Cellulose-starch Hybrid Films Plasticized by Aqueous ZnCl2 Solution
Abstract
:1. Introduction
2. Results and Discussion
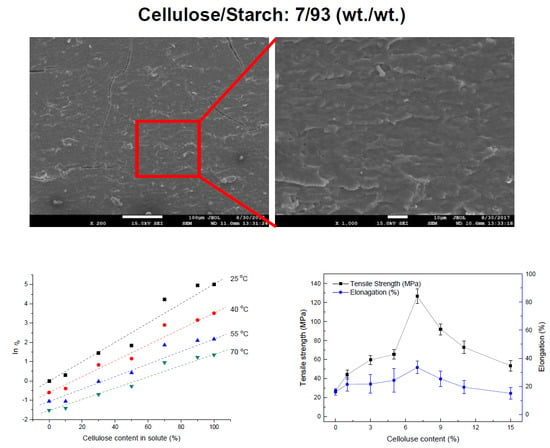
2.1. Rheological Study of Cellulose/Starch/ZnCl2 Solutions
2.2. Structural Characterization of Cellulose-starch Hybrid Films
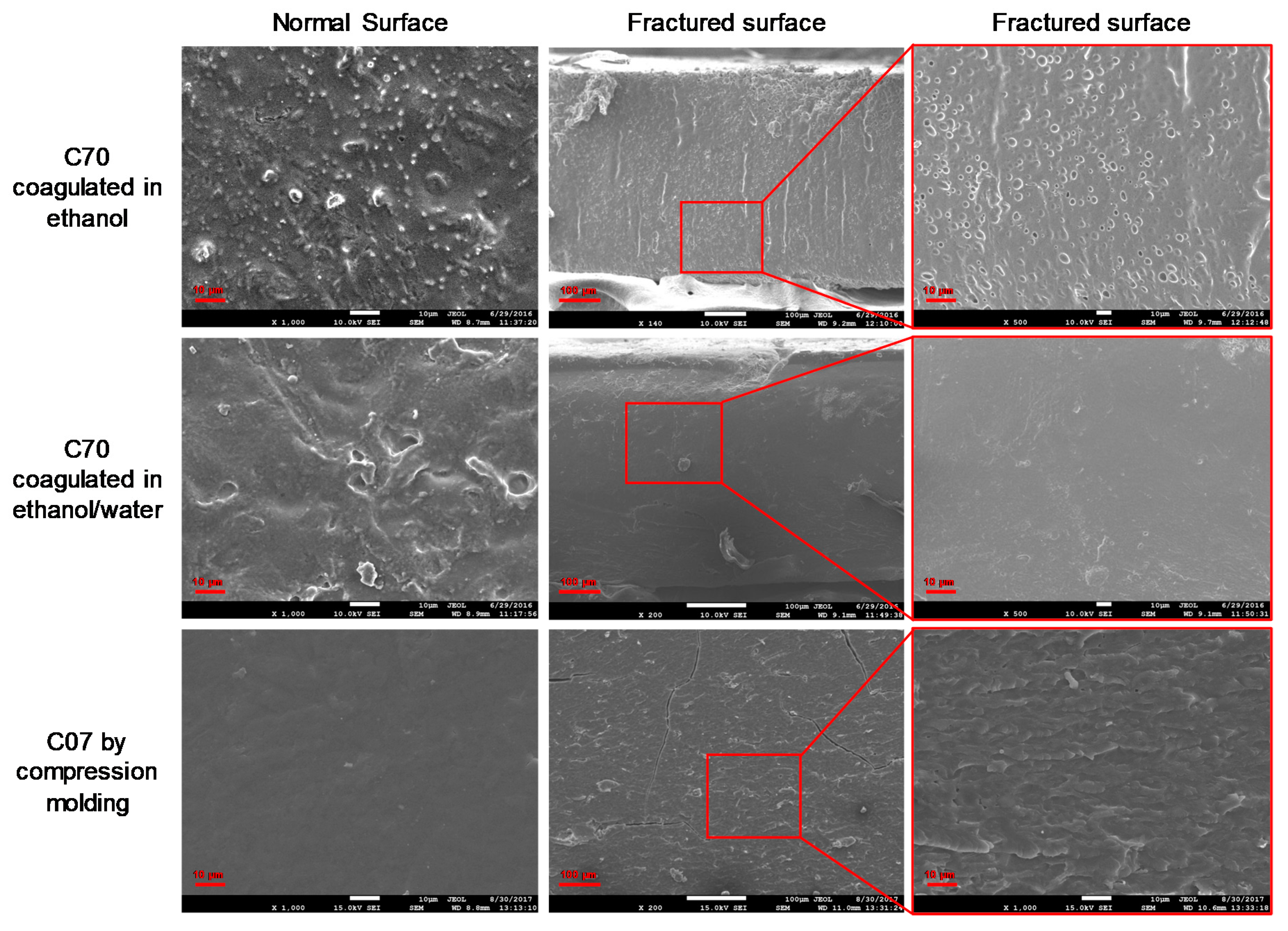
2.2.1. Morphology
2.2.2. XRD Analysis
2.2.3. FTIR Analysis
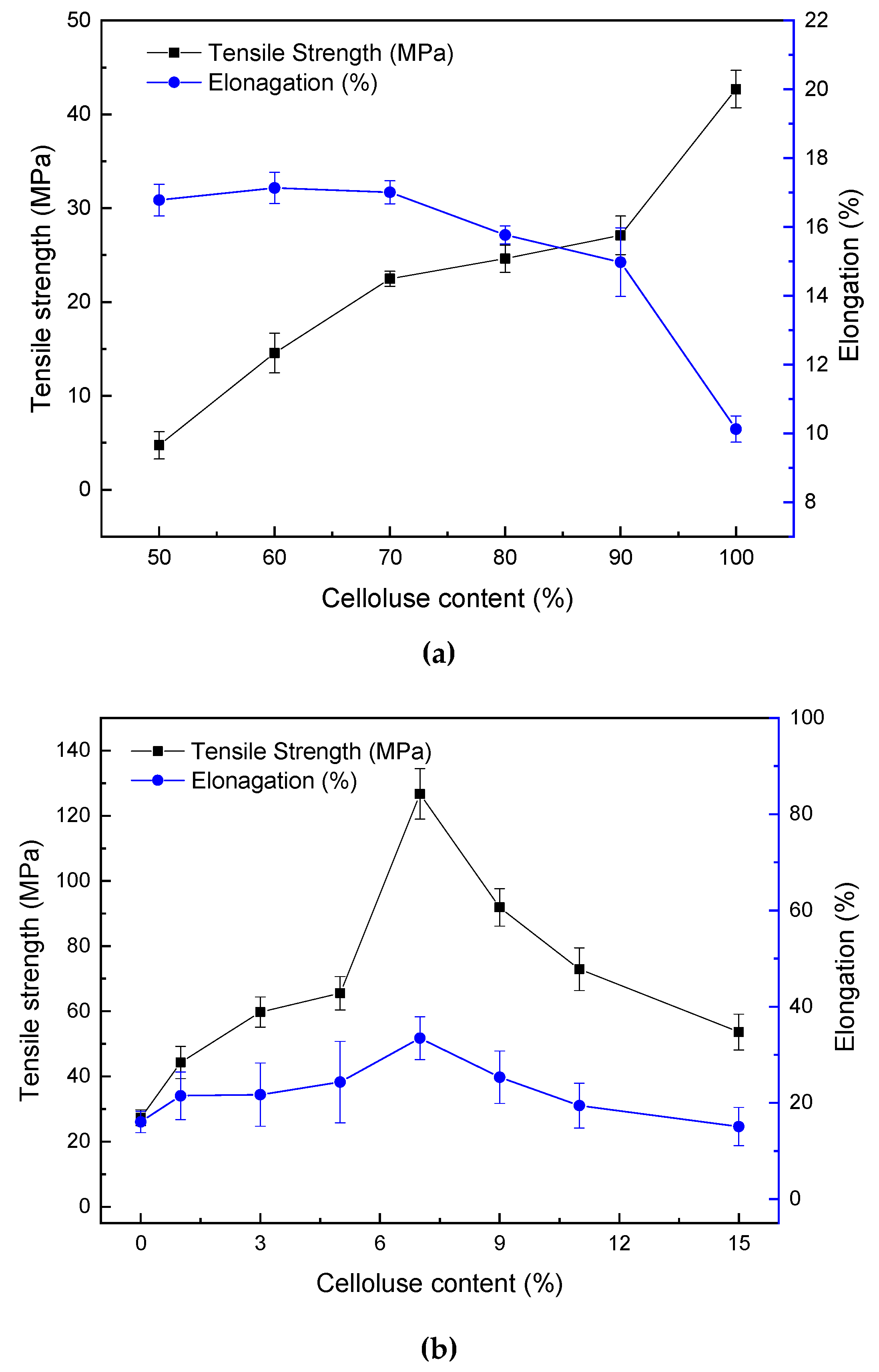
2.3. Mechanical Properties of Blend Films Plasticized by ZnCl2 Solution
3. Materials and Methods
3.1. Materials
3.2. Materials Preparation
3.3. Characterization
3.3.1. Rheological Properties
3.3.2. Scanning Electron Microscopy
3.3.3. X-ray Diffraction (XRD)
3.3.4. Fourier-Transform Infrared Spectroscopy
3.3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MCC | Microcrystalline cellulose |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier-transform infrared |
| ATR | Attenuated Total Reflectance |
| [Amim][Cl] | 1-allyl-3-methylimidazolium chloride |
| [Emin][OAc] | 1-ethyl-3-methylimidazolium acetate |
| [Bmim][OAc] | 1-butyl-3-methylimidazolium chloride |
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Xie, F.; Halley, P.J.; Avérous, L. Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 2012, 37, 595–623. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Su, B.; Liu, P.; Wang, J.; Liu, H.; Chen, L. Rheological properties of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2009, 49, 371–377. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion processing and characterization of edible starch films with different amylose contents. J. Food Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Bie, P.; Liu, P.; Yu, L.; Li, X.; Chen, L.; Xie, F. The properties of antimicrobial films derived from poly(lactic acid)/starch/chitosan blended matrix. Carbohydr. Polym. 2013, 98, 959–966. [Google Scholar] [CrossRef]
- Dean, K.; Sangwan, P.; Way, C.; Zhang, X.; Martino, V.P.; Xie, F.; Halley, P.J.; Pollet, E.; Avérous, L. Glycerol plasticised chitosan: A study of biodegradation via carbon dioxide evolution and nuclear magnetic resonance. Polym. Degrad. Stab. 2013, 98, 1236–1246. [Google Scholar] [CrossRef]
- Xie, D.F.; Martino, V.P.; Sangwan, P.; Way, C.; Cash, G.A.; Pollet, E.; Dean, K.M.; Halley, P.J.; Avérous, L. Elaboration and properties of plasticised chitosan-based exfoliated nano-biocomposites. Polymer 2013, 54, 3654–3662. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, M.; Arab-Tehrany, E.; Kahn, C.J.F.; Cleymand, F.; Fleutot, S.; Desobry, S.; Sánchez-González, L. Bioactive Films Containing Alginate-Pectin Composite Microbeads with Lactococcus lactis subsp. lactis: Physicochemical Characterization and Antilisterial Activity. Int. J. Mol. Sci. 2018, 19, 574. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Pollet, E.; Avérous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- López, O.V.; Ninago, M.D.; Lencina, M.M.S.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villar, M.A. Thermoplastic starch plasticized with alginate–glycerol mixtures: Melt-processing evaluation and film properties. Carbohydr. Polym. 2015, 126, 83–90. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Gurau, G.; Block, L.E.; Hansen, L.K.; Dingee, C.; Walters, A.; Rogers, R.D. Chitin-calcium alginate composite fibers for wound care dressings spun from ionic liquid solution. J. Mater. Chem. A 2014, 2, 3924–3936. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules I. In Starch (Third Edition); James, B., Roy, W., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 149–192. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef] [Green Version]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline celluloseby acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Zografi, G.; Kontny, M.J.; Yang, A.Y.S.; Brenner, G.S. Surface area and water vapor sorption of macrocrystalline cellulose. Int. J. Pharm. 1984, 18, 99–116. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Chen, L.; Xie, F. Solubility of starch and microcrystalline cellulose in 1-ethyl-3-methylimidazolium acetate ionic liquid and solution rheological properties. Phys. Chem. Chem. Phys. 2016, 18, 27584–27593. [Google Scholar] [CrossRef]
- Wang, X.-L.; Yang, K.-K.; Wang, Y.-Z. Properties of starch blends with biodegradable polymers. Polym. Rev. 2003, 43, 385–409. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Amaro, L.; Correia, D.M.; Marques-Almeida, T.; Martins, P.M.; Pérez, L.; Vilas, J.L.; Botelho, G.; Lanceros-Mendez, S.; Ribeiro, C. Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. Int. J. Mol. Sci. 2018, 19, 2149. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes sandwiched between chitosan layers: Novel bionanocomposites with multilayer structures. New J. Chem. 2018, 42, 8384–8390. [Google Scholar] [CrossRef]
- Meng, L.; Xie, F.; Zhang, B.; Wang, D.K.; Yu, L. Natural Biopolymer Alloys with Superior Mechanical Properties. ACS Sustain. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Mohammadi, N. Starch-based nanocomposites: A comparative performance study of cellulose whiskers and starch nanoparticles. Carbohydr. Polym. 2014, 106, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.; Pereira, A.; Fajardo, A.; Rubira, A.; Muniz, E. Superabsorbent hydrogel nanocomposites based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef]
- Moreira, F.; Marconcini, J.; Mattoso, L. Solid state ball milling as a green strategy to improve the dispersion of cellulose nanowhiskers in starch-based thermoplastic matrices. Cellulose 2012, 19, 2049–2056. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Xie, F.; Zhang, L.; Liao, L.; Liu, H.; Chen, L. Morphology and properties of thermal/cooling-gel bi-phasic systems based on hydroxypropyl methylcellulose and hydroxypropyl starch. Compos. Part B Eng. 2016, 101, 46–52. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Xie, F.; Li, S.; Sun, Q.; Liu, H.; Chen, L. On the investigation of thermal/cooling-gel biphasic systems based on hydroxypropyl methylcellulose and hydroxypropyl starch. Ind. Crops Prod. 2018, 124, 418–428. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, H.; Yu, L.; Simon, G.P.; Zhang, N.; Chen, L. Relationship between morphologies and mechanical properties of hydroxypropyl methylcellulose/hydroxypropyl starch blends. Carbohydr. Polym. 2016, 153, 329–335. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of starch–hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef]
- Liu, D.; Chang, P.R.; Deng, S.; Wang, C.; Zhang, B.; Tian, Y.; Huang, S.; Yao, J.; Ma, X. Fabrication and characterization of zirconium hydroxide-carboxymethyl cellulose sodium/plasticized Trichosanthes Kirilowii starch nanocomposites. Carbohydr. Polym. 2011, 86, 1699–1704. [Google Scholar] [CrossRef]
- Wu, D.; Chang, P.R.; Ma, X. Preparation and properties of layered double hydroxide-carboxymethylcellulose sodium/glycerol plasticized starch nanocomposites. Carbohydr. Polym. 2011, 86, 877–882. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Stevenson, D.G.; Willett, J.L.; Bhowmik, P.K. Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66, 546–550. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Spychaj, T. Ionic liquids: Media for starch dissolution, plasticization and modification. Carbohydr. Polym. 2011, 86, 424–428. [Google Scholar] [CrossRef]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic starch plasticized by an ionic liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Leroy, E.; Jacquet, P.; Coativy, G.; Reguerre, A.l.; Lourdin, D. Compatibilization of starch–zein melt processed blends by an ionic liquid used as plasticizer. Carbohydr. Polym. 2012, 89, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Mateyawa, S.; Xie, D.F.; Truss, R.W.; Halley, P.J.; Nicholson, T.M.; Shamshina, J.L.; Rogers, R.D.; Boehm, M.W.; McNally, T. Effect of the ionic liquid 1-ethyl-3-methylimidazolium acetate on the phase transition of starch: Dissolution or gelatinization? Carbohydr. Polym. 2013, 94, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.V.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of starch-based films plasticised by glycerol and by the ionic liquid 1-ethyl-3-methylimidazolium acetate: A comparative study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Flanagan, B.M.; Li, M.; Truss, R.W.; Halley, P.J.; Gidley, M.J.; McNally, T.; Shamshina, J.L.; Rogers, R.D. Characteristics of starch-based films with different amylose contents plasticised by 1-ethyl-3-methylimidazolium acetate. Carbohydr. Polym. 2015, 122, 160–168. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Zhang, T.; Chen, L.; Li, X.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; McNally, T.; Rogers, R.D. Different characteristic effects of ageing on starch-based films plasticised by 1-ethyl-3-methylimidazolium acetate and by glycerol. Carbohydr. Polym. 2016, 146, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Chen, L.; Li, X.; Xie, F. Effect of anti-solvents on the characteristics of regenerated cellulose from 1-ethyl-3-methylimidazolium acetate ionic liquid. Int. J. Biol. Macromol. 2019, 124, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tan, X.; Li, X.; Chen, L.; Xie, F. Characterization of regenerated starch from 1-ethyl-3-methylimidazolium acetate ionic liquid with different anti-solvents. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1231–1238. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Shamshina, J.L.; Rogers, R.D.; McNally, T.; Halley, P.J.; Truss, R.W.; Chen, L.; Zhao, S. Dissolution of Starch with Aqueous Ionic Liquid under Ambient Conditions. ACS Sustain. Chem. Eng. 2017, 5, 3737–3741. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xie, F.; Shamshina, J.L.; Rogers, R.D.; McNally, T.; Wang, D.K.; Halley, P.J.; Truss, R.W.; Zhao, S.; Chen, L. Facile Preparation of Starch-Based Electroconductive Films with Ionic Liquid. ACS Sustain. Chem. Eng. 2017, 5, 5457–5467. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef]
- Taheri, N.; Abdolmaleki, A.; Fashandi, H. Pyridinium-based ionic liquid/water mixture intended for efficient dissolution of cellulose, chitosan and chitin: The pivotal contribution of water. Carbohydr. Polym. 2018, 195, 413–419. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, G. From cellulose fibrils to single chains: Understanding cellulose dissolution in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 31592–31607. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Zhang, X.; Xu, L.; Wu, J.; Yu, J.; He, J.; Zhang, J. All-Cellulose Nanocomposites Reinforced with in Situ Retained Cellulose Nanocrystals during Selective Dissolution of Cellulose in an Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Murakami, M.-A.; Takegawa, A.; Kaneko, Y. Preparation of cellulose-starch composite gel and fibrous material from a mixture of the polysaccharides in ionic liquid. Carbohydr. Polym. 2009, 75, 180–183. [Google Scholar] [CrossRef]
- Liu, W.; Budtova, T. Ionic liquid: A powerful solvent for homogeneous starch–cellulose mixing and making films with tuned morphology. Polymer 2012, 53, 5779–5787. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. Inorganic molten salts as solvents for cellulose. Cellulose 2003, 10, 227–236. [Google Scholar] [CrossRef]
- Lin, M.; Shang, X.; Liu, P.; Xie, F.; Chen, X.; Sun, Y.; Wan, J. Zinc chloride aqueous solution as a solvent for starch. Carbohydr. Polym. 2016, 136, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, P.; Shang, X.; Xie, F.; Jiang, H.; Wang, J. Investigation of rheological properties and conformation of cassava starch in zinc chloride solution. Starch Stärke 2017, 69, 1600384. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Shang, X.; Xie, F. Starch–zinc complex and its reinforcement effect on starch-based materials. Carbohydr. Polym. 2019, 206, 528–538. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Lu, X.; Shen, X. Solubility of bacteria cellulose in zinc chloride aqueous solutions. Carbohydr. Polym. 2011, 86, 239–244. [Google Scholar] [CrossRef]
- Richards, N.J.; Williams, D.G. Complex formation between aqueous zinc chloride and cellulose-related d-glucopyranosides. Carbohydr. Res. 1970, 12, 409–420. [Google Scholar] [CrossRef]
- Cao, N.-J.; Xu, Q.; Chen, C.-S.; Gong, C.S.; Chen, L.F. Cellulose hydrolysis using zinc chloride as a solvent and catalyst. Appl. Biochem. Biotechnol. 1994, 45, 521–530. [Google Scholar] [CrossRef]
- Cao, N.J.; Xu, Q.; Chen, L.F. Acid hydrolysis of cellulose in zinc chloride solution. Appl. Biochem. Biotechnol. 1995, 51, 21. [Google Scholar] [CrossRef]
- Luo, Z.; Zou, J.; Chen, H.; Cheng, W.; Fu, X.; Xiao, Z. Synthesis and characterization of amylose–zinc inclusion complexes. Carbohydr. Polym. 2016, 137, 314–320. [Google Scholar] [CrossRef]
- Luo, Z.; Cheng, W.; Chen, H.; Fu, X.; Peng, X.; Luo, F.; Nie, L. Preparation and Properties of Enzyme-Modified Cassava Starch–Zinc Complexes. J. Agric. Food Chem. 2013, 61, 4631–4638. [Google Scholar] [CrossRef] [PubMed]
- Koganti, N.; Mitchell, J.R.; Ibbett, R.N.; Foster, T.J. Solvent effects on starch dissolution and gelatinization. Biomacromolecules 2011, 12, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Budtova, T. Dissolution of unmodified waxy starch in ionic liquid and solution rheological properties. Carbohydr. Polym. 2013, 93, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological Properties of Cellulose/Ionic Liquid Solutions: From Dilute to Concentrated States. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef]
- Sescousse, R.; Le, K.A.; Ries, M.E.; Budtova, T. Viscosity of Cellulose−Imidazolium-Based Ionic Liquid Solutions. J. Phys. Chem. B 2010, 114, 7222–7228. [Google Scholar] [CrossRef]
- Tajuddin, S.; Xie, F.; Nicholson, T.M.; Liu, P.; Halley, P.J. Rheological properties of thermoplastic starch studied by multipass rheometer. Carbohydr. Polym. 2011, 83, 914–919. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L.; Della Valle, G. In-line determination of plasticized wheat starch viscoelastic behavior: Impact of processing. Carbohydr. Polym. 2003, 53, 169–182. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and regeneration of cellulose in NaOH/thiourea aqueous solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A method for estimating the nature and relative proportions of amorphous, single, and double-helical components in starch granules by 13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crops Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, J.J.G.; Borger, D.B. Structure and properties of compression-molded thermoplastic starch materials from normal and high-amylose maize starches. J. Appl. Polym. Sci. 1997, 64, 631–644. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: Consequences for material properties. Trends Biotechnol. 1997, 15, 208–213. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.-F.; Yue, F.-X.; Sun, R.-C.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011, 86, 672–677. [Google Scholar] [CrossRef]
- Ching, T.W.; Haritos, V.; Tanksale, A. Ultrasound-assisted conversion of cellulose into hydrogel and functional carbon material. Cellulose 2018, 25, 2629–2645. [Google Scholar] [CrossRef]





| Samples | Method | Starch (g) | Cellulose (g) | Concentration of ZnCl2 Solution (wt %) | ZnCl2 Solution (g) |
|---|---|---|---|---|---|
| C00 | CM | 100.0 | 0.0 | 25 | 35 |
| C01 | CM | 99.0 | 1.0 | 25 | 35 |
| C03 | CM | 97.0 | 2.0 | 25 | 35 |
| C05 | CM | 95.0 | 5.0 | 25 | 35 |
| C07 | CM | 93.0 | 7.0 | 25 | 35 |
| C09 | CM | 91.0 | 9.0 | 25 | 35 |
| C11 | CM | 89.0 | 11.0 | 25 | 35 |
| C13 | CM | 87.0 | 13.0 | 25 | 35 |
| C15 | CM | 85.0 | 15.0 | 25 | 35 |
| C50 | SC | 2.5 | 2.5 | 65 | 95 |
| C60 | SC | 2.0 | 3.0 | 65 | 95 |
| C70 | SC | 1.5 | 3.5 | 65 | 95 |
| C80 | SC | 1.0 | 4.0 | 65 | 95 |
| C90 | SC | 0.5 | 4.5 | 65 | 95 |
| C100 | SC | 0.0 | 5.0 | 65 | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Jiang, H.; Wang, Q.; Liu, P.; Xie, F. Cellulose-starch Hybrid Films Plasticized by Aqueous ZnCl2 Solution. Int. J. Mol. Sci. 2019, 20, 474. https://doi.org/10.3390/ijms20030474
Shang X, Jiang H, Wang Q, Liu P, Xie F. Cellulose-starch Hybrid Films Plasticized by Aqueous ZnCl2 Solution. International Journal of Molecular Sciences. 2019; 20(3):474. https://doi.org/10.3390/ijms20030474
Chicago/Turabian StyleShang, Xiaoqin, Huihua Jiang, Qingling Wang, Peng Liu, and Fengwei Xie. 2019. "Cellulose-starch Hybrid Films Plasticized by Aqueous ZnCl2 Solution" International Journal of Molecular Sciences 20, no. 3: 474. https://doi.org/10.3390/ijms20030474