Protective Strategies of Haberlea rhodopensis for Acquisition of Freezing Tolerance: Interaction between Dehydration and Low Temperature
Abstract
:1. Introduction
2. Results
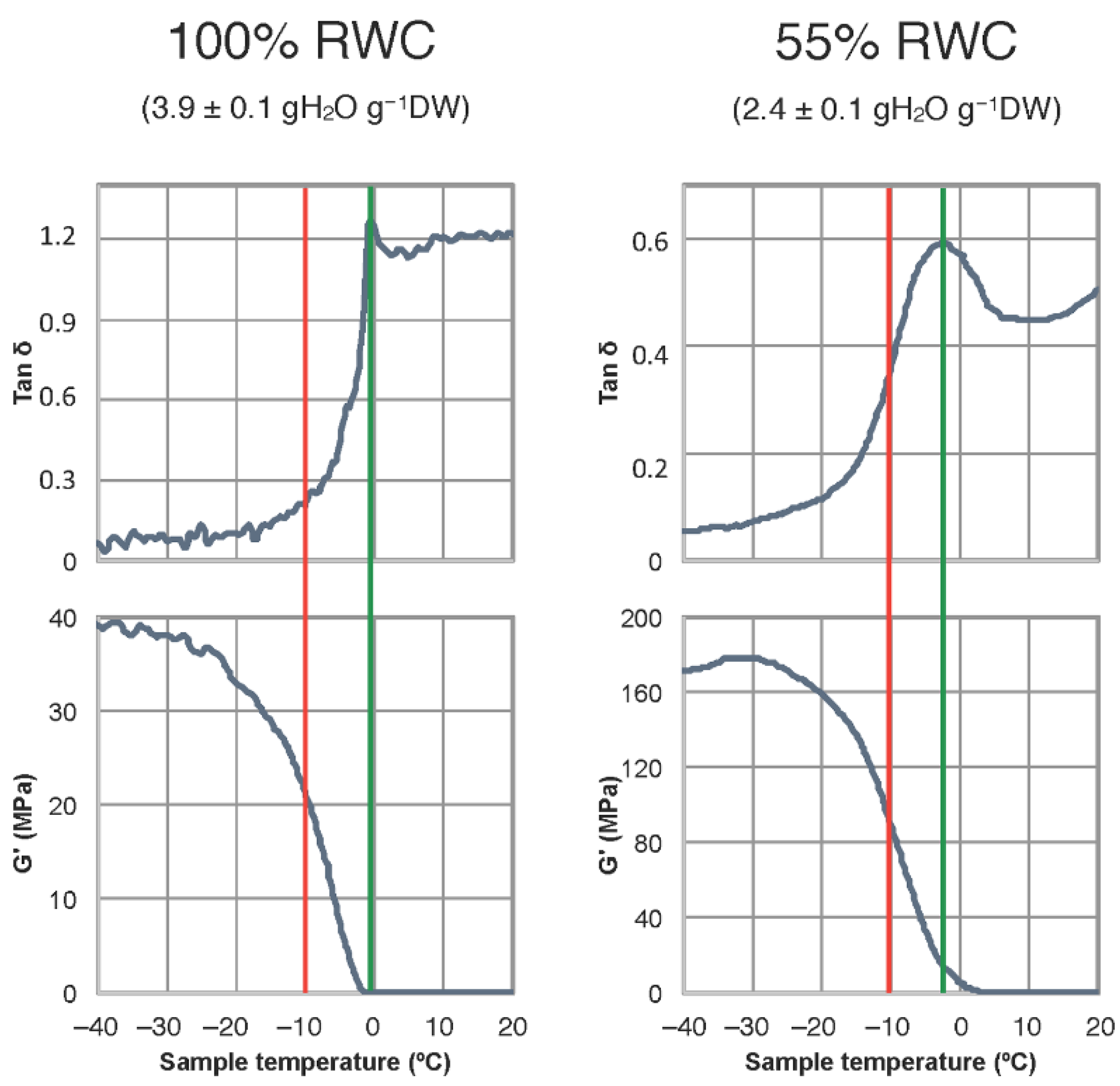
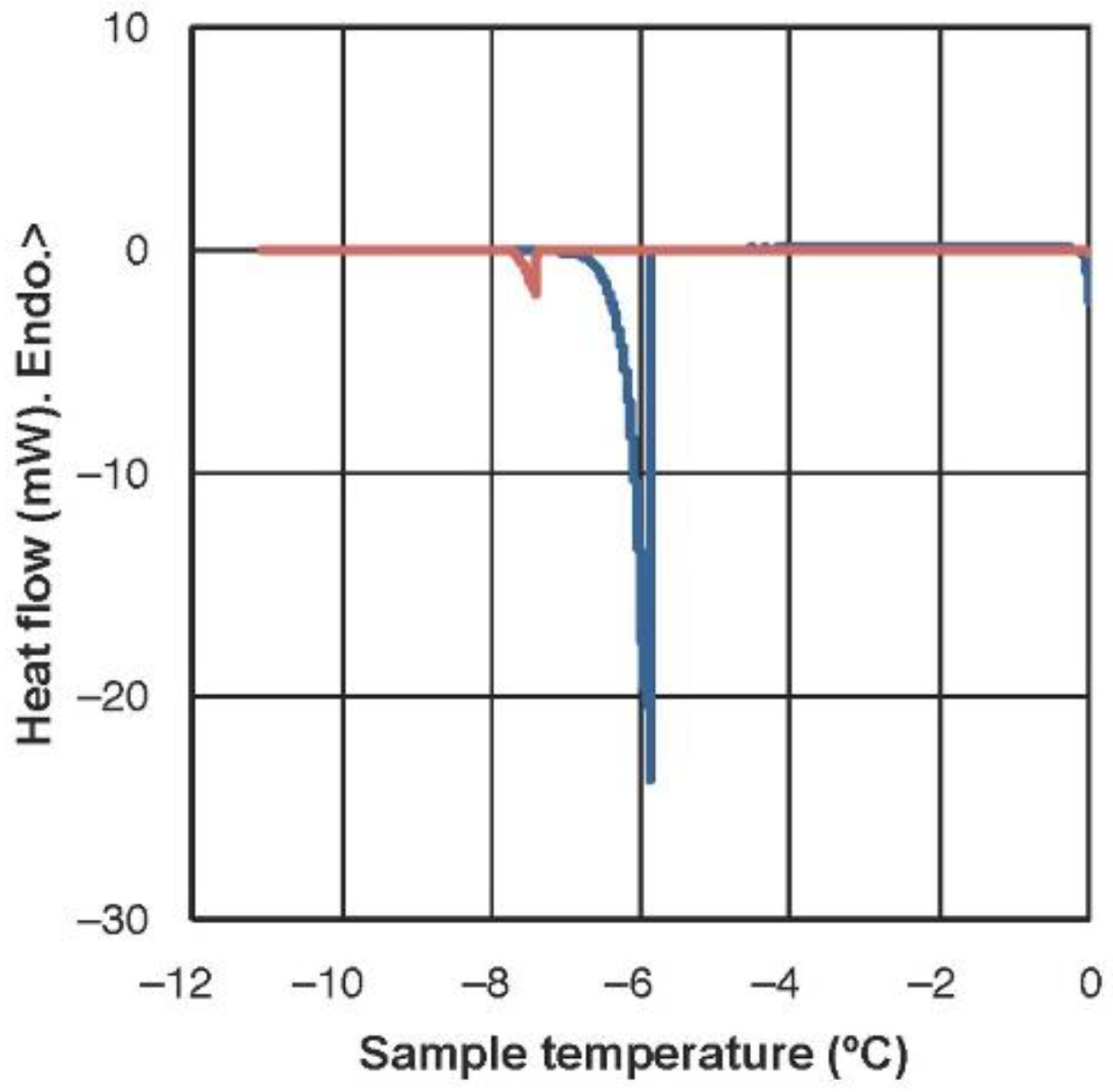
2.1. Freezing Temperature and Molecular Mobility in Winter Leaves of Haberlea Rhodopensis
2.2. Changes in Electrolyte Leakage and MDA Content during Cold Acclimation, Freezing Stress and Freezing-Induced Desiccation
2.2.1. Electrolyte Leakage (EL)
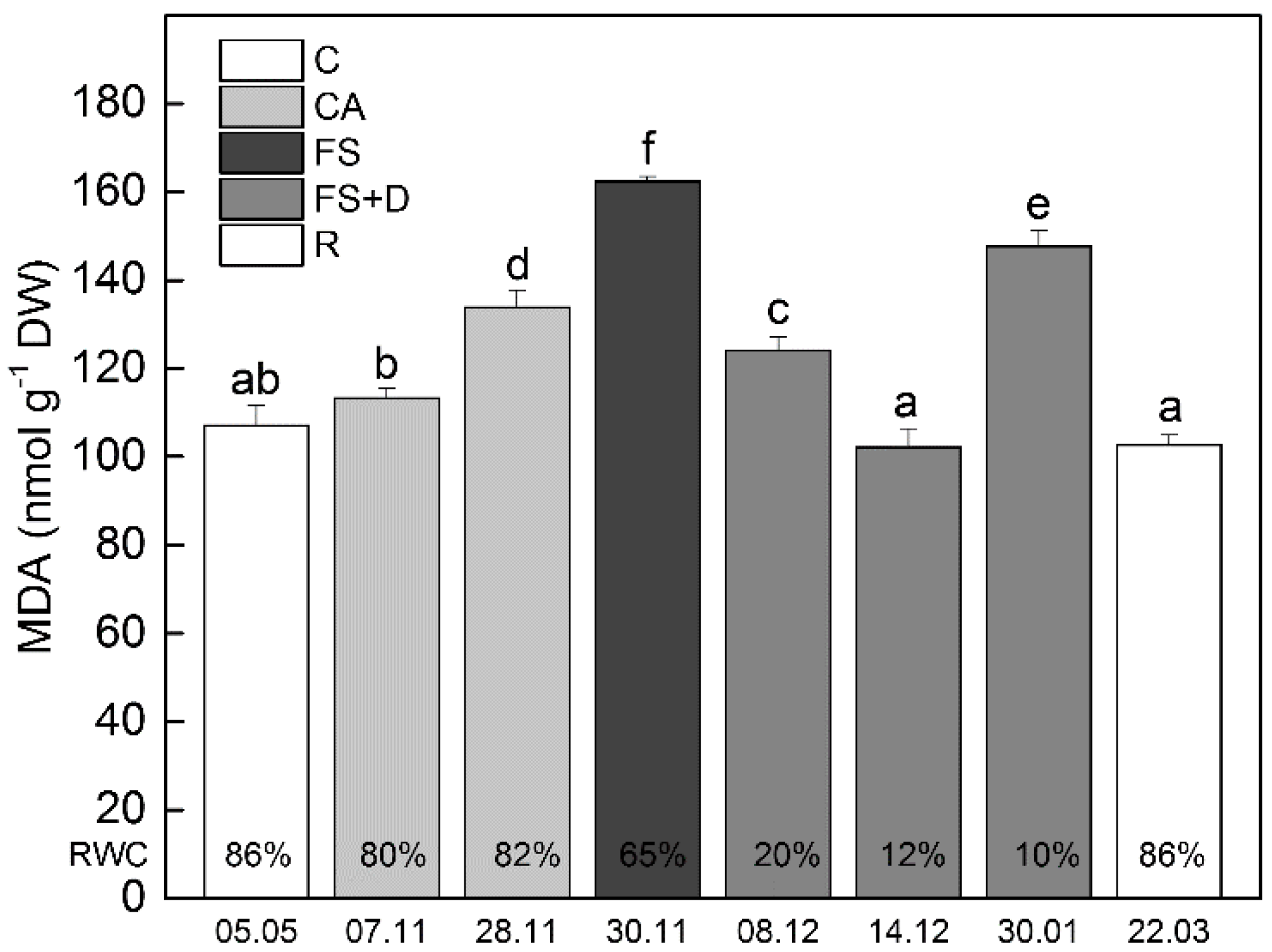
2.2.2. Malondialdehyde (MDA)
2.3. Changes in Fatty Acids Profile under CA, Freezing Stress, and Freezing-Induced Desiccation
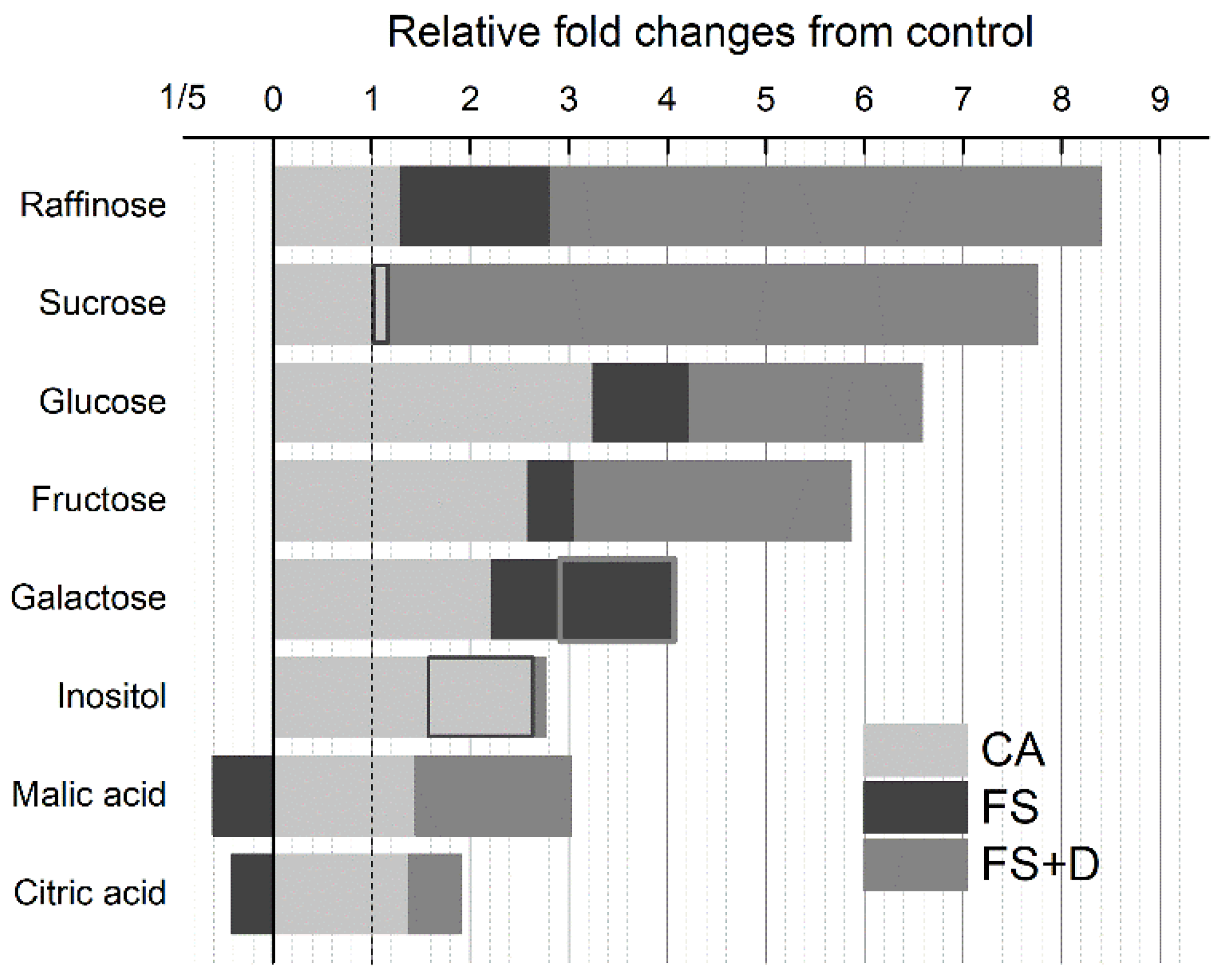
2.4. Importance of Proline and Carbohydrates for Freezing Tolerance
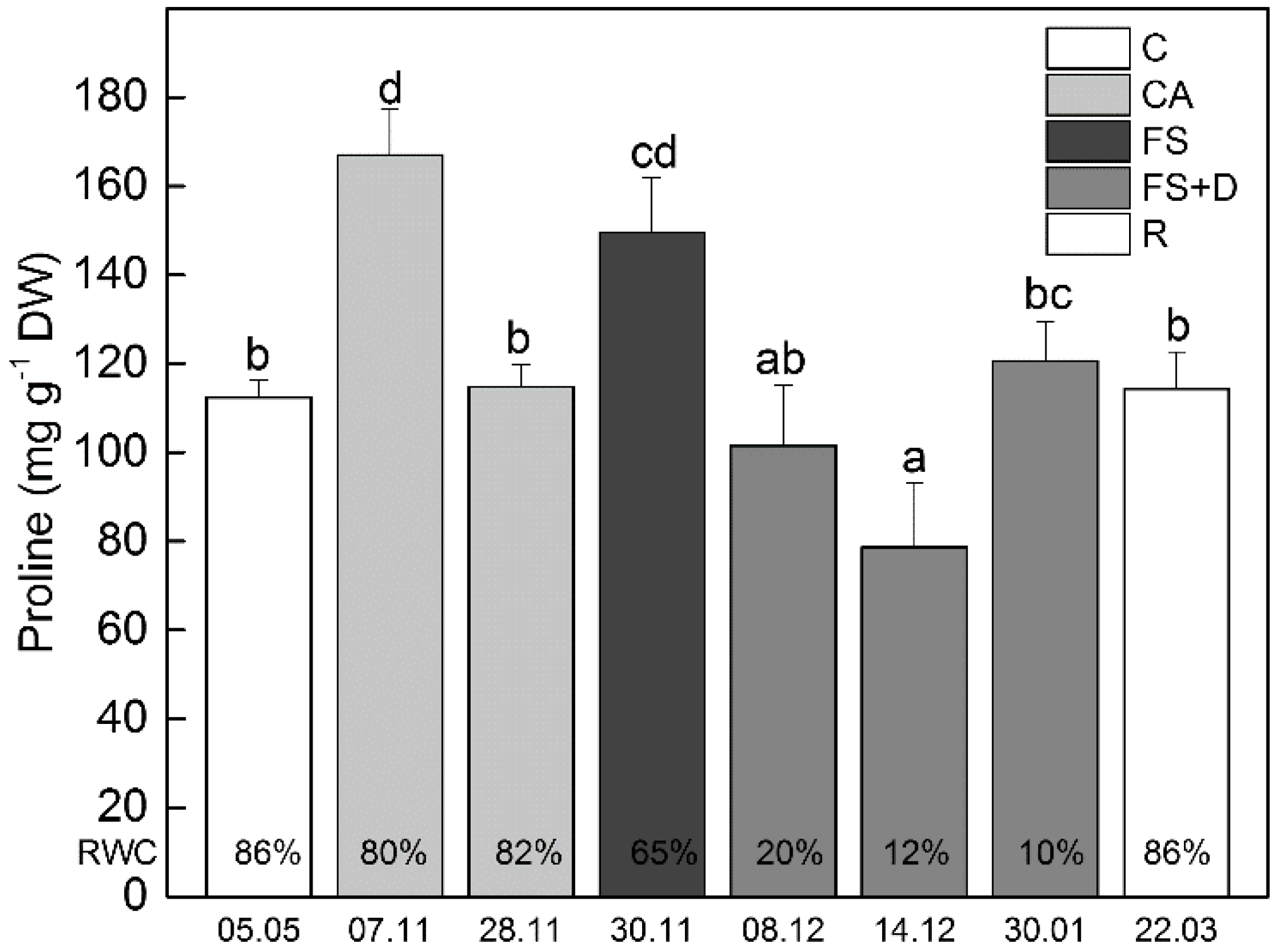
2.4.1. Proline
2.4.2. Carbohydrates
2.5. The Role of Protective Proteins for Freezing Tolerance of H. Rhodopensis
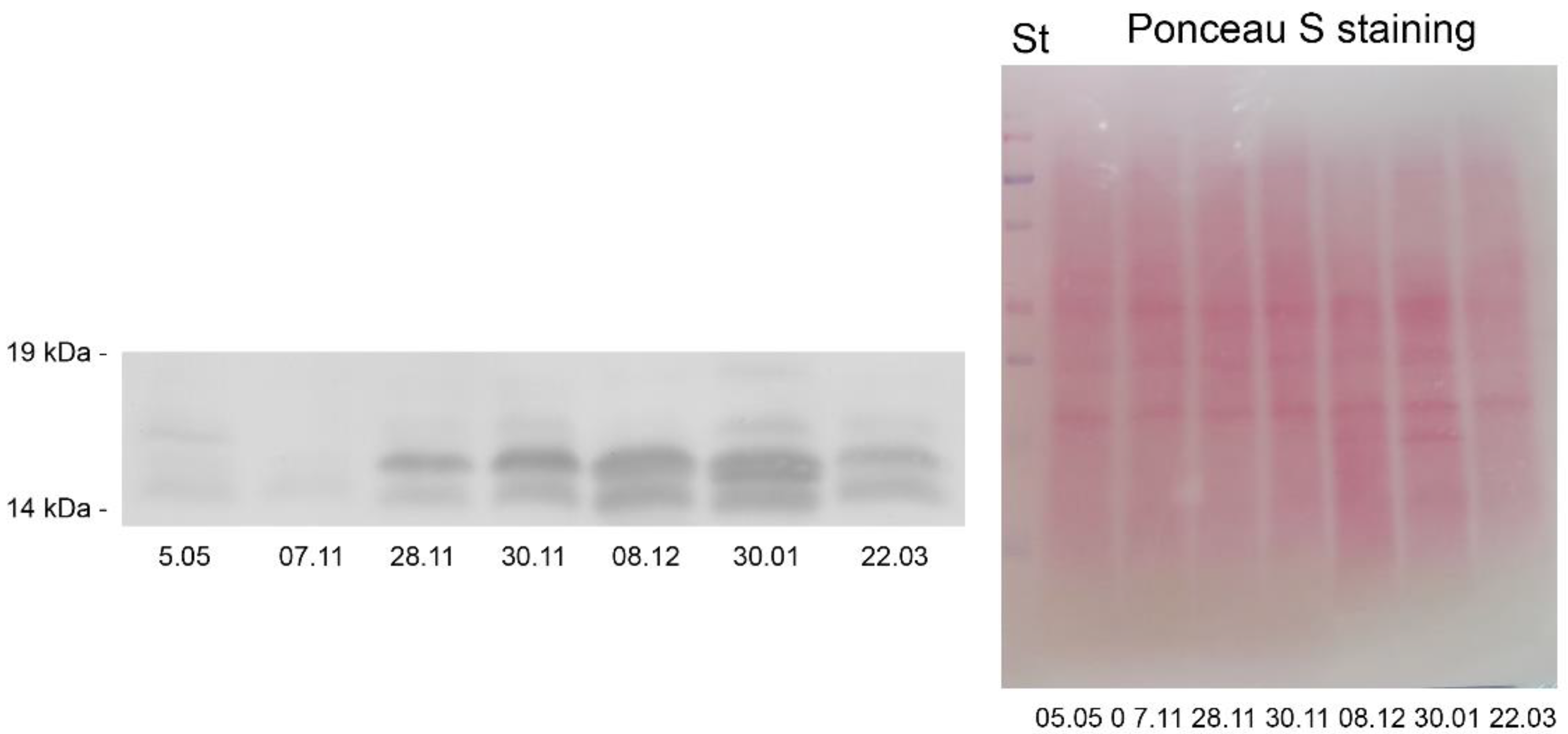
2.5.1. Dehydrins
2.5.2. ELIPs
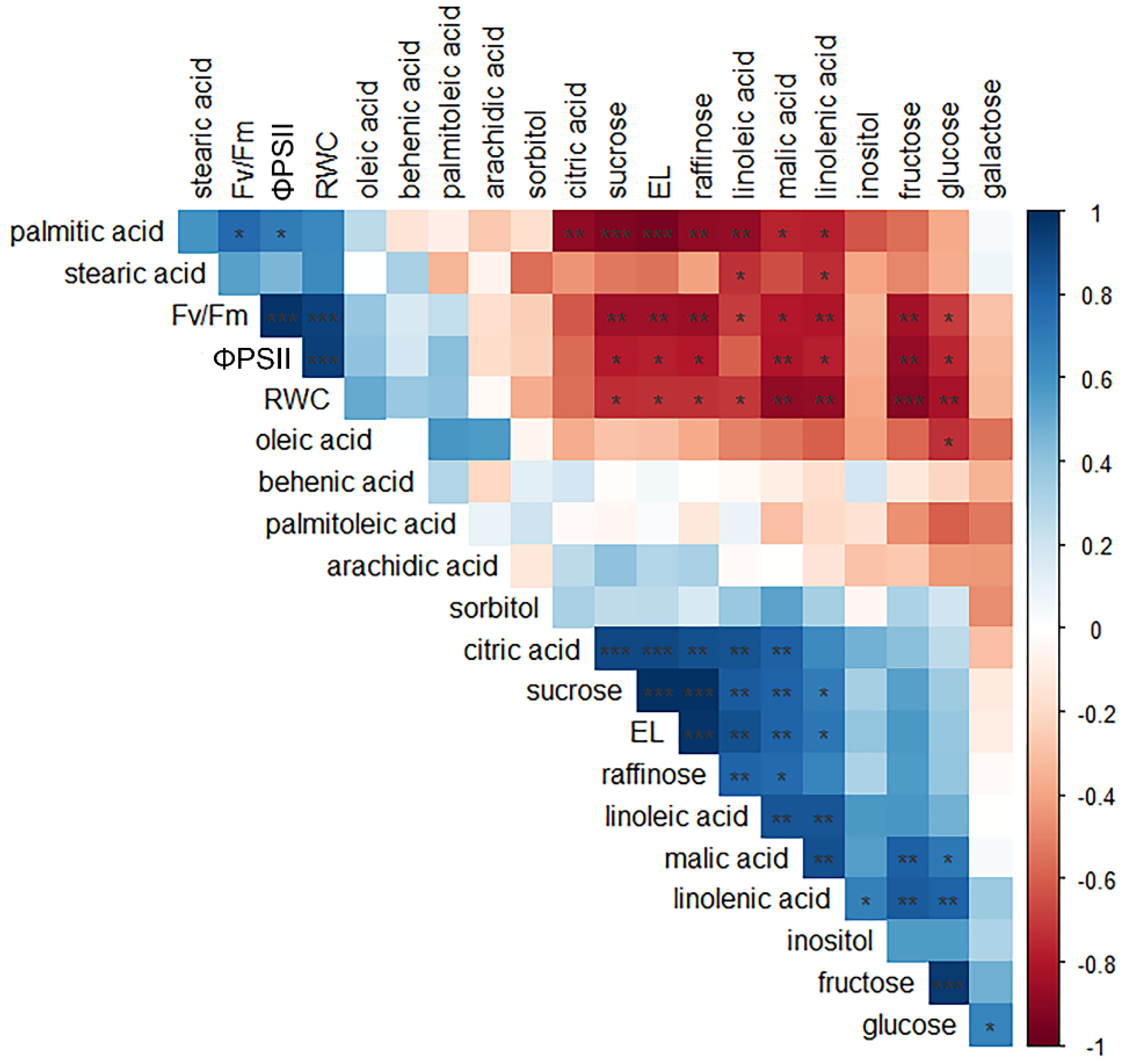
2.6. Correlation Patterns among Physiological and Biochemical Parameters
3. Discussion
3.1. Changes in Membrane Lipids Composition Contribute to Freezing Tolerance
3.2. The Role of Proline and Soluble Sugars in Freezing Tolerance
3.3. The Protective Role of Dehydrins and ELIPs during Freezing Stress
4. Materials and Methods
4.1. Plant Material and Temperature Treatments
- (1)
- Cold acclimation (CA): plants were sampled during gradual acclimation to low daily mean temperatures ranging from +1 °C to +7 °C (dates 7–28 November; CA).
- (2)
- Freezing stress (FS): after an overnight freezing wave exposure to ca. −10 °C, resulting in a slight desiccation of the leaves (ca. 25% reduction in RWC compared to the control) on the sampling day (date 30 November).
- (3)
- Freezing stress + desiccation (FS + D): plants were sampled during long-term exposure to freezing temperatures when significant desiccation of the leaves occurred (RWC ranging from 10 to 20%) from the beginning of December 2016 (date 1 December) till the end of January (date 30 January); During December, nighttime temperatures were negative, and the average daily temperature was ca. +5 °C, while in January for more than 20 days minimum temperatures reached values in the range of −8 °C to −17 °C. As a consequence, sampling on date 30 January is representative of long-term exposure to freezing temperatures compared to December samplings.
- (4)
- Recovery (R): plants were sampled in March 2017 as representative of plant recovery from freezing-induced desiccation.
4.2. Relative Water Content (RWC)
4.3. Dynamic Mechanical Thermal Analysis (DMTA)
4.4. Differential Scanning Calorimetry (DSC)
4.5. Electrolyte Leakage
4.6. Malondialdehyde Content (MDA)
4.7. Proline Content
4.8. Carbohydrate Analysis
4.9. Fatty Acid Analysis
4.10. Total Leaf Protein Extraction, SDS-PAGE and Western Blot Analysis
4.11. Statistical Analysis
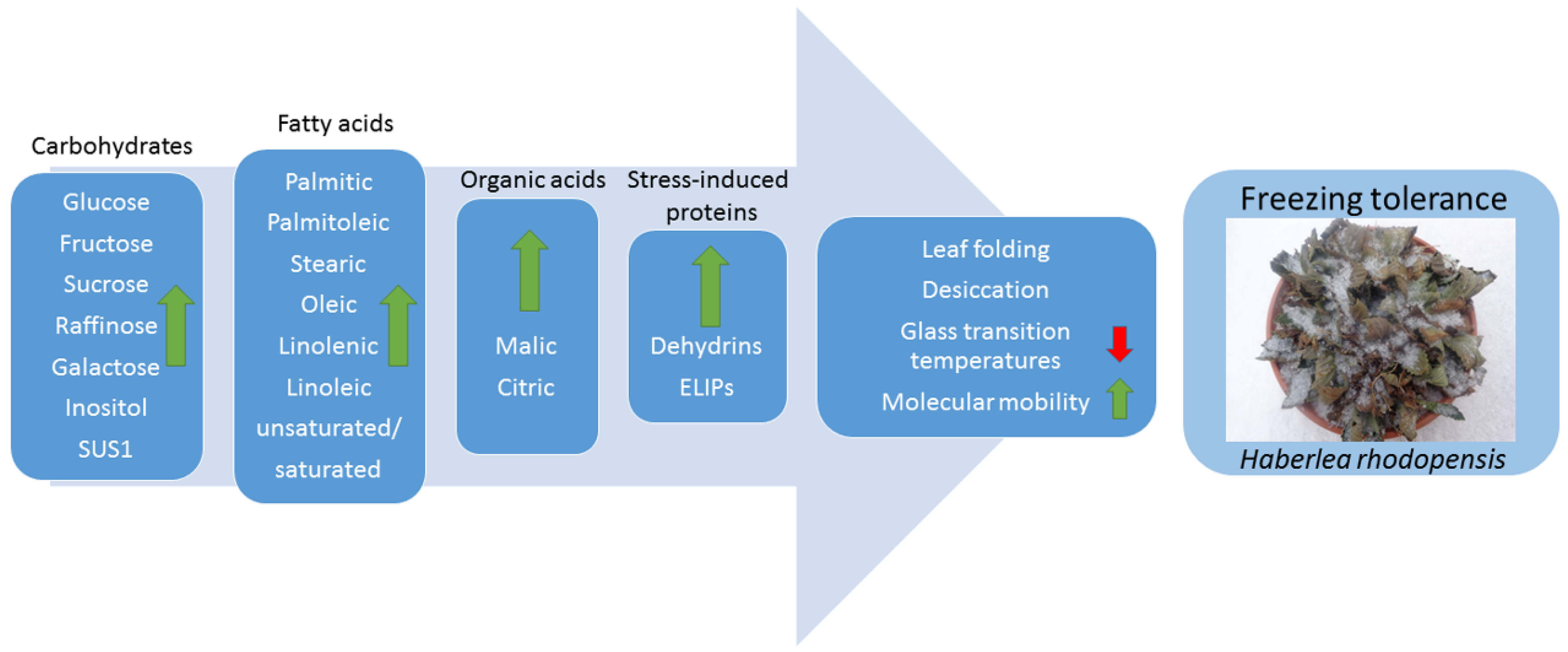
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomashow, M.F. Plant cold acclimation, freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar] [CrossRef]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation–what is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, P.C.; Singh, S. A review report: Low temperature stress for crop production. Int. J. Pure Appl. Biosci. 2018, 6, 575–598. [Google Scholar] [CrossRef]
- Takahashi, D.; Li, B.; Nakayama, T.; Kawamura, Y.; Uemura, M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Pogány, M.; Harrach, B.; Bozsó, Z.; Künstler, A.; Janda, T.; von Rad, U.; Vida, G.; Veisz, O. Cold hardening protects cereals from oxidative stress and necrotrophic fungal pathogenesis. Open Life Sci. 2016, 11, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowska-Pikania, G.; Kopeć, P.; Surówka, E.; Krzewska, M.; Dubas, E.; Nowicka, A.; Rapacz, M.; Wójcik-Jagła, M.; Malaga, S.; Żur, I. Changes in protein abundance and activity involved in freezing tolerance acquisition in winter barley (Hordeum vulgare L.). J. Proteom. 2017, 169, 58–72. [Google Scholar] [CrossRef]
- Verhoeven, A. Sustained energy dissipation in winter evergreens. New Phytol. 2014, 201, 57–65. [Google Scholar] [CrossRef]
- Míguez, F.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Diversity of winter photoinhibitory responses: A case study in co-occurring lichens, mosses, herbs and woody plants from subalpine environments. Physiol. Plant. 2017, 160, 282–296. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. Does the touch of cold make evergreen leaves tougher? Tree Physiol. 2016, 36, 267–272. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Freezing, drying, and/or vitrification of membrane–solute–water systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.S.; Bucci, S.J.; Scholz, F.G.; Goldstein, G. Freezing avoidance by supercooling in Olea europaea cultivars: The role of apoplastic water, solute content and cell wall rigidity. Plant Cell Environ. 2015, 38, 2061–2070. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Kong, R.S.; Henry, H.A. Prior exposure to freezing stress enhances the survival and recovery of Poa pratensis exposed to severe drought. Am. J. Bot. 2016, 103, 1890–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, J.S.; Pockman, W.T. Drought increases freezing tolerance of both leaves and xylem of Larrea tridentata. Plant Cell Environ. 2011, 34, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; DaCosta, M.; Ebdon, J.S.; Zhao, J. Effects of drought preconditioning on freezing tolerance of perennial ryegrass. Environ. Exp. Bot. 2012, 79, 11–20. [Google Scholar] [CrossRef]
- Verhoeven, A.; García-Plazaola, J.I.; Fernández-Marín, B. Shared mechanisms of photoprotection in photosynthetic organisms tolerant to desiccation or to low temperature. Environ. Exp. Bot. 2018, 154, 66–79. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Neuner, G.; Kuprian, E.; Laza, J.M.; García-Plazaola, J.I.; Verhoeven, A. First evidence of freezing tolerance in a resurrection plant: Insights into molecular mobility and zeaxanthin synthesis in the dark. Physiol. Plant. 2018, 163, 472–489. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Nadal, M.; Gago, J.; Fernie, A.R.; López-Pozo, M.; Artetxe, U.; García-Plazaola, J.I.; Verhoeven, A. Born to revive: Molecular and physiological mechanisms of double tolerance in a paleotropical and resurrection plant. New Phytol. 2020, 226, 741–759. [Google Scholar] [CrossRef]
- Mihailova, G.; Solti, Á.; Sárvári, É.; Keresztes, Á.; Rapparini, F.; Velitchkova, M.; Simova-Stoilova, L.; Aleksandrov, V.; Georgieva, K. Freezing tolerance of photosynthetic apparatus in the homoiochlorophyllous resurrection plant Haberlea rhodopensis. Environ. Exp. Bot. 2020, 178, 104157. [Google Scholar] [CrossRef]
- Georgieva, K.; Mihailova, G.; Gigova, L.; Dagnon, S.; Simova-Stoilova, L.; Velitchkova, M. The role of antioxidant defense in freezing tolerance of resurrection plant Haberlea rhodopensis. Physiol. Mol. Biol. Plants 2021, 27, 1119–1133. [Google Scholar] [CrossRef]
- Markovska, Y.K.; Tsonev, T.D.; Kimenov, G.P.; Tutekova, A.A. Physiological changes in higher poikilohydric plants–Haberlea rhodopensis Friv. and Ramonda serbica Panč. during drought and rewatering at different light regimes. J. Plant Physiol. 1994, 144, 100–108. [Google Scholar] [CrossRef]
- Georgieva, K.; Röding, A.; Büchel, C. Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. J. Plant Physiol. 2009, 166, 1520–1528. [Google Scholar] [CrossRef]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef] [Green Version]
- Farrant, J.M.; Cooper, K.; Dace, H.J.; Bentley, J.; Hilgart, A. Desiccation Tolerance. In Plant Stress Physiology, 2nd ed.; Shabala, S., Ed.; CAB International: Oxfordshire, UK, 2017; pp. 217–252. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Sprenger, N.; Bortlik, K.; Boller, T.; Wiemken, A. Desiccation increases sucrose levels in Ramonda and Haberlea, two genera of resurrection plants in the Gesneriaceae. Physiol. Plant. 1997, 100, 153–158. [Google Scholar] [CrossRef]
- Djilianov, D.; Ivanov, S.; Moyankova, D.; Miteva, L.; Kirova, E.; Alexieva, V.; Joudi, M.; Peshev, D.; Van den Ende, W. Sugar ratios, glutathione redox status and phenols in the resurrection species Haberlea rhodopensis and the closely related non-resurrection species Chirita eberhardtii. Plant Biol. 2011, 13, 767–776. [Google Scholar] [CrossRef]
- Moyankova, D.; Mladenov, P.; Berkov, S.; Peshev, D.; Georgieva, D.; Djilianov, D. Metabolic profiling of the resurrection plant Haberlea rhodopensis during desiccation and recovery. Physiol. Plant. 2014, 152, 675–687. [Google Scholar] [CrossRef]
- Georgieva, K.; Rapparini, F.; Bertazza, G.; Mihailova, G.; Sárvári, É.; Solti, Á.; Keresztes, Á. Alterations in the sugar metabolism and in the vacuolar system of mesophyll cells contribute to the desiccation tolerance of Haberlea rhodopensis ecotypes. Protoplasma 2017, 254, 193–201. [Google Scholar] [CrossRef]
- Déjardin, A.; Sokolov, L.N.; Kleczkowski, L.A. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem. J. 1999, 344, 503–509. [Google Scholar] [CrossRef]
- Bieniawska, Z.; Paul Barratt, D.H.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007, 49, 810–828. [Google Scholar] [CrossRef]
- Montané, M.H.; Kloppstech, K. The family of light-harvesting-related proteins (LHCs, ELIPs, HLIPs): Was the harvesting of light their primary function? Gene 2000, 258, 1–8. [Google Scholar] [CrossRef]
- Jan, N.; Andrabi, K.I. Cold resistance in plants: A mystery unresolved. Electron. J. Biotechnol. 2009, 12, 14–15. [Google Scholar] [CrossRef]
- Mihailova, G.; Abakumov, D.; Büchel, C.; Dietzel, L.; Georgieva, K. Drought-responsive gene expression in sun and shade plants of Haberlea rhodopensis under controlled environment. Plant Mol. Biol. Rep. 2017, 35, 313–322. [Google Scholar] [CrossRef]
- Mihailova, G.; Kocheva, K.; Goltsev, V.; Kalaji, H.M.; Georgieva, K. Application of a diffusion model to measure ion leakage of resurrection plant leaves undergoing desiccation. Plant Physiol. Biochem. 2018, 125, 185–192. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. The role of dehydrins in plant response to cold. Biol. Plant. 2007, 51, 601–617. [Google Scholar] [CrossRef]
- John, R.; Anjum, N.A.; Sopory, S.K.; Akram, N.A.; Ashraf, M. Some key physiological and molecular processes of cold acclimation. Biol. Plant. 2016, 60, 603–618. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Ricci, F.; Vazzana, C.; Quartacci, M.F. Unusual composition of thylakoid membranes of the resurrection plant Boea hygroscopica: Changes in lipids upon dehydration and rehydration. Physiol. Plant. 1995, 94, 135–142. [Google Scholar] [CrossRef]
- Yobi, A.; Wone, B.W.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef]
- Farrant, J.M.; Brandt, W.; Lindsey, G. An overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress 2007, 1, 72–84. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0706/PS_1(1)/PS_1(1)72-84o.pdf (accessed on 20 September 2022).
- Stitt, M.; Hurry, V. A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Benina, M.; Obata, T.; Mehterov, N.; Ivanov, I.; Petrov, V.; Toneva, V.; Fernie, A.; Gechev, T.S. Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front. Plant Sci. 2013, 4, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strimbeck, G.R.; Schaberg, P.G.; Fossdal, C.G.; Schröder, W.P.; Kjellsen, T.D. Extreme low temperature tolerance in woody plants. Front. Plant Sci. 2015, 6, 884. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Van den Ende, W. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant Sci. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Scott, P. Resurrection plants and the secrets of eternal leaf. Ann. Bot. 2000, 85, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a role of raffinose in stabilizing photosystem II during freeze–thaw cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef]
- Lehner, A.; Chopera, D.R.; Peters, S.W.; Keller, F.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M. Protection mechanisms in the resurrection plant Xerophyta viscosa: Cloning, expression, characterisation and role of XvINO1, a gene coding for a myo-inositol 1-phosphate synthase. Funct. Plant Biol. 2008, 35, 26–39. [Google Scholar] [CrossRef]
- Farrant, J.M.; Cooper, K.; Hilgart, A.; Abdalla, K.O.; Bentley, J.; Thomson, J.A.; Dace, H.J.; Peton, N.; Mundree, S.G.; Rafudeen, M.S. A molecular physiological review of vegetative desiccation tolerance in the resurrection plant Xerophyta viscosa (Baker). Planta 2015, 242, 407–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, K.L. Glass formation and desiccation tolerance in seeds. Plant Physiol. 1991, 96, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): Both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot. 2007, 58, 1947–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.R.; Marriott, A.S.; Bergström, E.; et al. Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Nägele, T.; Kandel, B.A.; Frana, S.; Meißner, M.; Heyer, A.G. A systems biology approach for the analysis of carbohydrate dynamics during acclimation to low temperature in Arabidopsis thaliana. FEBS J. 2011, 278, 506–518. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and barley dehydrins under cold, drought, and salinity–what can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, M.; Zolla, L.; Rinalducci, S. Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 2015, 117, 76–89. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [Green Version]
- Amme, S.; Matros, A.; Schlesier, B.; Mock, H.P. Proteome analysis of cold stress response in Arabidopsis thaliana using DIGE-technology. J. Exp. Bot. 2006, 57, 1537–1546. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášilová, P.; Prášil, I.T. Accumulation of WCS120 and DHN5 proteins in differently frost-tolerant wheat and barley cultivars grown under a broad temperature scale. Biol. Plant. 2013, 57, 105–112. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Anders, I.; Yuperlieva-Mateeva, B.; Nenkova, R.; Kostadinova, A.; Feller, U. Dehydrin expression as a potential diagnostic tool for cold stress in white clover. Plant Physiol. Biochem. 2014, 78, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.; Bartels, D.; Kalbitzer, H.R.; Jaenicke, R. The recombinant dehydrin-like desiccation stress protein from the resurrection plant Craterostigma plantagineum displays no defined three-dimensional structure in its native state. Biol. Chem. 1996, 377, 555–561. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Rakić, T.; Stevanović, B.; Radović, S. Characterization of oxidative and antioxidative events during dehydration and rehydration of resurrection plant Ramonda nathaliae. Plant Growth Regul. 2011, 64, 231–240. [Google Scholar] [CrossRef]
- Rakić, T.; Lazarević, M.; Jovanović, Ž.S.; Radović, S.; Siljak-Yakovlev, S.; Stevanović, B.; Stevanović, V. Resurrection plants of the genus Ramonda: Prospective survival strategies–unlock further capacity of adaptation, or embark on the path of evolution? Front. Plant Sci. 2014, 4, 550. [Google Scholar] [CrossRef] [Green Version]
- Zarter, C.R.; Adams III, W.W.; Ebbert, V.; Cuthbertson, D.J.; Adamska, I.; Demmig-Adams, B. Winter down-regulation of intrinsic photosynthetic capacity coupled with up-regulation of Elip-like proteins and persistent energy dissipation in a subalpine forest. New Phytol. 2006, 172, 272–282. [Google Scholar] [CrossRef]
- Zarter, C.R.; Adams, W.W.; Ebbert, V.; Adamska, I.; Jansson, S.; Demmig-Adams, B. Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ. 2006, 29, 869–878. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Singer, J.W.; Fessehaie, A.; Krebs, S.L.; Arora, R. Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: A comparison of photoprotective strategies in overwintering plants. Plant Sci. 2009, 177, 607–617. [Google Scholar] [CrossRef]
- Adamska, I. The Elip family of stress proteins in the thylakoid membranes of pro- and eukaryota. In Regulation of Photosynthesis. Advances in Photosynthesis and Respiration; Aro, E.M., Andersson, B., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 11, pp. 487–505. [Google Scholar] [CrossRef]
- Alamillo, J.M.; Bartels, D. Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci. 2001, 160, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Neale, A.D.; Blomstedt, C.K.; Bronson, P.; Le, T.N.; Guthridge, K.; Evans, J.; Gaff, D.F.; Hamill, J.D. The isolation of genes from the resurrection grass Sporobolus stapfianus which are induced during severe drought stress. Plant Cell Environ. 2000, 23, 265–277. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, X.; Wood, A.J. Two early light-inducible protein (ELIP) cDNAs from the resurrection plant Tortula ruralis are differentially expressed in response to desiccation, rehydration, salinity, and high light. J. Exp. Bot. 2002, 53, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Dinakar, C.; Bartels, D. Desiccation tolerance in resurrection plants: New insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci. 2013, 4, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Yang, G.; Zhang, L.; Yang, X.; Zhao, S.; Ji, Z.; Zhou, Q.; Hu, M.; Wang, Y.; Chen, M.; et al. The resurrection genome of Boea hygrometrica: A blueprint for survival of dehydration. Proc. Natl. Acad. Sci. USA 2015, 112, 5833–5837. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Marín, B.; Kranner, I.; Sebastián, M.S.; Artetxe, U.; Laza, J.M.; Vilas, J.L.; Pritchard, H.W.; Nadajaran, J.; Míguez, F.; Becerril, J.M.; et al. Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. J. Exp. Bot. 2013, 64, 3033–3043. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; López-Pozo, M.; Perera-Castro, A.V.; Arzac, M.I.; Sáenz-Ceniceros, A.; Colesie, C.; De Los Ríos, A.; Sancho, L.G.; Pintado, A.; Laza, J.M.; et al. Symbiosis at its limits: Ecophysiological consequences of lichenization in the genus Prasiola in Antarctica. Ann. Bot. 2019, 124, 1211–1226. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zanetti, F.; Chieco, C.; Alexopoulou, E.; Vecchi, A.; Bertazza, G.; Monti, A. Comparison of new castor (Ricinus communis L.) genotypes in the mediterranean area and possible valorization of residual biomass for insect rearing. Ind. Crops Prod. 2017, 107, 581–587. [Google Scholar] [CrossRef]
- Liu, K.S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. JAOCS 1994, 71, 1179–1187. [Google Scholar] [CrossRef]
- Mladenov, P.; Zasheva, D.; Djilianov, D.; Tchorbadjieva, M. Towards proteomics of desiccation tolerance in the resurrection plant Haberlea rhodopensis. Compt. Rend. Acad. Bulg. Sci. 2015, 68, 59–64. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Anders, I.; Feller, U. Identification and expression of different dehydrin subclasses involved in the drought response of Trifolium repens. J. Plant Physiol. 2014, 171, 213–224. [Google Scholar] [CrossRef] [PubMed]



| Control | CA | FS | FS + D | R | ||||
|---|---|---|---|---|---|---|---|---|
| Fatty acid | 5 May | 7 November | 28 November | 30 November | 5 December | 8 December | 14 December | 22 March |
| RWC 86% | RWC 80% | RWC 82% | RWC 65% | RWC 35% | RWC 20% | RWC 12% | RWC 86% | |
| Palmitic (16:0) | 40.36 ± 1.54 d | 38.84 ± 0.65 cd | 36.45 ± 1.02 bc | 36.77 ± 2.73 bc | 36.73 ± 0.11 bc | 36.38 ± 0.90 bc | 27.61 ± 2.74 a | 35.47 ± 0.60 b |
| Palmitoleic (16:1) | 0.34 ± 0.01 d | 0.12 ± 0.02 ab | 0.15 ± 0.01 b | 0.34 ± 0.08 d | 0.06 ± 0.01 a | 0.16 ± 0.01 b | 0.24 ± 0.04 c | 0.59 ± 0.04 e |
| Stearic (18:0) | 28.81 ± 1.63 b | 29.88 ± 1.14 b | 32.30 ± 1.70 b | 23.82 ± 1.02 a | 24.42 ± 1.25 a | 24.53 ± 0.49 a | 22.93 ± 3.23 a | 24.67 ± 2.84 a |
| Oleic (18:1) | 12.87 ± 1.56 d | 9.61 ± 0.31 bc | 9.61 ± 0.63 bc | 16.57 ± 1.84 e | 5.97 ± 0.80 a | 6.06 ± 0.15 a | 7.58 ± 1.32 ab | 10.32 ± 1.52 c |
| Linoleic (18:2) | 12.51 ± 1.43 a | 14.22 ± 0.88 ab | 12.50 ± 1.14 a | 12.60 ± 1.91 a | 16.61 ± 2.13 bc | 18.23 ± 0.30 c | 23.20 ± 4.23 d | 17.21 ± 1.33 bc |
| Linolenic (18:3) | 4.20 ± 0.48 a | 6.74 ± 0.75 ab | 8.01 ± 1.61 b | 9.17 ± 1.62 bc | 15.68 ± 2.24 de | 14.28 ± 0.78 d | 17.57 ± 2.43 e | 11.09 ± 1.96 c |
| Arachidic (20:0) | 0.53 ± 0.07 cd | 0.51 ± 0.03 c | 0.45 ± 0.02 bc | 0.66 ± 0.07 e | 0.44 ± 0.01 bc | 0.13 ± 0.02 a | 0.64 ± 0.10 de | 0.36 ± 0.05 b |
| Behenic (22:0) | 0.28 ± 0.05 b | 0.09 ± 0.01 a | 0.53 ± 0.08 c | 0.07 ± 0.02 a | 0.08 ± 0.01 a | 0.23 ± 0.01 b | 0.23 ± 0.05 b | 0.31 ± 0.10 b |
| Control | CA | FS* | FS + D | R | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Compounds | 5 May | 7 November | 28 November | 30 November | 5 December | 8 December | 14 December | 22 March |
| RWC 86% | RWC 80% | RWC 82% | RWC 65% | RWC 35% | RWC 20% | RWC 12% | RWC 86% | ||
| Carbohydrates | Raffinose | 1.00 | 1.16 | 1.44 | 2.81 | 1.84 | 6.84 | 16.53 | 0.71 |
| Sucrose | 1.00 | 0.85 | 1.54 | 1.17 | 2.93 | 4.36 | 15.87 | 1.13 | |
| Glucose | 1.00 | 2.58 | 3.91 | 4.22 | 6.09 | 8.56 | 5.07 | 2.79 | |
| Fructose | 1.00 | 1.76 | 3.41 | 3.06 | 4.42 | 8.00 | 5.16 | 2.23 | |
| Galactose | 1.00 | 2.12 | 2.31 | 4.07 | 3.31 | 3.31 | 2.14 | 2.74 | |
| Inositol | 1.00 | 2.44 | 2.87 | 1.72 | 2.58 | 2.89 | 2.83 | 2.81 | |
| Organic acids | Malic acid | 1.00 | 1.41 | 1.48 | 0.61 | 2.43 | 3.12 | 3.51 | 1.03 |
| Citric acid | 1.00 | 1.38 | 1.37 | 0.42 | 1.29 | 1.28 | 3.15 | 1.10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, K.; Mihailova, G.; Fernández-Marín, B.; Bertazza, G.; Govoni, A.; Arzac, M.I.; Laza, J.M.; Vilas, J.L.; García-Plazaola, J.I.; Rapparini, F. Protective Strategies of Haberlea rhodopensis for Acquisition of Freezing Tolerance: Interaction between Dehydration and Low Temperature. Int. J. Mol. Sci. 2022, 23, 15050. https://doi.org/10.3390/ijms232315050
Georgieva K, Mihailova G, Fernández-Marín B, Bertazza G, Govoni A, Arzac MI, Laza JM, Vilas JL, García-Plazaola JI, Rapparini F. Protective Strategies of Haberlea rhodopensis for Acquisition of Freezing Tolerance: Interaction between Dehydration and Low Temperature. International Journal of Molecular Sciences. 2022; 23(23):15050. https://doi.org/10.3390/ijms232315050
Chicago/Turabian StyleGeorgieva, Katya, Gergana Mihailova, Beatriz Fernández-Marín, Gianpaolo Bertazza, Annalisa Govoni, Miren Irati Arzac, José Manuel Laza, José Luis Vilas, José Ignacio García-Plazaola, and Francesca Rapparini. 2022. "Protective Strategies of Haberlea rhodopensis for Acquisition of Freezing Tolerance: Interaction between Dehydration and Low Temperature" International Journal of Molecular Sciences 23, no. 23: 15050. https://doi.org/10.3390/ijms232315050





