Scholar] [CrossRef]
- Nighojkar, A.; Srivastava, S.; Kumar, A. Pectin methylesterase from germinating Vigna sinensis seeds. Plant Sci. 1994, 103, 115–120. [Google Scholar] [CrossRef]
- Ren, C.; Kermode, A.R. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol. 2000, 124, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Scheler, C.; Weitbrecht, K.; Pearce, S.P.; Hampstead, A.; Büttner-Mainik, A.; Lee, K.J.; Voegele, A.; Oracz, K.; Dekkers, B.J.; Wang, X.; et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015, 167, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013, 161, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sitrit, Y.; Hadfield, K.A.; Bennett, A.B.; Bradford, K.J.; Downie, A.B. Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol. 1999, 121, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Peretto, R.; Favaron, F.; Bettini, V.; De Lorenzo, G.; Marini, S.; Alghisi, P.; Cervone, F.; Bonfante, P. Expression and localization of polygalacturonase during the outgrowth of lateral roots in Allium porrum L. Planta 1992, 188, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Beeckman, T.; Graham, N.; Bhalerao, R.; Zhang, H.; Casero, P.; Sandberg, G.; Bennett, M.J. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003, 8, 165–171. [Google Scholar] [CrossRef]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2008, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Rybel, B.D.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Comparison Of The Effects of A Localised Supply of Phosphate, Nitrate, Ammonium and Potassium on the Growth of the Seminal Root System, and the Shoot, in Barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Zhang, H.; Forde, B.G. Regulation of Arabidopsis root development by nitrate availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, S.; Meng, L.; Xue, R.; Wang, C.; Liu, G.; Dong, C.; Wang, S.; Dong, J.; Zhang, Y. Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil 2015, 396, 163–173. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Filleur, S.; Diatloff, E.; Mounier, E.; Tillard, P.; Forde, B.G.; Gojon, A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitraterich patches. Proc. Natl. Acad. Sci. USA 2006, 103, 19206–19211. [Google Scholar] [CrossRef] [PubMed]
- Raviv, B.; Grafi, G. Dead floral bracts of the wheat dispersal unit store and release upon hydration multiple phytohormones: Implications for seedling vigor. 2018; in press. [Google Scholar]
- Leigh, R.A.; Wynn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Havlin, J.L. Soil and fertilizer nitrogen. Soil Fertil. Fertil. 1993, 4, 112–183. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Hendry, G.A.F.; Thompson, K.; Moss, C.J.; Edwards, E.; Thorpe, P.C. Seed persistence: A correlation between seed longevity in the soil and ortho-dihydroxyphenol concentration. Funct. Ecol. 1994, 8, 658–664. [Google Scholar] [CrossRef]
- De Bolle, M.F.; Eggermont, K.; Duncan, R.E.; Osborn, R.W.; Terras, F.R.; Broekaert, W.F. Cloning and characterization of two cDNA clones encoding seed-specific antimicrobial peptides from Mirabilis jalapa L. Plant Mol. Biol. 1995, 28, 713–721. [Google Scholar] [CrossRef]
- Rose, T.L.; Conceicao, A.D.S.; Jose, X.F.; Okorokov, L.A.; Fernandes, K.V.S.; Marty, F.; Marty-Mazars, D.; Carvalho, A.O.; Gomes, V.M. Defense proteins from Vigna ungaiculata seed exudates: Characterization and inhibitory activity against Fusarium oxysporum. Plant Soil 2006, 286, 181–191. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Yasseen, Y.; Barringer, S.A.; Splittstoesser, W.E.; Costanza, S. The role of seed coats in seed viability. Bot. Rev. 1994, 60, 426–439. [Google Scholar] [CrossRef]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [PubMed]
- Gijzen, M.; Kuflu, K.; Qutob, D.; Chernys, J.T. A class I chitinase from soybean seed coat. J. Exp. Bot. 2001, 52, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Schlumbaum, A.; Mauch, F.; Vogeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Brogue, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic plants with enhanced resistance to the pathogen Rhizoctonia solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Pieterse, C.M. Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).