Chitosan Hydrogel Beads Supported with Ceria for Boron Removal
Abstract
:1. Introduction
2. Results and Discussions
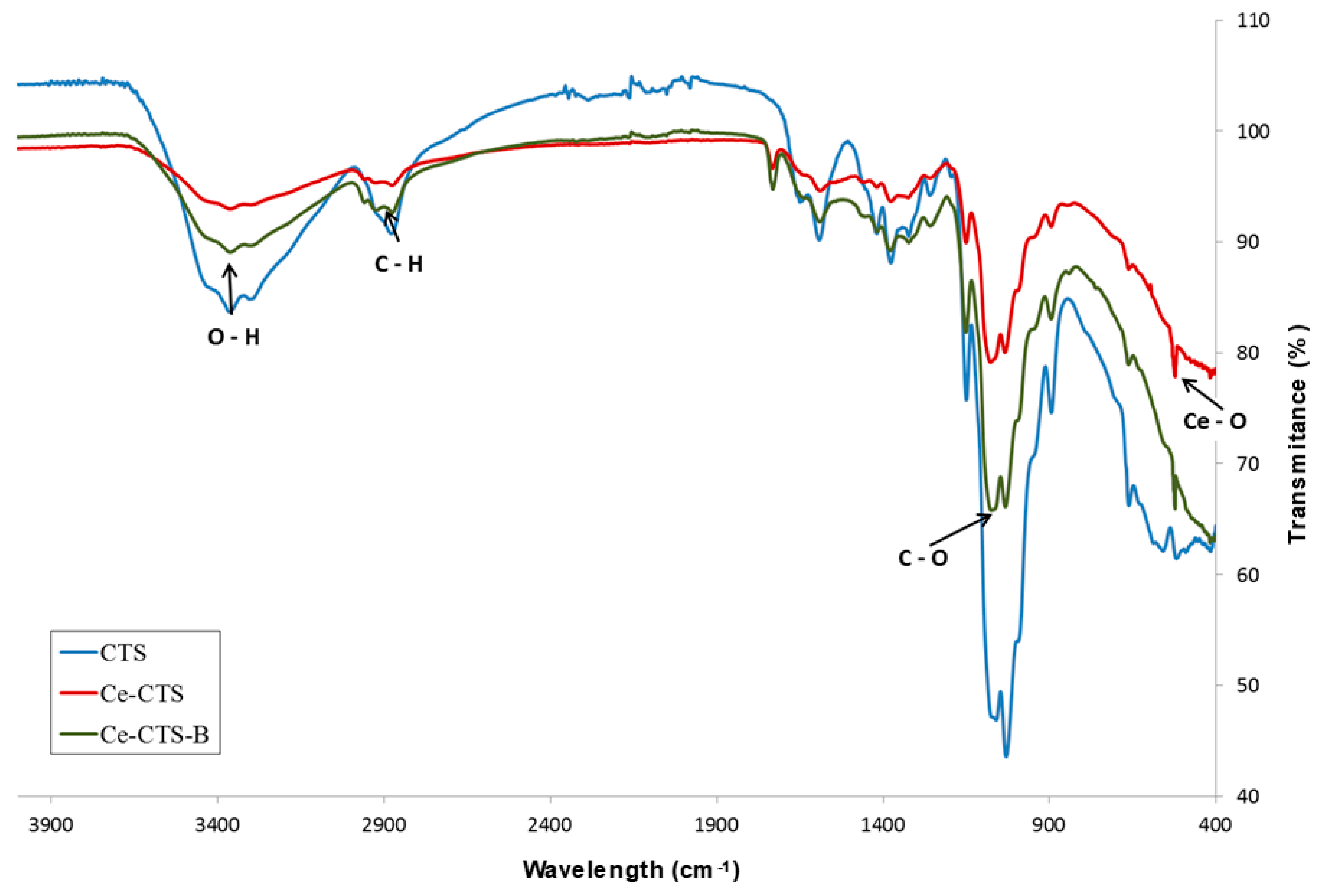
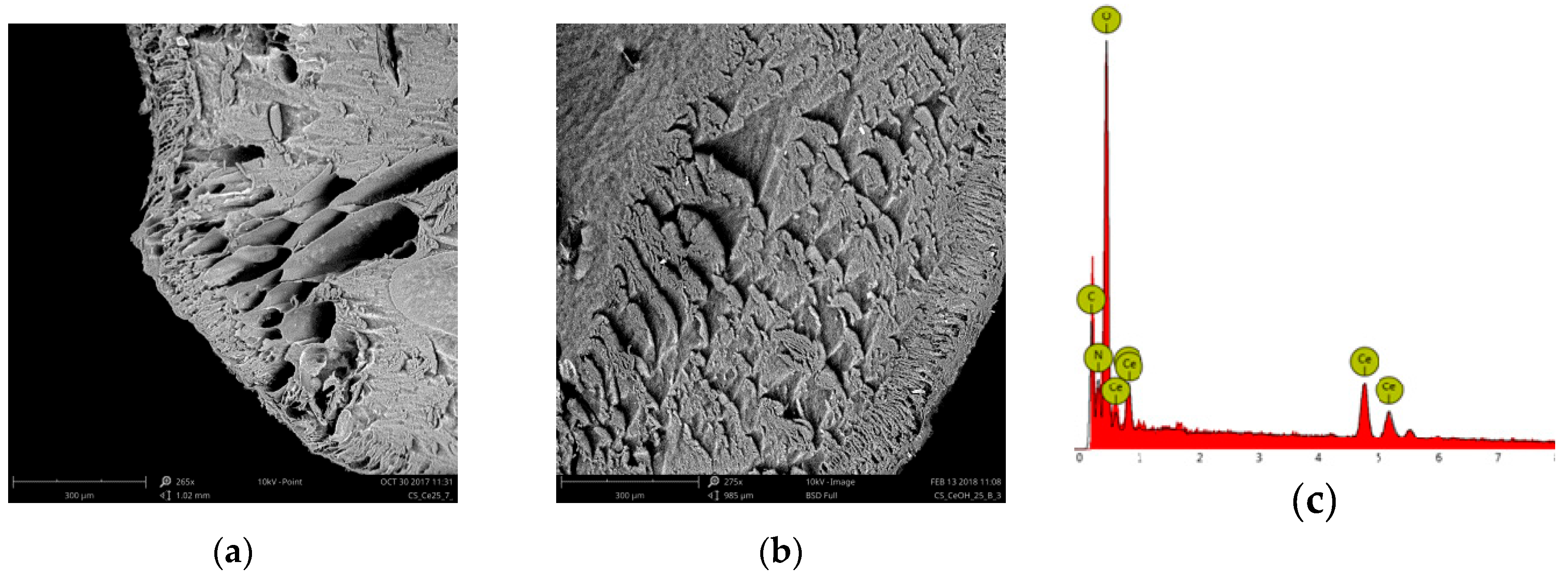
2.1. Ce-CTS Composite Characteristics
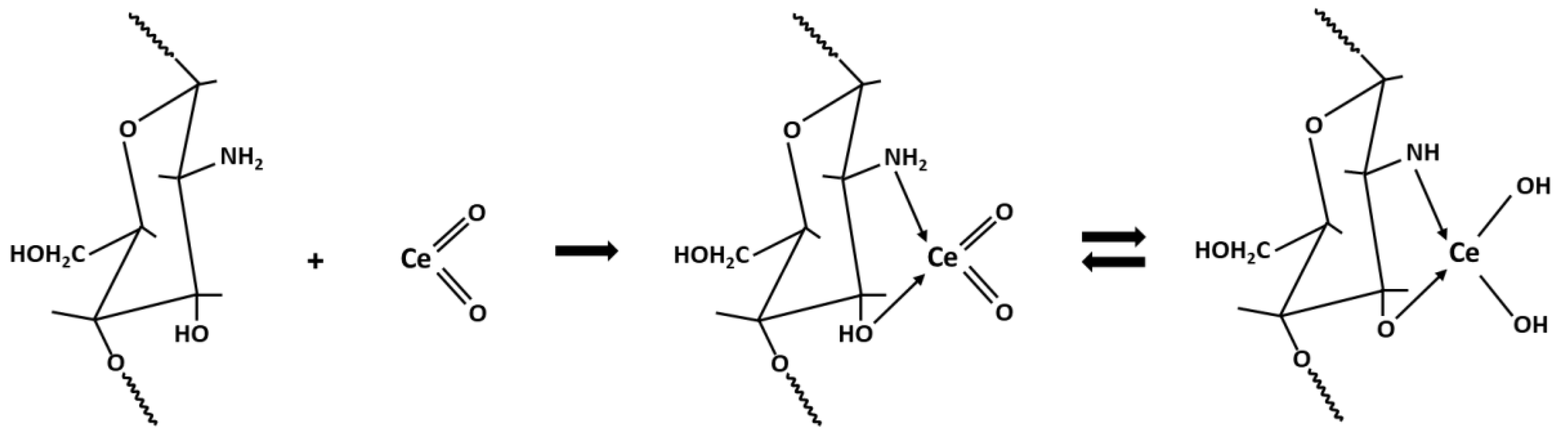
2.2. The Possible Mechanism of Ce-CTS Composite Formation
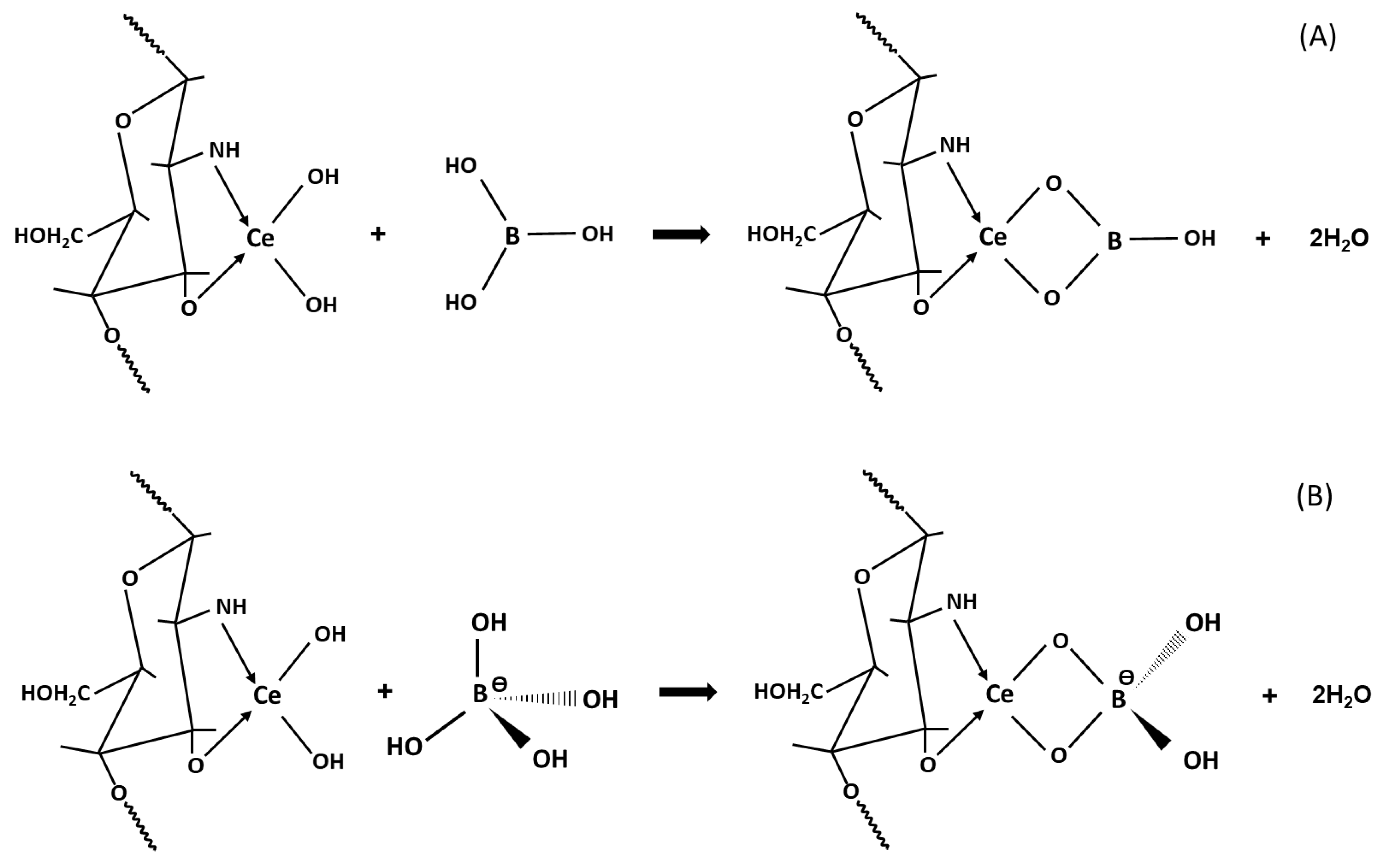
2.3. pH Study and the Mechanism of B Adsorption
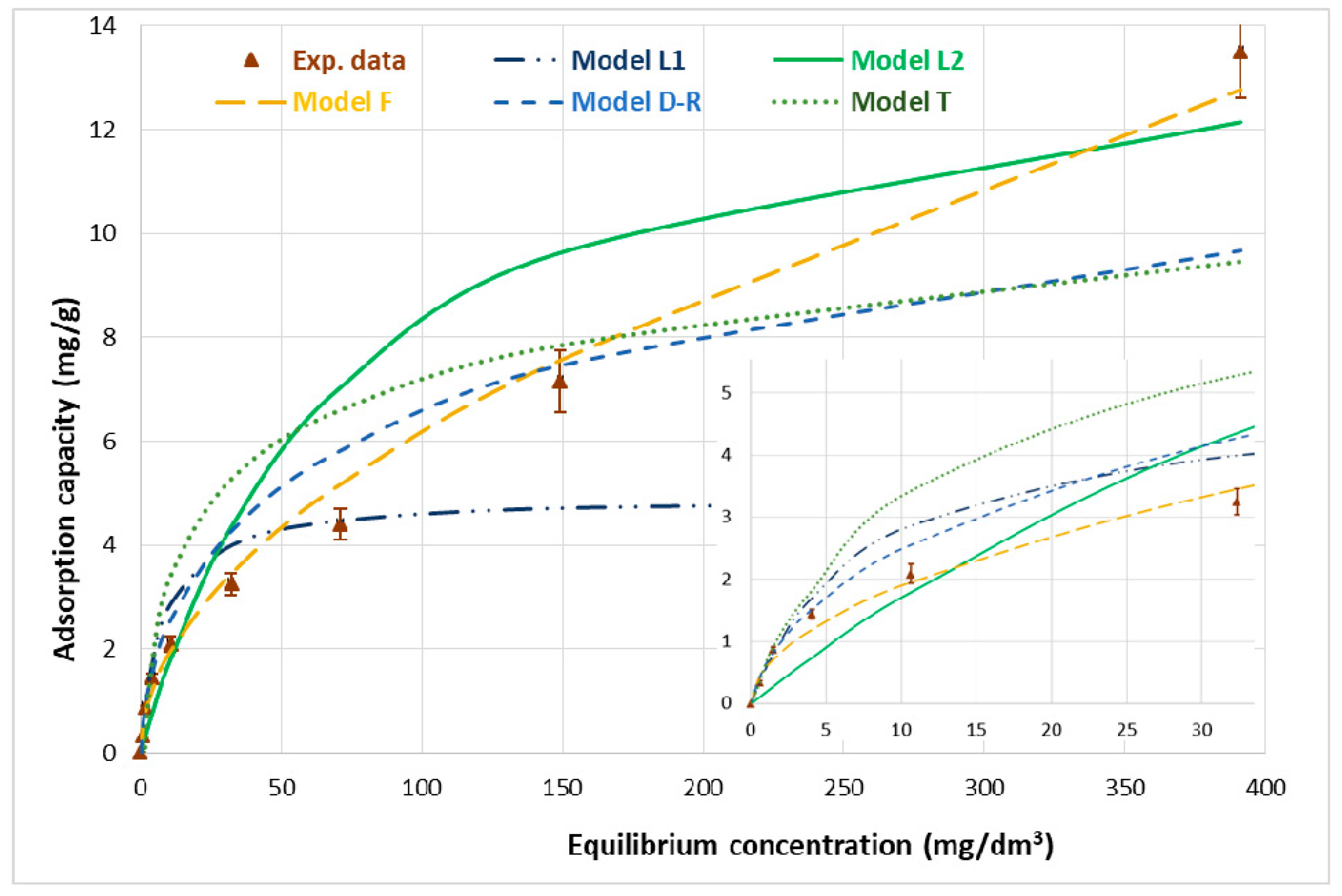
2.4. Isotherm Models
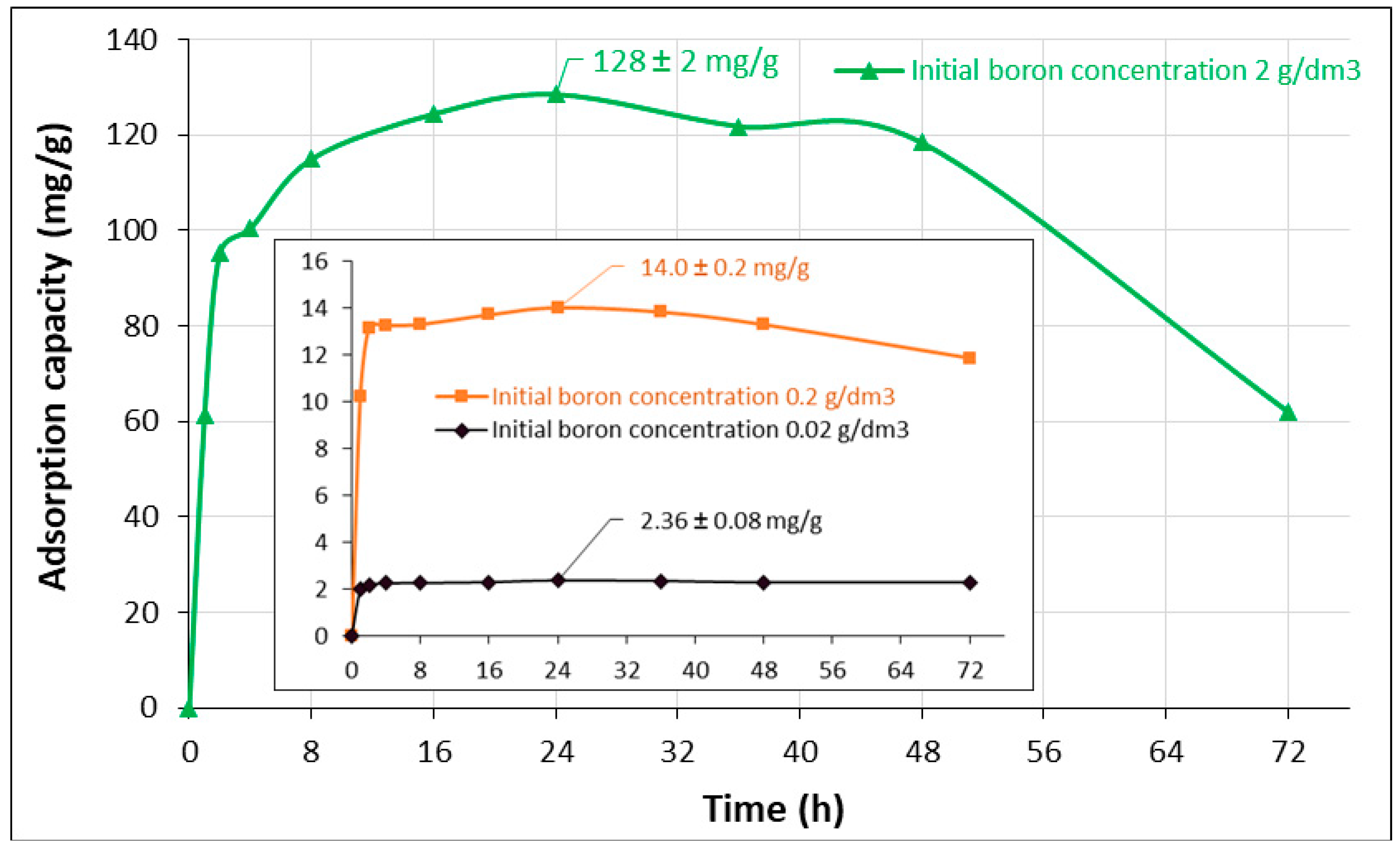
2.5. Kinetic Models
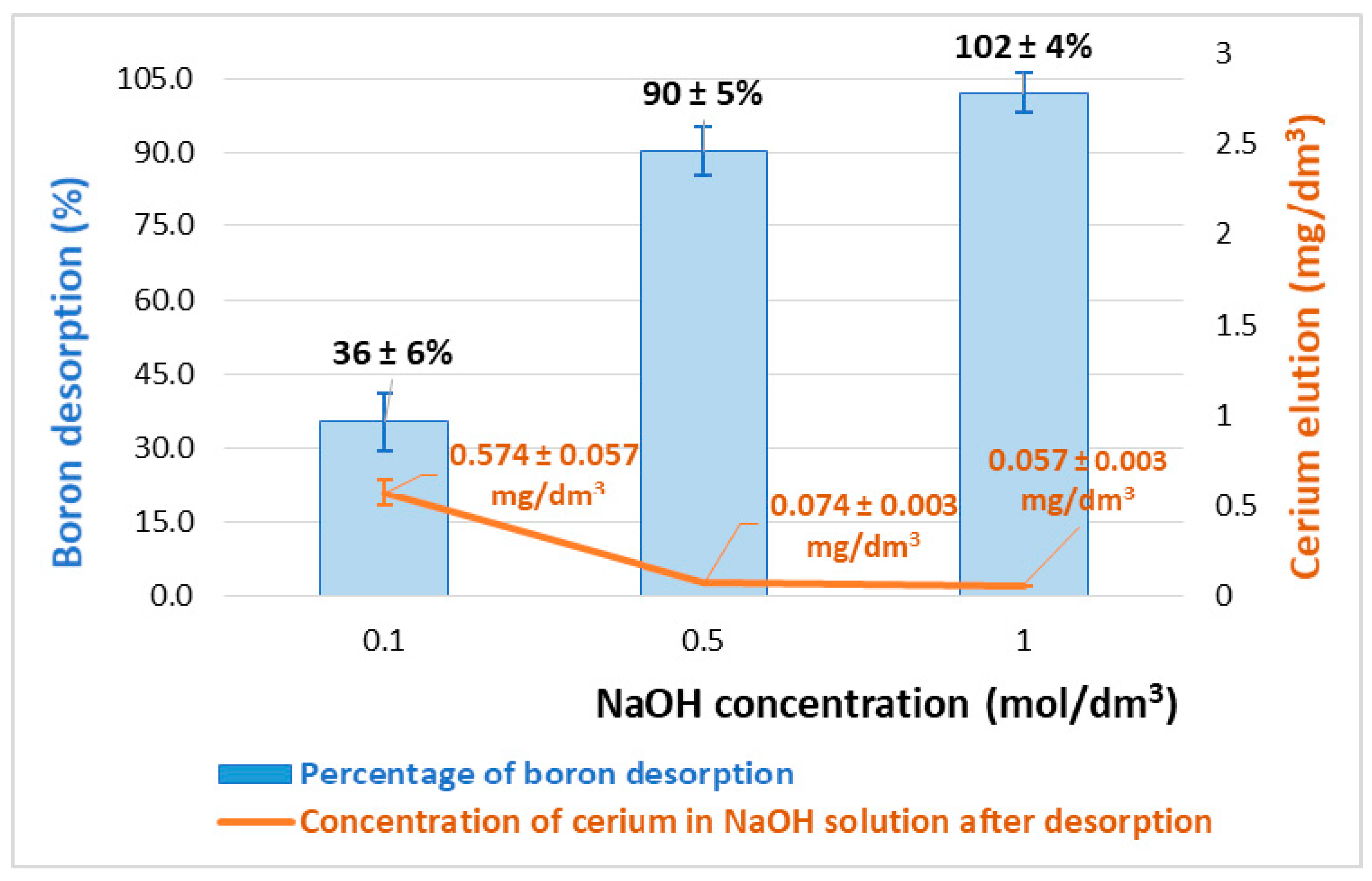
2.6. The Stability of the Ce-CTS and Desorption Tests
3. Materials and Methods
3.1. Reagents
3.2. Analytical Procedures and Apparatus
3.3. Ce-CTS Composite Preparation
3.4. Adsorption and Desorption Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTS | Unmodified chitosan |
| Ce-CTS | Chitosan composite supported with Ce(IV) |
| Ce-CTS-B | Chitosan composite supported with Ce(IV) after boron adsorption |
| FTIR | Fourier-transform infrared spectrometer |
| SEM-EDS | Scanning electron microscope with energy dispersive spectrometer |
| XRD | X-ray diffractometer |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| L model | Langmuir model |
| F model | Freundlich model |
| T model | Temkin model |
References
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska-Grzesik, E.; Ciba, J.; Grossman, A.; Kluczka, J.; Trojanowska, J.; Zołotajkin, M. Chemical Elements. Compendium; 2 Theta: Cesky Tesin, Czech Republic, 2013. [Google Scholar]
- Guan, Z.; Lv, J.; Bai, P.; Guo, X. Boron removal from aqueous solutions by adsorption—A review. Desalination 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Shang, X.; Jiang, H.; Wang, Q.; Liu, P.; Xie, F. Cellulose-starch Hybrid Films Plasticized by Aqueous ZnCl2 Solution. Int. J. Mol. Sci. 2019, 20, 474. [Google Scholar] [CrossRef]
- Jakobik-Kolon, A.; Mitko, K.; Bok-Badura, J. Zinc Sorption Studies on Pectin-Based Biosorbents. Materials 2017, 10, 84. [Google Scholar] [CrossRef]
- Kluczka, J. Reactive Polymers in Mercury Removal from Electrolytic Brine. Sep. Sci. Technol. 2009, 44, 3698–3716. [Google Scholar] [CrossRef]
- Smolik, M.; Zolotajkin, M.; Kluczka, J. Distribution of trace amounts of impurities during manganese(II) sulfate crystallization at 20-degrees-c and 2-degrees-c. Pol. J. Chem. 1995, 69, 1322–1327. [Google Scholar]
- Celik, Z.; Can, B.; Kocakerim, M. Boron removal from aqueous solutions by activated carbon impregnated with salicylic acid. J. Hazard. Mater. 2008, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Roslan, N.; Yaacub, N.; Latif, M. Boron Removal from Aqueous Solution Using Curcumin-impregnated Activated Carbon. Sains Malays. 2013, 42, 1293–1300. [Google Scholar]
- Zohdi, N.; Mahdavi, F.; Abdullah, L.; Choong, T. Removal of boron from aqueous solution using magnetic carbon nanotube improved with tartaric acid. J. Environ. Health Sci. Eng. 2014, 12. [Google Scholar] [CrossRef]
- Karahan, S.; Yurdakoc, M.; Seki, Y.; Yurdakoc, K. Removal of boron from aqueous solution by clays and modified clays. J. Colloid Interface Sci. 2006, 293, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Trojanowska, J.; Zolotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin. Water Treat. 2015, 54, 1839–1849. [Google Scholar] [CrossRef]
- Demetriou, A.; Pashalidis, I.; Nicolaides, A.; Kumke, M. Surface mechanism of the boron adsorption on alumina in aqueous solutions. Desalin. Water Treat. 2013, 51, 6130–6136. [Google Scholar] [CrossRef]
- Kluczka, J. Boron Removal from Aqueous Solutions using an Amorphous Zirconium Dioxide. Int. J. Environ. Res. 2015, 9, 711–720. [Google Scholar]
- Kluczka, J.; Gnus, M.; Dudek, G.; Turczyn, R. Removal of boron from aqueous solution by composite chitosan beads. Sep. Sci. Technol. 2017, 52, 1559–1571. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Boron removal from water and its recovery using iron (Fe+3) oxide/hydroxide-based nanoparticles (NanoFe) and NanoFe-impregnated granular activated carbon as adsorbent. Desalination 2014, 333, 107–117. [Google Scholar] [CrossRef]
- Yuksel, S.; Yurum, Y. Removal of Boron from Aqueous Solutions by Adsorption Using Fly Ash, Zeolite, and Demineralized Lignite. Sep. Sci. Technol. 2010, 45, 105–115. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Salih, N. Application of eggshell wastes for boron remediation from water. J. Mol. Liq. 2018, 256, 599–610. [Google Scholar] [CrossRef]
- Elwakeel, K. Environmental Application of Chitosan Resins for the Treatment of Water and Wastewater: A Review. J. Dispers. Sci. Technol. 2010, 31, 273–288. [Google Scholar] [CrossRef]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.; Sivasurian, N.; Meenakshi, S. Efficacy of La3+ entrapped chitosan bio-polymeric matrix for the recovery of oil from oil-in-water emulsion. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Desbrieres, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Barbusiński, K.; Salwiczek, S.; Paszewska, A. The use of chitosan for removing selected pollutants from water and wastewater—short review. Archit. Civ. Eng. Environ. 2016, 9, 107–115. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. Effective removal of nitrate and phosphate anions from aqueous solutions using functionalised chitosan beads. Desalin. Water Treat. 2014, 52, 2583–2593. [Google Scholar] [CrossRef]
- Bursali, E.A.; Seki, Y.; Seyhan, S.; Delener, M.; Yurdakoc, M. Synthesis of Chitosan Beads as Boron Sorbents. J. Appl. Polym. Sci. 2011, 122, 657–665. [Google Scholar] [CrossRef]
- Pakdel, P.; Peighambardoust, S. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhang, Y.; Wang, D.; Xu, Y.; Luo, L. Preparation of mixed rare earths modified chitosan for fluoride adsorption. J. Rare Earths 2013, 31, 817–822. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Huang, R.; Wang, G. Synthesis of a novel Ce(III)-incorporated cross-linked chitosan and its effective removal of fluoride from aqueous solution. J. Rare Earths 2016, 34, 1053–1061. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.; Meenakshi, S. Facile synthesis of metal incorporated chitin for the recovery of oil from oil-in-water emulsion using adsorptive method. J. Clean. Prod. 2016, 139, 1339–1350. [Google Scholar] [CrossRef]
- Zhu, T.; Zhu, T.; Gao, J.; Zhang, L.; Zhang, W. Enhanced adsorption of fluoride by cerium immobilized cross-linked chitosan composite. J. Fluor. Chem. 2017, 194, 80–88. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Q.; Wang, J.; Huang, R. Phosphate removal by Ce(III)-impregnated crosslinked chitosan complex from aqueous solutions. Polym. Eng. Sci. 2017, 57, 44–51. [Google Scholar] [CrossRef]
- Wang, F.; Ge, M. Fibrous mat of chitosan/polyvinyl alcohol/containing cerium(III) for the removal of chromium(VI) from aqueous solution. Text. Res. J. 2013, 83, 628–637. [Google Scholar] [CrossRef]
- Ansari, A.A.; Kaushik, A. Synthesis and optical properties of nanostructured Ce(OH)4. J. Semicond. 2010, 31, 033001. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayaman, V.; Chandarshekar, B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1987. [Google Scholar]
- Elanchezhiyan, S.; Prabhu, S.M.; Meenakshi, S. Effective adsorption of oil droplets from oil-in-water emulsion using metal ions encapsulated biopolymers: Role of metal ions and their mechanism in oil removal. Int. J. Biol. Macromol. 2018, 112, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Liu, X.; Zhang, W. Simultaneous oxidation and adsorption of As(III) from water by cerium modified chitosan ultrafine nanobiosorbent. J. Hazard. Mater. 2016, 308, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gazi, M.; Shahmohammadi, S. Removal of trace boron from aqueous solution using iminobis-(propylene glycol) modified chitosan beads. React. Funct. Polym. 2012, 72, 680–686. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Sastre, A.; Guibal, E. Development of a new chitosan/Ni(OH)(2)-based sorbent for boron removal. Chem. Eng. J. 2014, 244, 576–586. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Paderewski, M.L. Adsorption Processes in Chemical Engineering; WNT: Warszawa, Poland, 1999. [Google Scholar]
- Oladipo, A.; Gazi, M. Hydroxyl-enhanced magnetic chitosan microbeads for boron adsorption: Parameter optimization and selectivity in saline water. React. Funct. Polym. 2016, 109, 23–32. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations; McGraw Hill: New York, NY, USA, 1980. [Google Scholar]
- Wei, Y.T.; Zheng, Y.M.; Chen, J.P. Design and fabrication of an innovative and environmental friendly adsorbent for boron removal. Water Res. 2011, 45, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, W.; Jia, C.-Y.; Wang, L.; Li, H.-Y. Effect of pH on biosorption of boron onto cotton cellulose. Desalination 2007, 207, 257–267. [Google Scholar] [CrossRef]
- Kluczka, J.; Korolewicz, T.; Zołotajkin, M.; Simka, W.; Raczek, M. A new adsorbent for boron removal from aqueous solutions. Environ. Technol. 2013, 34, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.; Nallappan, M.; Ujang, Z. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review. React. Funct. Polym. 2014, 85, 54–68. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore and solid diffusion kinetics in fixed bed adsorption order constant pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Kluczka, J.; Torz, A.; Lacka, D.; Kazek-Kesik, A.; Adamek, J. Boron Removal by Adsorption on Cobalt(II) Doped Chitosan Bio-composite. J. Polym. Environ. 2018, 26, 2039–2048. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Rogacki, G.; Sujka, W.; Zarzycki, R. Sorption of copper by chitosan hydrogel: Kinetics and equilibrium. Chem. Eng. Process. 2016, 109, 104–113. [Google Scholar] [CrossRef]



| Sorbent/Parameter | Temp.(C) | pH | C0(B) (mg/dm3) | Adsorbent Dosage (g/dm3) | t (min) | qexpt (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Filler | Chitosan | ||||||
| NanoTiO2 | 25 | 4 | 20 | 50 | 5 | 4.3 | [15] |
| NanoCr2O3 | 25 | 4 | 20 | 50 | 5 | 3.5 | [15] |
| NanoFe3O4 | 25 | 4 | 20 | 50 | 5 | 4.4 | [15] |
| Fe(OH)3 | 25 | 4 | 20 | 50 | 5 | 7.8 | [15] |
| Without filler | 25 | 8 | 10 | 2 | 30 | 3.5 | [26] |
| Glycidol | 25 | 7 | 5.2 | 8.3 | 45 | 23.8 | [39] |
| Ni(OH)2 | 25 | 7 | 50 | 1 | 4320 | 61.4 *** | [40] |
| NMDG * | 20 | 7 | 4.8 | 0.5 | 480 | 35.1 | [45] |
| Co(OH)2 | 25 | 8.5 | 20 | 50 | 60 | 2.5 | [46] |
| Ce(OH)4 | 20 | 7 | 500 | 100 | 2880 | 13.5 | This paper |
| Other sorbents | |||||||
| Amberlite IRA-743 | 30 | 9.5 | 40 | 10 | 7.5 | [3] | |
| AC ** and Salicylic Acid | 25 | 8 | 25 | 20 | 30 | 0.325 | [8] |
| AC ** and Curcumin | 25 | 5.5 | 1000 | 40 | 120 | 5.05* | [9] |
| Fly ash zeolite | 25 | 7 | 50 | 20 | 30 | 2.3 | [12] |
| Cotton cellulose | 25 | 7 | 500 | 20 | 480 | 11.3 | [47] |
| Clinoptylolite | 25 | 8 | 50 | 20 | 30 | 0.155 | [48] |
| Clinoptylolite and ZrO2 | 25 | 8 | 50 | 20 | 30 | 1.03 | [48] |
| Purolite S 108 | 30 | 9.2 | **** | 2 | **** | 6.2 | [49] |
| Isotherm Model | Parameters of the Isotherm Models (qexpt = 13.5 ± 0.9 mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| L | qm (mg/g) | B (dm3/mg) | R2 | ARE (%) | ||||
| 4.95 | 0.129 | 0.9897 | 23.9 | |||||
| RL for initial boron concentration (mg/dm3): | ||||||||
| 2 | 5 | 10 | 20 | 50 | 100 | 200 | 500 | |
| 0.795 | 0.608 | 0.437 | 0.279 | 0.134 | 0.072 | 0.037 | 0.015 | |
| F | KF (mg/g) | n | R2 | ARE (%) | ||||
| 0.584 | 1.95 | 0.9810 | 13.2 | |||||
| D–R | Xm (mg/g) | E (kJ/mol) | R2 | ARE (%) | ||||
| 14.4 | −9.13 | 0.9709 | 26.7 | |||||
| T | AT | bT × 10−3 | R2 | ARE (%) | ||||
| 0.727 | 1.48 | 0.7651 | 41.0 | |||||
| Initial Boron Concentration | Experimental Capacity | Parameters of the Kinetic Models | |||
|---|---|---|---|---|---|
| Pseudo-first-order | |||||
| C0(B) (mg/dm3) | qexpt (mg/g) | q1 (mg/g) | K1 (g/(mg∙h)) | R12 | |
| 20 | 2.36 | 0.148 | −0.0368 | 0.1234 | |
| 200 | 14.0 | 1.026 | 0.0394 | 0.1927 | |
| 2000 | 128.5 | 58.425 | 0.1697 | 0.9864 | |
| Pseudo-second-order | |||||
| C0(B) (mg/dm3) | qexpt (mg/g) | q2 (mg/g) | k2 (g/(mg∙h)) | R22 | r (mg/(g∙h)) |
| 20 | 2.36 | 2.37 | 1.540 | 0.9996 | 8.65 |
| 200 | 14.0 | 14.1 | 0.2167 | 0.9997 | 43.1 |
| 2000 | 128.5 | 133.3 | 0.0065 | 0.9995 | 114.9 |
| Intraparticle diffusion model | |||||
| C0(B) (mg/dm3) | qexpt (mg/g) | q3 (mg/g) | Kp (mg/(g∙(h)1/2)) | R32 | |
| 20 | 2.36 | 2.01 | 0.076 | 0.7273 | |
| 200 | 14.0 | 11.2 | 0.650 | 0.5269 | |
| 2000 | 128.5 | 65.0 | 14.5 | 0.8018 | |
| Model combining the chemical reaction with intraparticle diffusion | |||||
| C0(B) (mg/dm3) | qexpt (mg/g) | q4 (mg/g) | K4 (dm3/(g∙h)) | R42 | |
| 20 | 2.36 | 2.95 | 0.0099 | 0.9287 | |
| 200 | 14.0 | 103.5 | 0.0060 | 0.9081 | |
| 2000 | 128.5 | 1577 | 0.0034 | 0.8925 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluczka, J.; Dudek, G.; Kazek-Kęsik, A.; Gnus, M. Chitosan Hydrogel Beads Supported with Ceria for Boron Removal. Int. J. Mol. Sci. 2019, 20, 1567. https://doi.org/10.3390/ijms20071567
Kluczka J, Dudek G, Kazek-Kęsik A, Gnus M. Chitosan Hydrogel Beads Supported with Ceria for Boron Removal. International Journal of Molecular Sciences. 2019; 20(7):1567. https://doi.org/10.3390/ijms20071567
Chicago/Turabian StyleKluczka, Joanna, Gabriela Dudek, Alicja Kazek-Kęsik, and Małgorzata Gnus. 2019. "Chitosan Hydrogel Beads Supported with Ceria for Boron Removal" International Journal of Molecular Sciences 20, no. 7: 1567. https://doi.org/10.3390/ijms20071567





