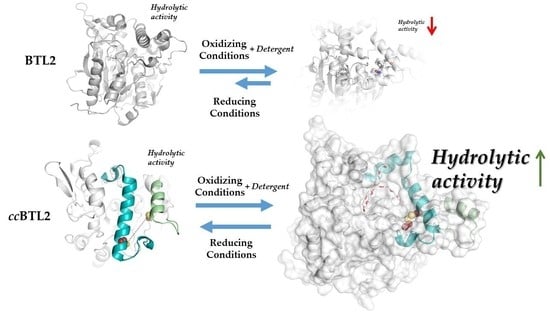
Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2
Abstract
:1. Introduction
2. Results and Discussion
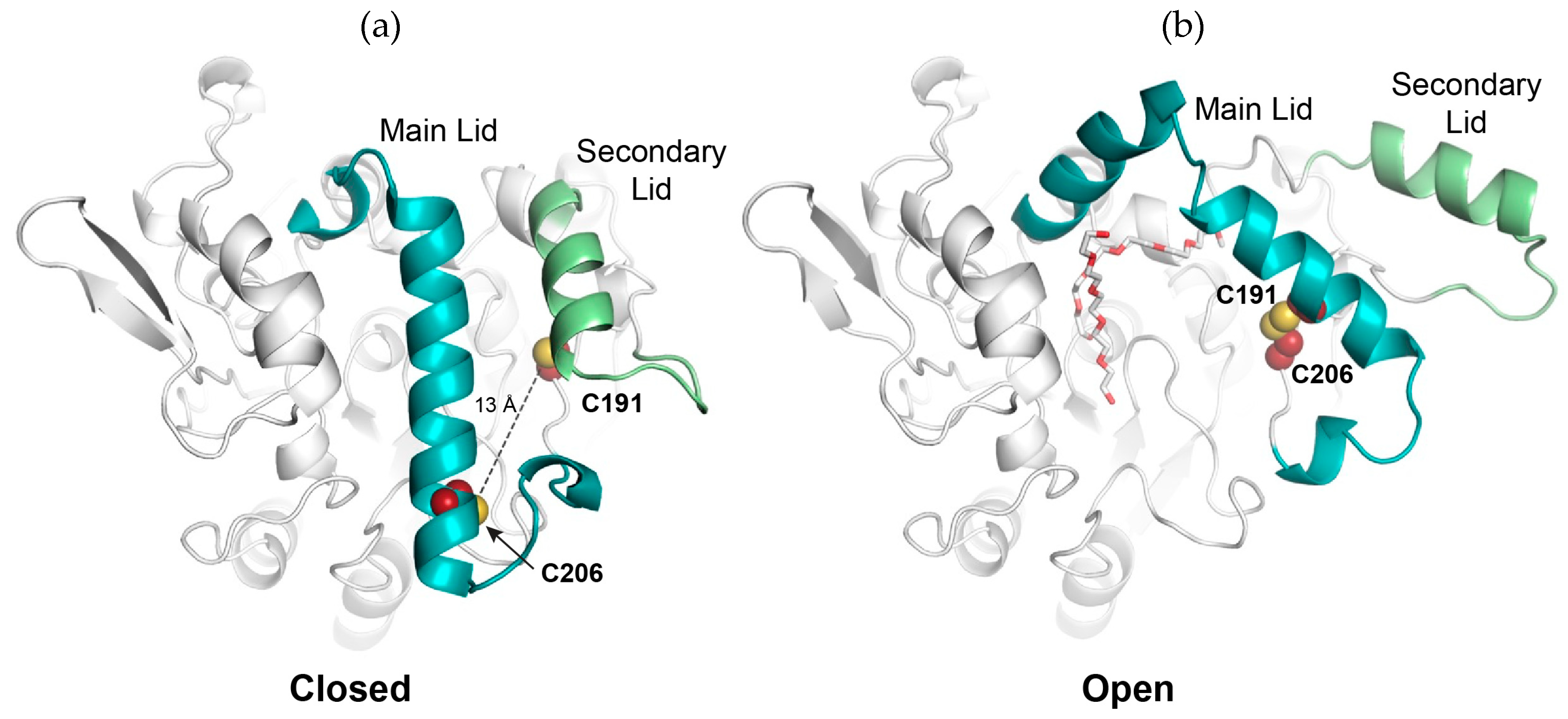
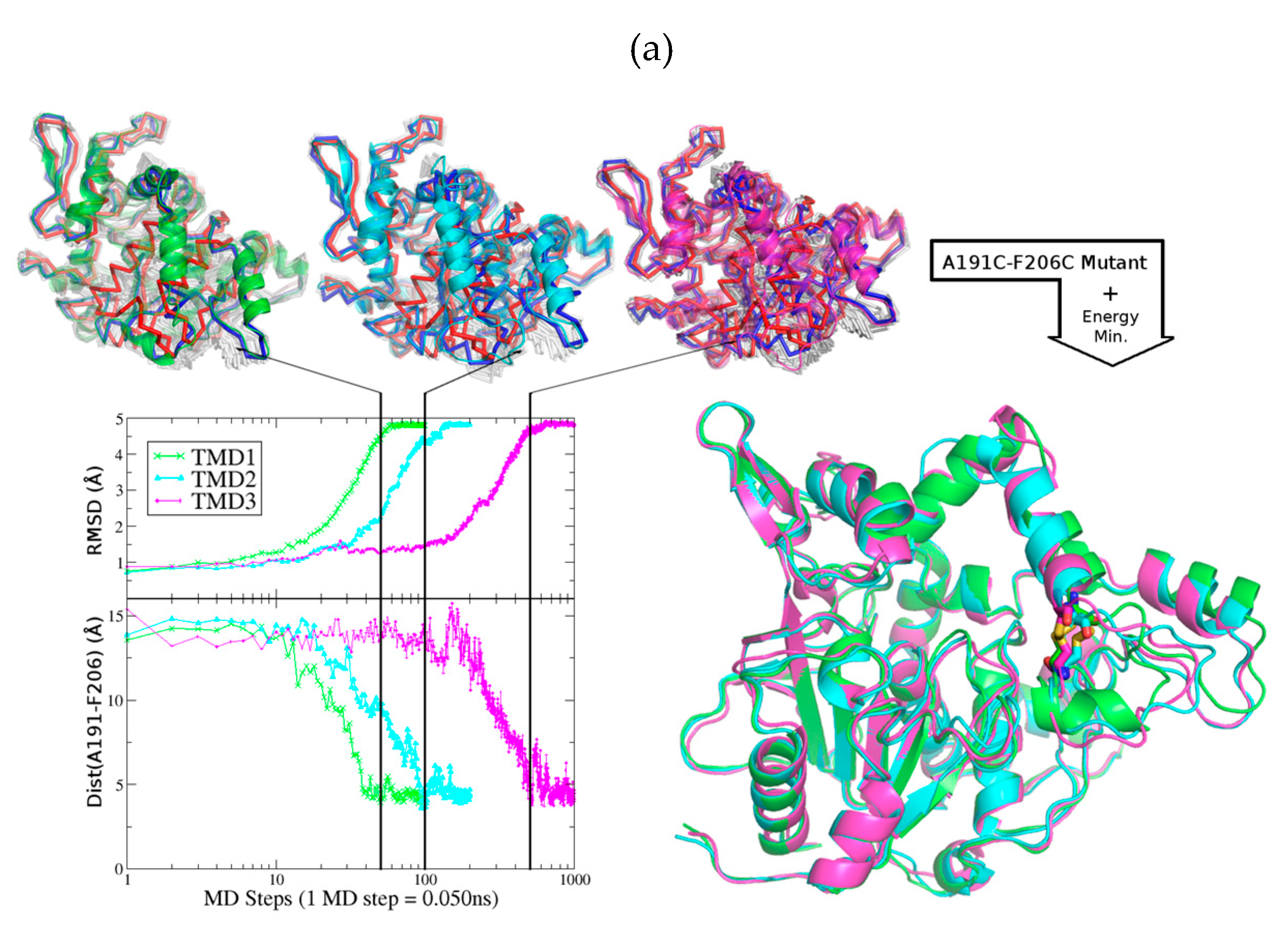
2.1. Design of An Engineered BTL2 Mutant (ccBTL2) to Lock the Open Conformation
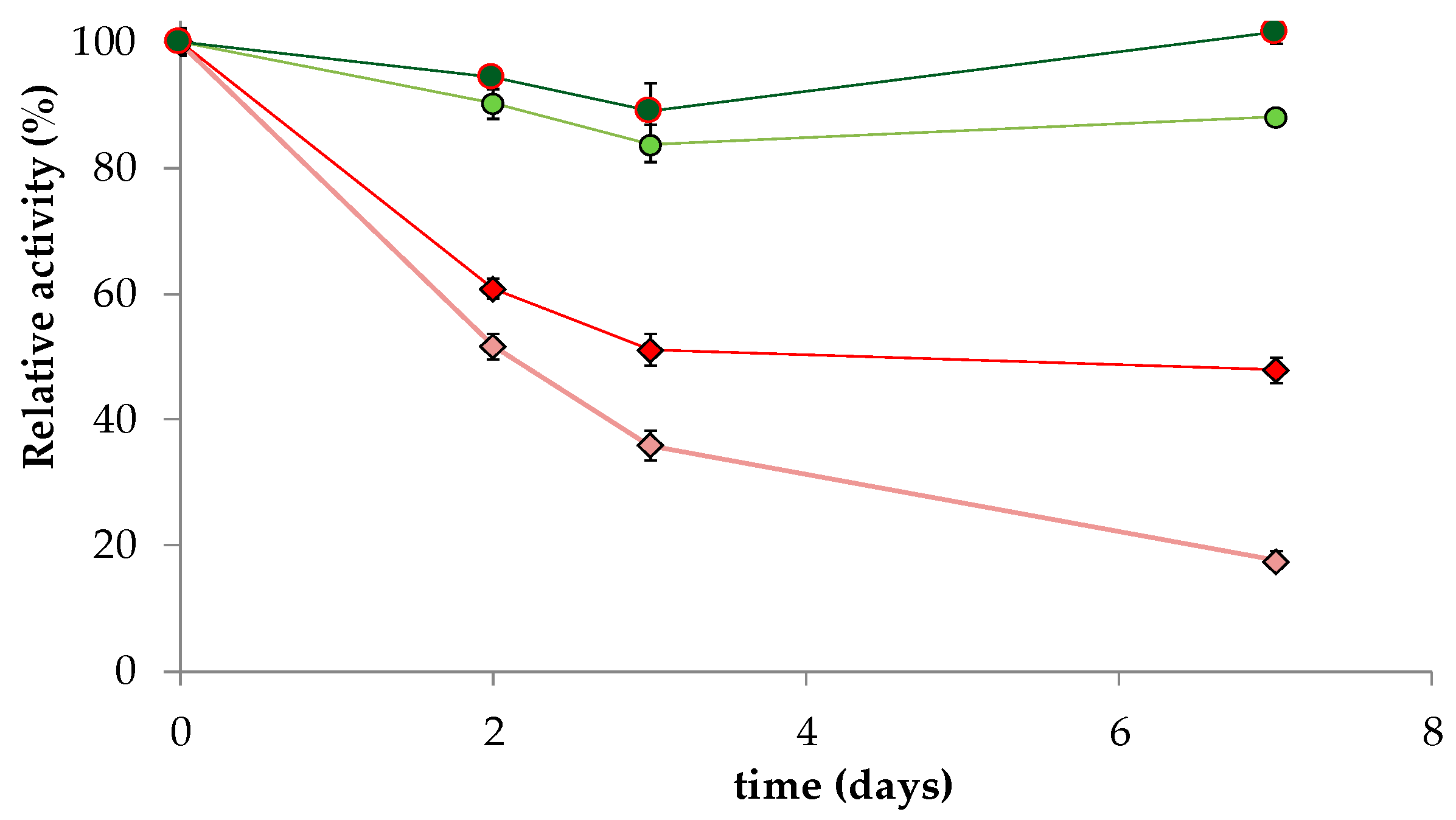
2.2. ccBTL2 Conserved Similar Expression and Optimal Properties
2.3. A Tunable Formation of the Disulfide Bond in ccBTL2
2.4. Activity of the Engineered ccBTL2 Is Enhanced in Presence of Detergent
2.4.1. Effect of Cu2+ Pretreatment in Enzymatic Activity in Absence of Detergents
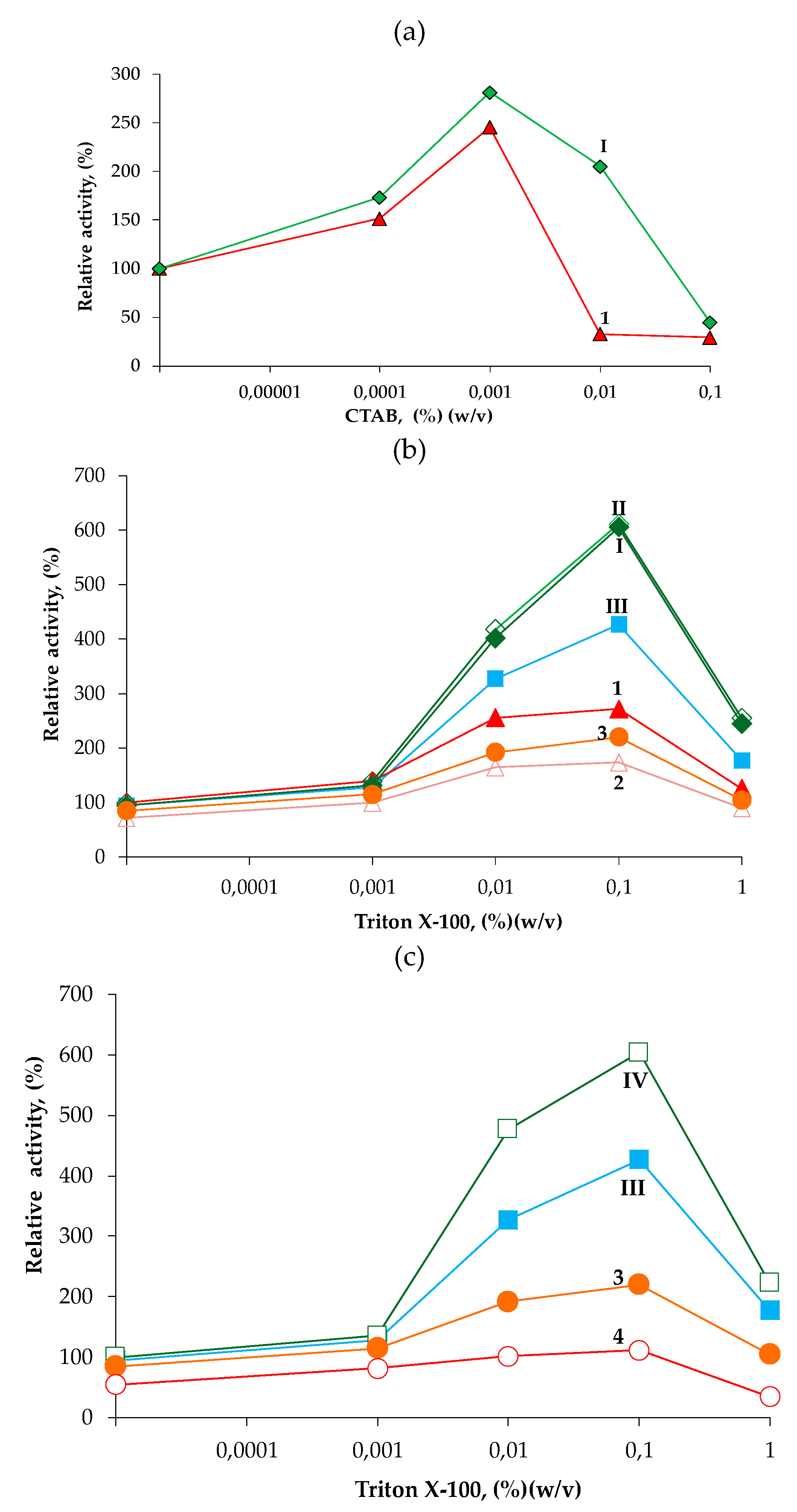
2.4.2. Hydrolysis of p-NPB in Presence of Detergents
2.4.3. Transformation of Natural Triglycerides
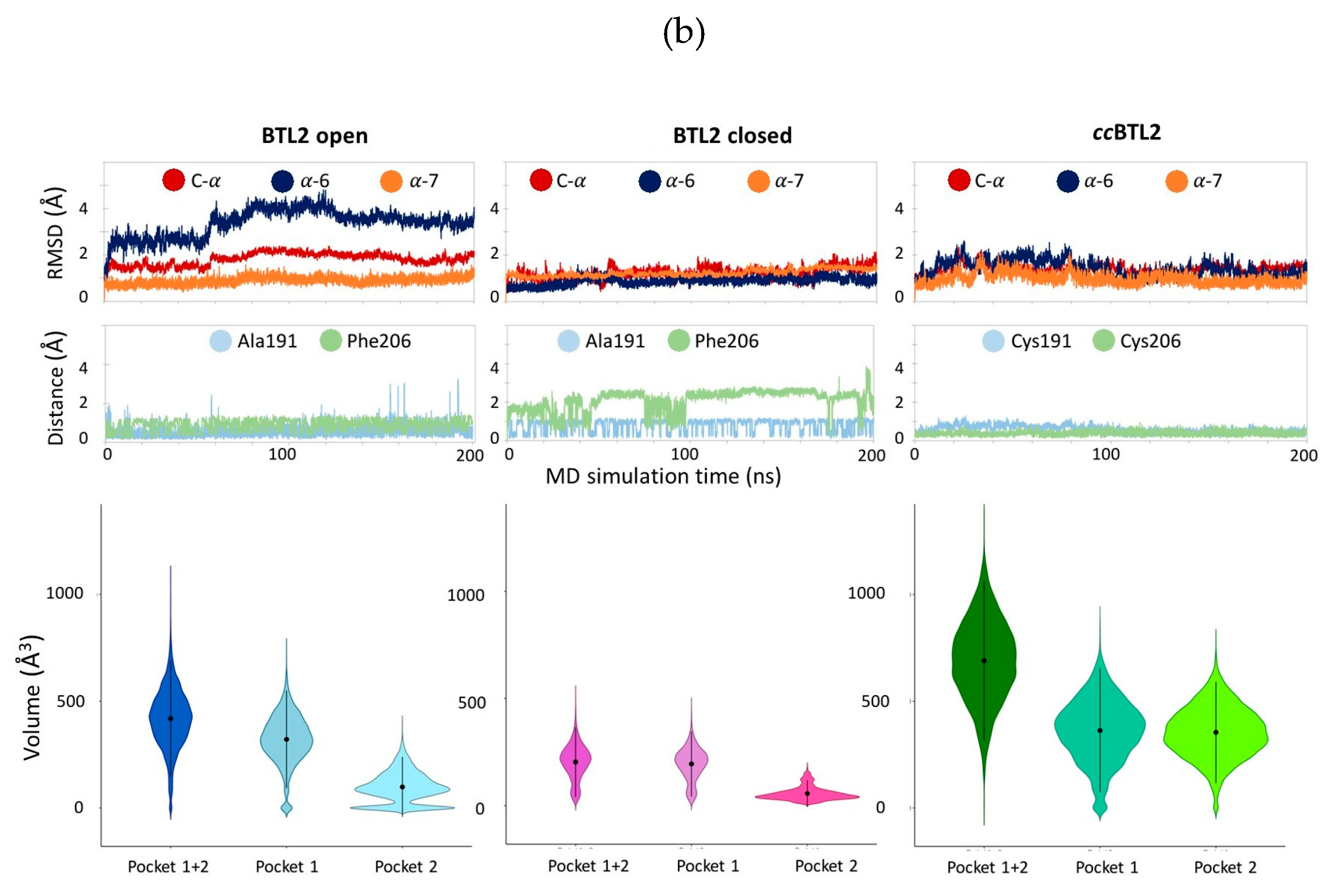
2.5. Exploring ccBTL2 Active Conformation by Molecular Dynamics
3. Materials and Methods
3.1. Materials
3.2. Site Directed Mutagenesis, Cloning, Expression and Purification of BTL2 Variants
3.3. Protein Determination, Enzymatic Activity Assay, Enzyme Immobilization and Characterization of Derivatives
3.4. Assay with 4-PDS for CNBr Derivatives
3.5. Incubation of CNBr Derivatives in Oxidizing/Reducing Conditions
3.5.1. Oxidizing Conditions
3.5.2. Reducing Conditions
3.6. Derivative Thermal Stability
3.7. Transformation of Natural Triglycerides
3.8. Targeted Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BTL2 | Wild type Geobacillus thermocatenulatus lipase 2 |
| øBTL2 | BTL2 mutant lacking native cysteine residues (C64S, C295S BTL2) |
| ccBTL2 | A191C, F206C, C64S, C295S BTL2 (engineered mutant) |
| CNBr | Cyanogen bromide Sepharose® support |
| CTAB | hexadecyltrimethylammonium bromide |
| 4-PDS | 4,4′-dipyridyldisulfide |
| p-NPB | p-nitrophenyl butyrate |
| p-NP | p-nitrophenyl ester |
| DTT | Dithiothreitol |
| GSH | Glutathione (reduced form) |
| GSSG | Glutathione (oxidized form) |
| PUFAs | Polyunsaturated fatty acids |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| TMD | Targeted Molecular Dynamics |
References
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A Broader View: Microbial Enzymes and Their Relevance in Industries, Medicine, and Beyond. Available online: https://www.hindawi.com/journals/bmri/2013/329121/ (accessed on 5 June 2019).
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef] [PubMed]
- Galante, Y.M.; Formantici, C. Enzyme Applications in Detergency and in Manufacturing Industries. Curr. Org. Chem. 2003, 7, 1399–1422. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S.S. Microbial detergent compatible lipases. J. Sci. Ind. Res. 2015, 74, 105–113. [Google Scholar]
- Verger, R. ‘Interfacial activation’ of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Carrasco-López, C. Activation of bacterial thermo alkalophilic lipases is spurred by dramatic structural rearrangements. J. Biolog. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Godoy, C.A.; Romero, O.; de las Rivas, B.; Mateo, C.; Fernandez-Lorente, G.; Guisan, J.M.; Palomo, J.M. Changes on enantioselectivity of a genetically modified thermophilic lipase by site-directed oriented immobilization. J. Mol. Catal. B Enzym. 2013, 87, 121–127. [Google Scholar] [CrossRef]
- Gao, B.; Xu, T.; Lin, J.; Zhang, L.; Su, E.; Jiang, Z.; Wei, D. Improving the catalytic activity of lipase LipK107 from Proteus sp. by site-directed mutagenesis in the lid domain based on computer simulation. J. Mol. Catal. B Enzym. 2011, 68, 286–291. [Google Scholar] [CrossRef]
- Quilles, J.C.J.; Brito, R.R.; Borges, J.P.; Aragon, C.C.; Fernandez-Lorente, G.; Bocchini-Martins, D.A.; Gomes, E.; da Silva, R.; Boscolo, M.; Guisan, J.M. Modulation of the activity and selectivity of the immobilized lipases by surfactants and solvents. Biochem. Eng. J. 2015, 93, 274–280. [Google Scholar] [CrossRef] [Green Version]
- López-Gallego, F.; Abian, O.; Guisán, J.M. Altering the interfacial activation mechanism of a lipase by solid-phase selective chemical modification. Biochemistry 2012, 51, 7028–7036. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Han, S.; Zheng, S.; Lin, Y. Enhancing thermostability of a Rhizomucor miehei lipase by engineering a disulfide bond and displaying on the yeast cell surface. Appl. Microbiol. Biotechnol. 2009, 85, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-W.; Tan, N.-J.; Xiao, R.; Xu, Y. Engineering a Disulfide Bond in the Lid Hinge Region of Rhizopus chinensis Lipase: Increased Thermostability and Altered Acyl Chain Length Specificity. PLoS ONE 2012, 7, e46388. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Wu, W.; Guan, W.; Deng, Z.; Qiao, H. Enhancing thermostability of Yarrowia lipolytica lipase 2 through engineering multiple disulfide bonds and mitigating reduced lipase production associated with disulfide bonds. Enzym. Microb. Technol. 2019, 126, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Dombkowski, A.A. Disulfide by Design 2.0: A web-based tool for disulfide engineering in proteins. BMC Bioinform. 2013, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Dannert, C.; Rúa, M.L.; Wahl, S.; Schmid, R.D. Bacillus thermocatenulatus lipase: A thermoalkalophilic lipase with interesting properties. Biochem. Soc. Trans. 1997, 25, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A. Enhanced activity of an immobilized lipase promoted by site-directed chemical modification with polymers. Process Biochem. 2010, 45, 534–541. [Google Scholar] [CrossRef]
- Godoy, C.A.; Fernández-Lorente, G.; de las Rivas, B.; Filice, M.; Guisan, J.M.; Palomo, J.M. Medium engineering on modified Geobacillus thermocatenulatus lipase to prepare highly active catalysts. J. Mol. Catal. B Enzym. 2011, 70, 144–148. [Google Scholar] [CrossRef]
- Khaleghinejad, S.H.; Motalleb, G.; Karkhane, A.A.; Aminzadeh, S.; Yakhchali, B. Study the effect of F17S mutation on the chimeric Bacillus thermocatenulatus lipase. J. Genet. Eng. Biotechnol. 2016, 14, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Yuan, C.; Hwang, H.-T.; Zhao, X.; Ramkrishna, D.; Liu, D.; Varma, A. Engineering surface hydrophobicity improves activity of Bacillus thermocatenulatus lipase 2 enzyme. Biotechnol. J. 2015, 10, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; de las Rivas, B.; Lopez-Tejedor, D.; Palomo, J.M. Effect of Site-Specific Peptide-Tag Labeling on the Biocatalytic Properties of Thermoalkalophilic Lipase from Geobacillus thermocatenulatus. ChemBioChem 2018, 19, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Karkhane, A.A. The effect of substitution of Phe181 and Phe182 with Ala on activity, substrate specificity and stabilization of substrate at the active site of Bacillus thermocatenulatus lipase. J. Mol. Catal. B Enzym. 2009, 61, 162. [Google Scholar] [CrossRef]
- Tyndall, J.D.A. Crystal structure of a thermostable lipase from bacillus stearothermophilus P1. J. Mol. Biol. 2002, 323, 859–869. [Google Scholar] [CrossRef]
- Rua, M.L.; Atomi, H.; Schmidt-Dannert, C.; Schmid, R.D. High-level expression of the thermoalkalophilic lipase from Bacillus thermocatenulatus in Escherichia coli. Appl. Microbiol. Biotechnol. 1998, 49, 405–410. [Google Scholar] [PubMed]
- Godoy, C.A.; de las Rivas, B.; Grazú, V.; Montes, T.; Guisán, J.M.; López-Gallego, F. Glyoxyl-disulfide agarose: A tailor-made support for site-directed rigidification of proteins. Biomacromolecules 2011, 12, 1800–1809. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General Trend of Lipase to Self-Assemble Giving Bimolecular Aggregates Greatly Modifies the Enzyme Functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schlieben, N.H. Expression, purification, and aggregation studies of his-tagged thermoalkalophilic lipase from bacillus thermocatenulatus. Protein Expr. Purif. 2004, 34, 103–110. [Google Scholar] [CrossRef]
- Timucin, E.; Sezerman, O.U. Zinc Modulates Self-Assembly of Bacillus thermocatenulatus Lipase. Biochemistry 2015, 54, 3901–3910. [Google Scholar] [CrossRef]
- Willems, N.; Lelimousin, M.; Skjold-Jørgensen, J.; Svendsen, A.; Sansom, M.S.P. The effect of mutations in the lid region of Thermomyces lanuginosus lipase on interactions with triglyceride surfaces: A multi-scale simulation study. Chem. Phys. Lipids 2018, 211, 4–15. [Google Scholar] [CrossRef]
- Lambert, C.; Léonard, N.; Bolle, X.D.; Depiereux, E. ESyPred3D: Prediction of proteins 3D structures. Bioinformatics 2002, 18, 1250–1256. [Google Scholar] [CrossRef]
- Winther, J.R.; Thorpe, C. Quantification of thiols and disulfides. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 838–846. [Google Scholar] [CrossRef]
- Calce, E.; Vitale, R.M.; Scaloni, A.; Amodeo, P.; De Luca, S. Air oxidation method employed for the disulfide bond formation of natural and synthetic peptides. Amino Acids 2015, 47, 1507–1515. [Google Scholar] [CrossRef]
- Meehan, B.M.; Landeta, C.; Boyd, D.; Beckwith, J. The Disulfide Bond Formation Pathway Is Essential for Anaerobic Growth of Escherichia coli. J. Bacteriol. 2017, 199, e00120-17. [Google Scholar] [CrossRef]
- Karlsson, M. Denaturant-assisted formation of a stabilizing disulfide bridge from engineered cysteines in nonideal conformations. Biochemistry 2005, 44, 3487–3493. [Google Scholar] [CrossRef]
- Kim, H. Gene cloning and characterization of thermostable lipase from bacillus stearothermophilus L1. Biosci. Biotechnol. Biochem. 1998, 62, 66–71. [Google Scholar] [CrossRef]
- Muhammad, G.; Croguennec, T.; Julien, J.; Michel, P.; Saïd, B. Copper modulates the heat-induced sulfhydryl/disulfide interchange reactions of β-Lactoglobulin. Food Chem. 2009, 116, 884–891. [Google Scholar] [CrossRef]
- Godoy, C.A.; de las Rivas, B.; Bezbradica, D.; Bolivar, J.M.; López-Gallego, F.; Fernandez-Lorente, G.; Guisan, J.M. Reactivation of a thermostable lipase by solid phase unfolding/refolding: Effect of cysteine residues on refolding efficiency. Enzym. Microb. Technol. 2011, 49, 388–394. [Google Scholar] [CrossRef]
- Salameh, M.A.; Wiegel, J. Effects of Detergents on Activity, Thermostability and Aggregation of Two Alkalithermophilic Lipases from Thermosyntropha lipolytica. Open Biochem. J. 2010, 4, 22–28. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G. Improved catalytic properties of immobilized lipases by the presence of very low concentrations of detergents in the reaction medium. J. Mol. Catal. B Enzym. 2007, 10, 385–393. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Pizarro, C.; López-Vela, D.; Betancor, L.; Carrascosa, A.V.; Pessela, B.; Guisan, J.M. Hydrolysis of Fish Oil by Lipases Immobilized Inside Porous Supports. J. Am. Oil. Chem. Soc. 2011, 88, 819–826. [Google Scholar] [CrossRef]
- Godoy, C.A. New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation. Int. J. Mol. Sci. 2017, 18, 2130. [Google Scholar] [CrossRef]
- Toro, E.C.; Rodríguez, D.F.; Morales, N.; García, L.M.; Godoy, C.A. Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production. Catalysts 2019, 9, 546. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.E.; Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Jeong, S. Novel zinc-binding center and a temperature switch in the bacillus stearothermophilus L1 lipase. J. Biolog. Chem. 2002, 277, 17041–17047. [Google Scholar] [CrossRef]
- Schlitter, J.; Engels, M.; Krüger, P. Targeted molecular dynamics: A new approach for searching pathways of conformational transitions. J. Mol. Graph. 1994, 12, 84–89. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G. Solid-Phase Chemical Amination of a Lipase from Bacillus thermocatenulatus To Improve Its Stabilization via Covalent Immobilization on Highly Activated Glyoxyl-Agarose. Biomacromolecules 2008, 9, 2553–2561. [Google Scholar] [CrossRef]
- Godoy, C.A.; de las Rivas, B.; Guisán, J.M. Site-directing an intense multipoint covalent attachment (MCA) of mutants of the Geobacillus thermocatenulatus lipase 2 (BTL2): Genetic and chemical amination plus immobilization on a tailor-made support. Process Biochem. 2014, 49, 1324–1331. [Google Scholar] [CrossRef]
- Riener, C.; Kada, G.; Gruber, H. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef]
| Enzyme | Specific p-NPB Esterase Activity (IU/mg) a | Activity Recovery (%) b | Optimal pH c | Optimal Temperature (°C) d | t1/2 at 75 °C (min) d |
|---|---|---|---|---|---|
| BTL2 | 660 ± 21 | 65± 2.3 | 8.5-9.5 | 64 ± 3.1 | 28 ± 1.3 |
| ccBTL2 | 630 ± 17 | 67 ± 3.1 | 8.5-9.5 | 66 ± 2.3 | 30 ± 1.1 |
| Pretreatment in Presence (+) or Absence (-) of Guanidine 8 M | nmol of Equivalent Free Cys Per mg of Protein a | |||
|---|---|---|---|---|
| BTL2 | ccBTL2 | |||
| As obtained | − | 35.0 ± 2.26 | 0.752 ± 0.62 | |
| + | 7.01 ± 1.25 | 2.01 ± 0.02 | ||
| Reducing incubation | 25 mM DTT | − | 34.8 ± 4.45 | 42.0 ± 1.75 |
| + | 42.9 ± 1.88 | 44.0 ±2.26 | ||
| GSSG 1 mM/ | − | 31.4 ±2.63 | 32.4± 1.31 | |
| GSH 10 mM | + | 34.5 ± 3.13 | 38.4 ± 2.57 | |
| Oxidizing incubation | 200 µM Cu2+ | − | 4.82 ± 1.38 | 6.08 ± 1.32 |
| + | 4.51 ± 1.88 | 1.00 ± 0.81 | ||
| GSSG 10 mM/ | − | 19.6 ± 1.13 | 12.4 ± 4.40 | |
| GSH 1 mM | + | 2.63 ± 0.69 | 7.02 ± 2.00 | |
| CNBr-Derivative | Treatment | Hydrolysis of Sardine Oil | Palm Olein Ethyl Esters Yield at 72 h (%) c | |
|---|---|---|---|---|
| Activity a(µmol PUFAs/min) | Specificity b (EPA/DHA) | |||
| BTL2 | As obtained | 0.86 ± 0.04 | 1.9 ± 0.1 | 22.1 ± 1.9 |
| 200 µM Cu2+ | 0.15 ± 0.02 | 2.0 ± 0.3 | <5 | |
| 25 mM DTT | 0.95 ± 0.06 | 1.8 ± 0.2 | 30.3 ± 2.9 | |
| ccBTL2 | As obtained | 0.96 ± 0.04 | 1.6 ± 0.2 | 29.5 ± 1.1 |
| 200 µM Cu2+ | 0.99 ± 0.06 | 1.7 ± 0.1 | 34.4 ± 1.3 | |
| 25 mM DTT | 0.85 ± 0.07 | 1.8 ± 0.2 | 25.7 ± 2.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, C.A.; Klett, J.; Di Geronimo, B.; Hermoso, J.A.; Guisán, J.M.; Carrasco-López, C. Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2. Int. J. Mol. Sci. 2019, 20, 5245. https://doi.org/10.3390/ijms20215245
Godoy CA, Klett J, Di Geronimo B, Hermoso JA, Guisán JM, Carrasco-López C. Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2. International Journal of Molecular Sciences. 2019; 20(21):5245. https://doi.org/10.3390/ijms20215245
Chicago/Turabian StyleGodoy, César A., Javier Klett, Bruno Di Geronimo, Juan A. Hermoso, José M. Guisán, and César Carrasco-López. 2019. "Disulfide Engineered Lipase to Enhance the Catalytic Activity: A Structure-Based Approach on BTL2" International Journal of Molecular Sciences 20, no. 21: 5245. https://doi.org/10.3390/ijms20215245