Antifibrotic TSG-6 Expression Is Synergistically Increased in Both Cells during Coculture of Mesenchymal Stem Cells and Macrophages via the JAK/STAT Signaling Pathway
Abstract
:1. Introduction
2. Results
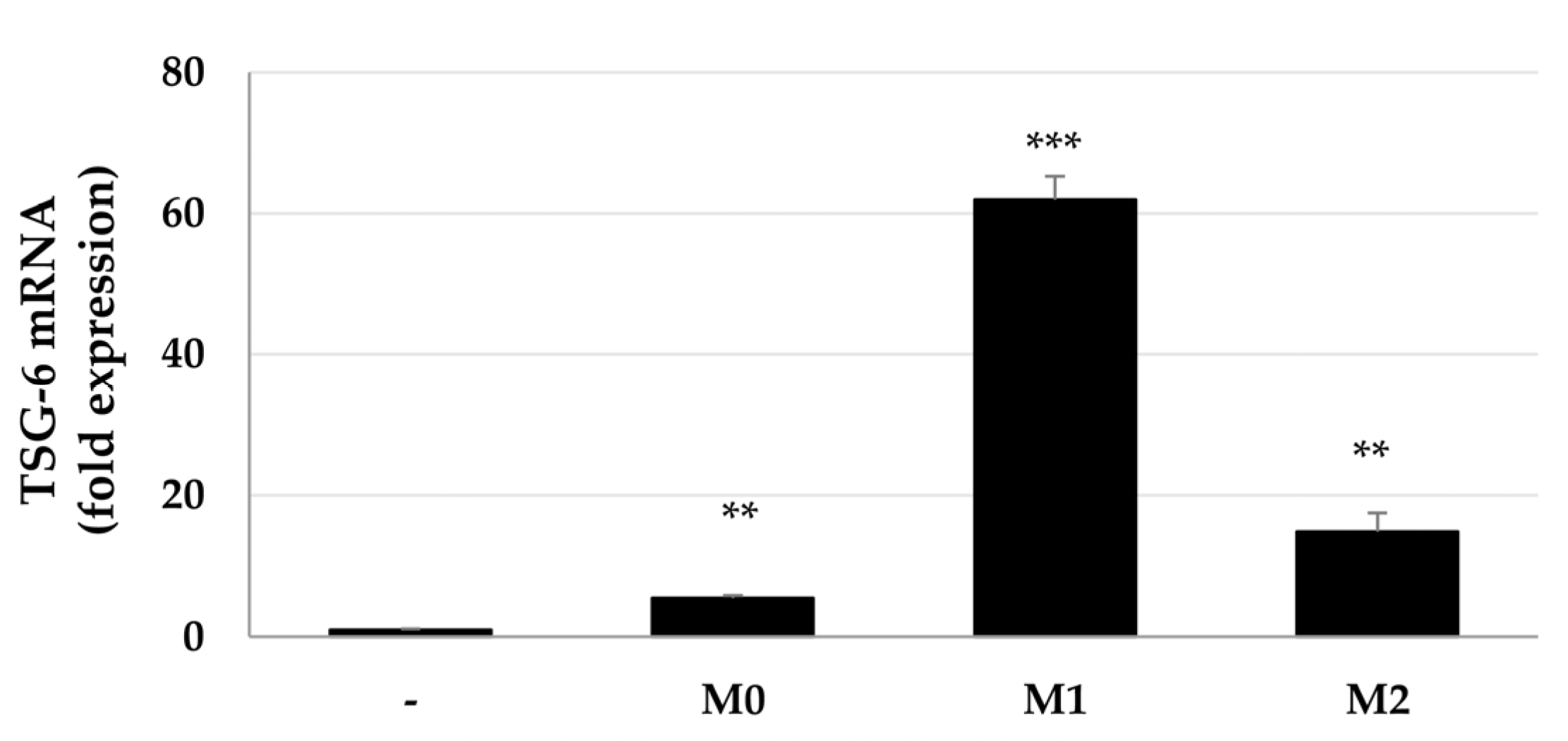
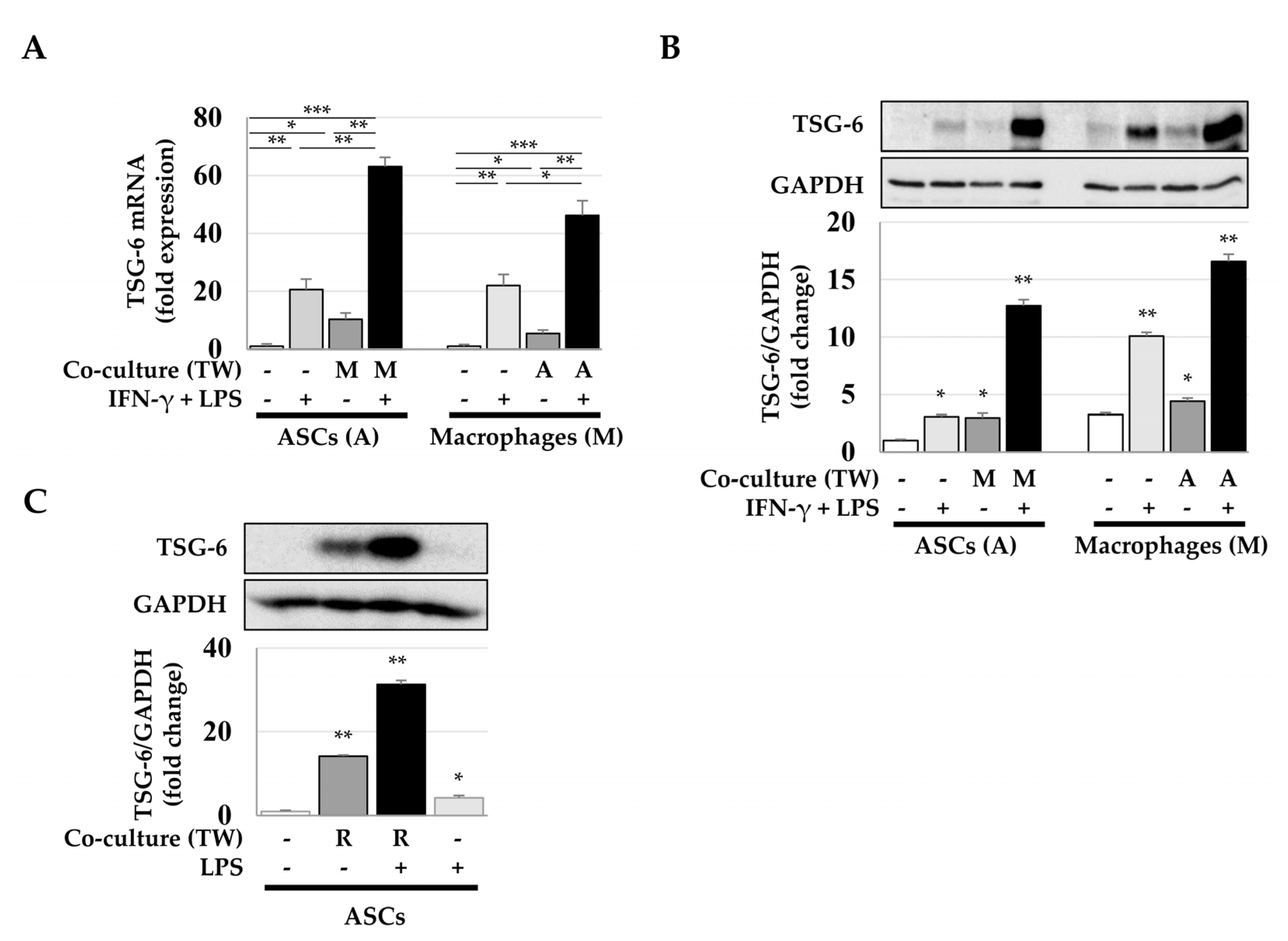
2.1. Increased Expression of TSG-6 mRNA in ASCs after Coculture with Macrophages
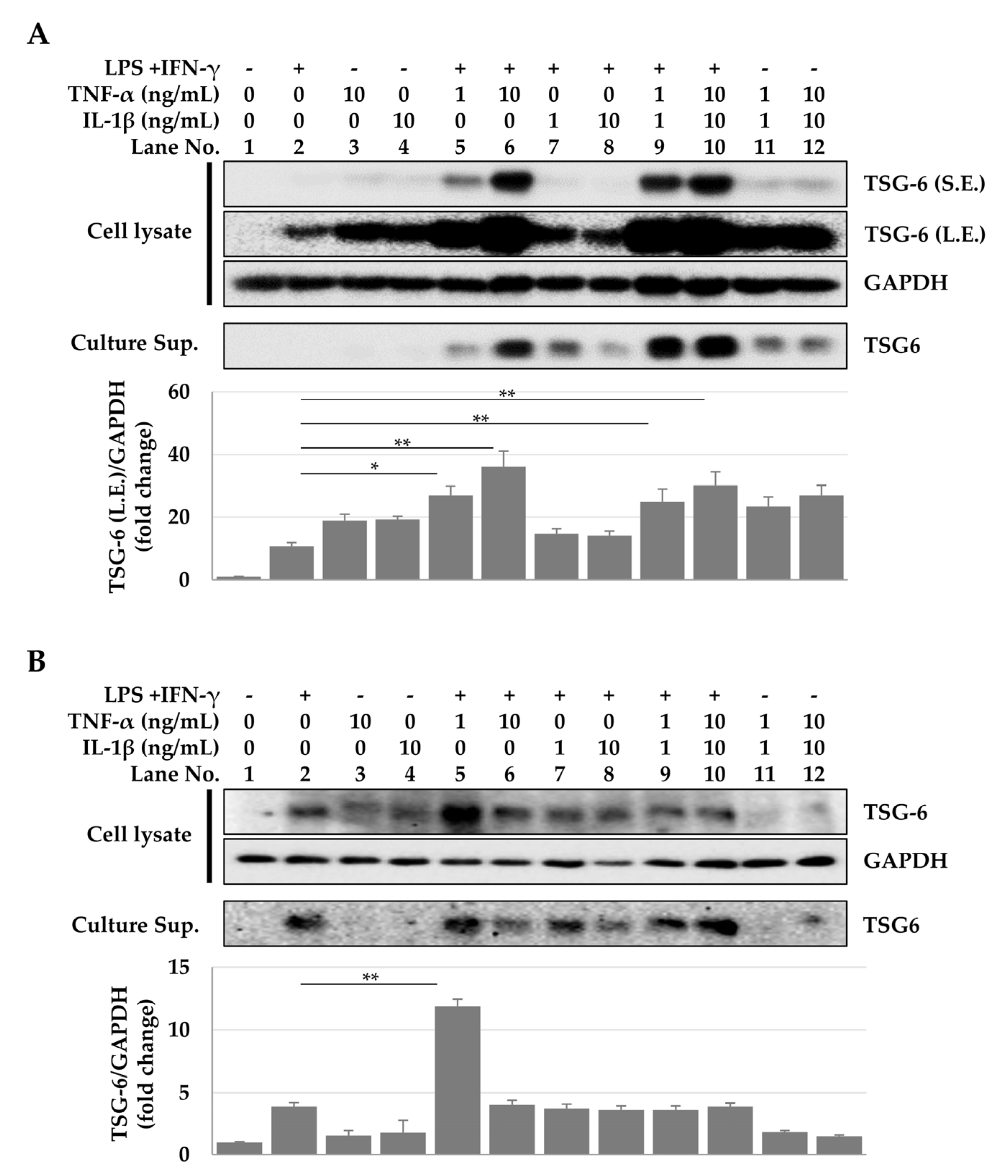
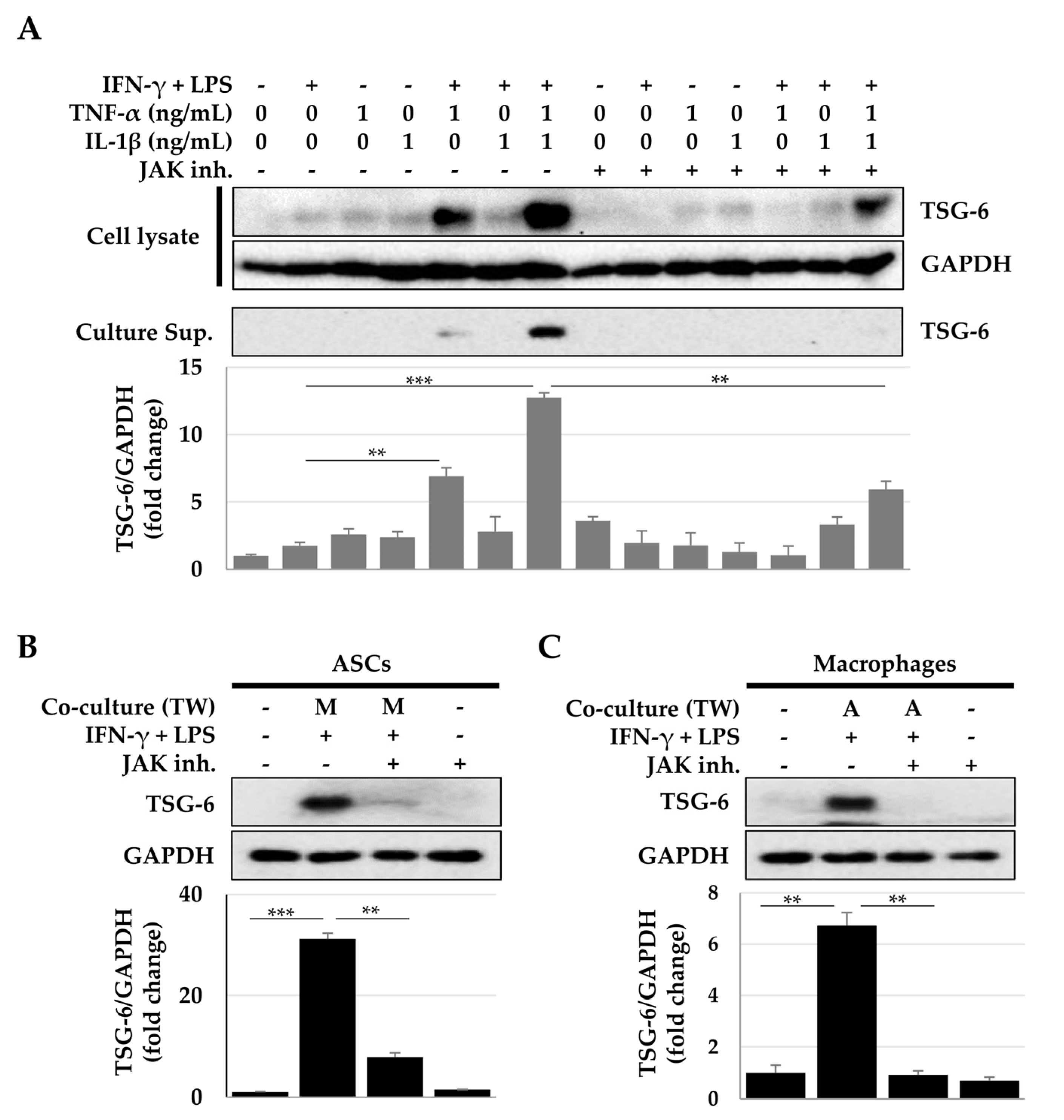
2.2. TSG-6 Expression in ASCs and Macrophages Treated with Cytokine Combinations
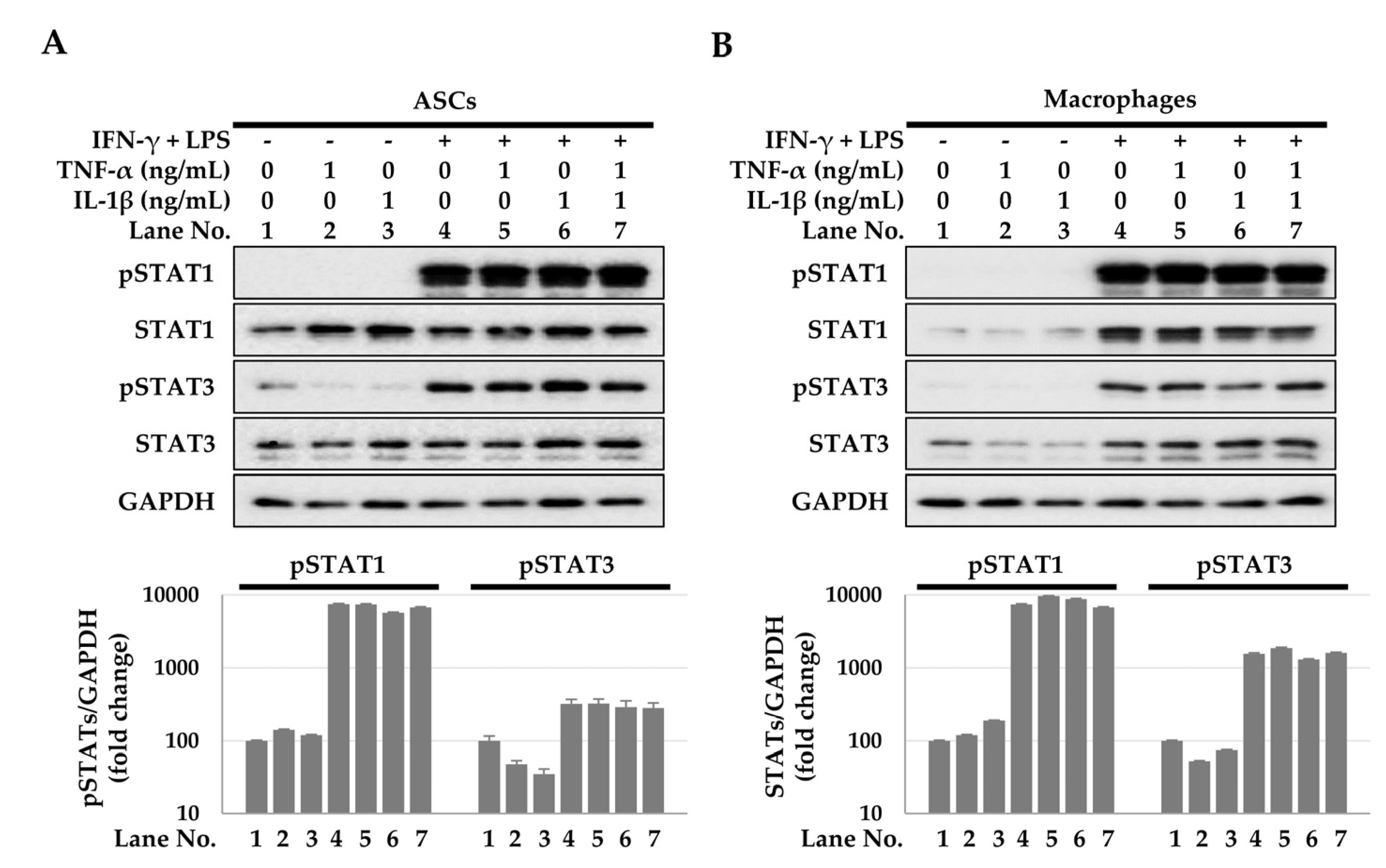
2.3. Activation of the Signal Transducer and Activator of Transcription (STAT) Signaling Pathway for TSG-6 Expression
2.4. Antifibrotic Effects of TSG-6 Expressed in ASCs and Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Macrophage Differentiation of THP-1 Monocytes
4.4. qPCR
4.5. Immunoblotting
4.6. Small Interfering RNA (siRNA) Treatment
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapel, A.; Bertho, J.M.; Bensidhoum, M.; Fouillard, L.; Young, R.G.; Frick, J.; Demarquay, C.; Cuvelier, F.; Mathieu, E.; Trompier, F.; et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003, 5, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Mouiseddine, M.; Francois, S.; Semont, A.; Sache, A.; Allenet, B.; Mathieu, N.; Frick, J.; Thierry, D.; Chapel, A. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br. J. Radiol 2007, 80, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Beynon, A.L.; Thome, J.; Coogan, A.N. Age and time of day influences on the expression of transforming growth factor-beta and phosphorylated SMAD3 in the mouse suprachiasmatic and paraventricular nuclei. Neuroimmunomodulation 2009, 16, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Roddy, G.W.; Choi, H.; Lee, R.H.; Ylostalo, J.H.; Rosa, R.H., Jr.; Prockop, D.J. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. USA 2010, 107, 16875–16880. [Google Scholar] [CrossRef] [Green Version]
- Roddy, G.W.; Oh, J.Y.; Lee, R.H.; Bartosh, T.J.; Ylostalo, J.; Coble, K.; Rosa, R.H., Jr.; Prockop, D.J. Action at a distance: Systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 2011, 29, 1572–1579. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef]
- Fujimoto, T.; Savani, R.C.; Watari, M.; Day, A.J.; Strauss, J.F., 3rd. Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2). Am. J. Pathol. 2002, 160, 1495–1502. [Google Scholar] [CrossRef]
- Ochsner, S.A.; Russell, D.L.; Day, A.J.; Breyer, R.M.; Richards, J.S. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology 2003, 144, 1008–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Liau, G. Identification of a novel serum and growth factor-inducible gene in vascular smooth muscle cells. J. Biol. Chem. 1993, 268, 21453. [Google Scholar] [CrossRef]
- Maier, R.; Wisniewski, H.G.; Vilcek, J.; Lotz, M. TSG-6 expression in human articular chondrocytes. Possible implications in joint inflammation and cartilage degradation. Arthritis Rheum 1996, 39, 552–559. [Google Scholar] [CrossRef]
- Ye, L.; Mora, R.; Akhayani, N.; Haudenschild, C.C.; Liau, G. Growth factor and cytokine-regulated hyaluronan-binding protein TSG-6 is localized to the injury-induced rat neointima and confers enhanced growth in vascular smooth muscle cells. Circ. Res. 1997, 81, 289–296. [Google Scholar] [CrossRef]
- Klampfer, L.; Lee, T.H.; Hsu, W.; Vilcek, J.; Chen-Kiang, S. NF-IL6 and AP-1 cooperatively modulate the activation of the TSG-6 gene by tumor necrosis factor alpha and interleukin-1. Mol. Cell. Biol. 1994, 14, 6561–6569. [Google Scholar] [CrossRef]
- Wisniewski, H.G.; Maier, R.; Lotz, M.; Lee, S.; Klampfer, L.; Lee, T.H.; Vilcek, J. TSG-6: A TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J. Immunol. 1993, 151, 6593–6601. [Google Scholar]
- Milner, C.M.; Higman, V.A.; Day, A.J. TSG-6: A pluripotent inflammatory mediator? Biochem. Soc. Trans. 2006, 34, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Choi, H.; Nishida, H.; Oh, J.Y.; Gregory, C.; Lee, R.H.; Yu, J.M.; Watanabe, J.; An, S.Y.; Bartosh, T.J.; et al. Scalable Production of a Multifunctional Protein (TSG-6) That Aggregates with Itself and the CHO Cells That Synthesize It. PLoS ONE 2016, 11, e0147553. [Google Scholar] [CrossRef]
- Lauer, M.E.; Cheng, G.; Swaidani, S.; Aronica, M.A.; Weigel, P.H.; Hascall, V.C. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J. Biol. Chem. 2013, 288, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Aprecio, R.M.; Zhou, X.; Wang, Q.; Zhang, W.; Ding, Y.; Li, Y. Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: A pilot study. PLoS ONE 2014, 9, e100285. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.S.; Hyun, J.; Kim, J.; Kim, S.U.; Cha, H.J.; Jung, Y. Tumor necrosis factor-inducible gene 6 promotes liver regeneration in mice with acute liver injury. Stem Cell Res. Ther. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, B.; Wang, H.; Tao, Q.; Ge, S.; Zhai, Z. Tumor necrosis factor alpha-stimulated gene-6 (TSG-6) inhibits the inflammatory response by inhibiting the activation of P38 and JNK signaling pathway and decreases the restenosis of vein grafts in rats. Heart Vessel. 2017, 32, 1536–1545. [Google Scholar] [CrossRef]
- Watanabe, R.; Sato, Y.; Ozawa, N.; Takahashi, Y.; Koba, S.; Watanabe, T. Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis. Int. J. Mol. Sci 2018, 19, 465. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Mindrescu, C.; Le, J.; Wisniewski, H.G.; Vilcek, J. Up-regulation of cyclooxygenase-2 expression by TSG-6 protein in macrophage cell line. Biochem. Biophys. Res. Commun. 2005, 330, 737–745. [Google Scholar] [CrossRef]
- Watanabe, R.; Watanabe, H.; Takahashi, Y.; Kojima, M.; Konii, H.; Watanabe, K.; Shirai, R.; Sato, K.; Matsuyama, T.A.; Ishibashi-Ueda, H.; et al. Atheroprotective Effects of Tumor Necrosis Factor-Stimulated Gene-6. JACC Basic Transl. Sci. 2016, 1, 494–509. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNFalpha-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Pei, Y.; Zeng, H.; Yang, Y.; Que, M.; Xiao, Y.; Wang, J.; Weng, X. Recombinant TSG-6 protein inhibits the growth of capsule fibroblasts in frozen shoulder via suppressing the TGF-beta/Smad2 signal pathway. J. Orthop. Surg. Res. 2021, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Usunier, B.; Brossard, C.; L’Homme, B.; Linard, C.; Benderitter, M.; Milliat, F.; Chapel, A. HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis. Int. J. Mol. Sci. 2021, 22, 1790. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Weng, X.J.; Li, X.J.; Tian, X.Y. TSG-6 Inhibits the Growth of Keloid Fibroblasts Via Mediating the TGF-beta1/Smad Signaling Pathway. J. Investig. Surg. 2021, 34, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Chan, C.K.; Braun, K.R.; Green, P.S.; O’Brien, K.D.; Chait, A.; Day, A.J.; Wight, T.N. Monocyte-to-macrophage differentiation: Synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 2012, 287, 14122–14135. [Google Scholar] [CrossRef] [Green Version]
- Yakymovych, I.; Ten Dijke, P.; Heldin, C.H.; Souchelnytskyi, S. Regulation of Smad signaling by protein kinase C. FASEB J. 2001, 15, 553–555. [Google Scholar] [CrossRef]
- Ryer, E.J.; Hom, R.P.; Sakakibara, K.; Nakayama, K.I.; Nakayama, K.; Faries, P.L.; Liu, B.; Kent, K.C. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 780–786. [Google Scholar] [CrossRef]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef] [Green Version]
- Milner, C.M.; Tongsoongnoen, W.; Rugg, M.S.; Day, A.J. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochem. Soc. Trans. 2007, 35, 672–676. [Google Scholar] [CrossRef]
- Heusinkveld, M.; de Vos van Steenwijk, P.J.; Goedemans, R.; Ramwadhdoebe, T.H.; Gorter, A.; Welters, M.J.; van Hall, T.; van der Burg, S.H. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J. Immunol. 2011, 187, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef]
- Kim, S.; Jung, P.Y.; Lee, J.S.; Hwang, S.; Sohn, J.H.; Yoon, Y.; Bae, K.S.; Eom, Y.W. Cultured human skeletal muscle satellite cells exhibit characteristics of mesenchymal stem cells and play anti-inflammatory roles through prostaglandin E2 and hepatocyte growth factors. Cell Biol. Int. 2021, 45, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, K.H.; Jang, I.K.; Lee, M.W.; Kim, H.E.; Yang, M.S.; Eom, Y.; Lee, J.E.; Kim, Y.J.; Yang, S.K.; Jung, H.L.; et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009, 259, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; O’Shea, J.J. Jaks and STATs: Biological implications. Annu. Rev. Immunol. 1998, 16, 293–322. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [Green Version]
- Damerau, A.; Gaber, T.; Ohrndorf, S.; Hoff, P. JAK/STAT Activation: A General Mechanism for Bone Development, Homeostasis, and Regeneration. Int. J. Mol. Sci. 2020, 21, 9004. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.C.; Yoon, Y.; Jung, P.Y.; Kim, M.Y.; Baik, S.K.; Ryu, H.; Eom, Y.W. Antifibrotic TSG-6 Expression Is Synergistically Increased in Both Cells during Coculture of Mesenchymal Stem Cells and Macrophages via the JAK/STAT Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 13122. https://doi.org/10.3390/ijms232113122
Gong SC, Yoon Y, Jung PY, Kim MY, Baik SK, Ryu H, Eom YW. Antifibrotic TSG-6 Expression Is Synergistically Increased in Both Cells during Coculture of Mesenchymal Stem Cells and Macrophages via the JAK/STAT Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(21):13122. https://doi.org/10.3390/ijms232113122
Chicago/Turabian StyleGong, Seong Chan, Yongdae Yoon, Pil Young Jung, Moon Young Kim, Soon Koo Baik, Hoon Ryu, and Young Woo Eom. 2022. "Antifibrotic TSG-6 Expression Is Synergistically Increased in Both Cells during Coculture of Mesenchymal Stem Cells and Macrophages via the JAK/STAT Signaling Pathway" International Journal of Molecular Sciences 23, no. 21: 13122. https://doi.org/10.3390/ijms232113122




