Antagonizing RARγ Drives Necroptosis of Cancer Stem Cells
Abstract
:1. Introduction
2. Why Target RARγ to Eliminate CSCs?
2.1. The Role of RARγ within Stem Cells
2.2. RARγ Is an Oncogene for a Number of Cancers
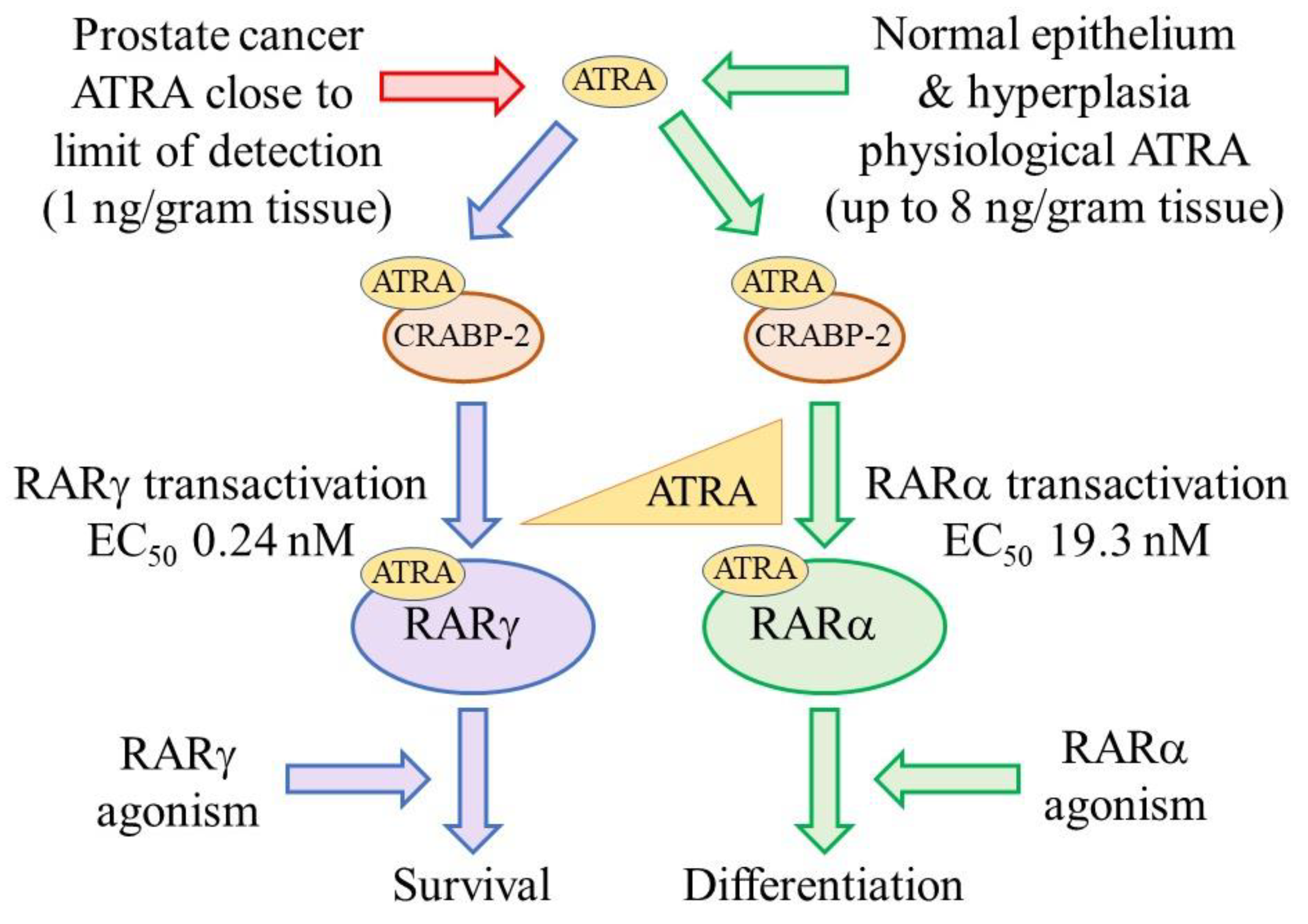
2.3. PCa Cells Are Dependent on Active RARγ for Their Survival
3. Agonists and Antagonists of RARs
4. Antagonizing RARγ Kills CSCs
5. Antagonizing All RARs Is Effective against Pediatric Brain Tumors
6. The Effect of Antagonizing RARs on Normal Cells
7. Concluding Remarks
8. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greaves, M. Evolutionary determinants of cancer. Cancer Discov. 2015, 5, 806–820. [Google Scholar] [CrossRef] [Green Version]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fialkow, P.J.; Denman, A.M.; Jacobson, G.J.; Lowenthal, M.N. Chronic myelocytic leukaemia: Origin of some lymphocytes from leukaemic stem cell. J. Clin. Investig. 1978, 62, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Holyoake, T.; Jiang, X.; Eaves, C.; Eaves, A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 1999, 94, 2056–2064. [Google Scholar] [CrossRef]
- Graham, S.M.; Jorgensen, H.G.; Allan, E.; Pearson, C.; Alcorn, M.J.; Richmond, L.; Holyoake, T.L. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002, 99, 319–325. [Google Scholar] [CrossRef]
- Corbin, A.S.; Agarwal, A.; Loriaux, M.; Cortes, J.; Deininger, M.W.; Druker, B.J. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Investig. 2011, 121, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Lim, Z.; Brand, R.; Martino, R.; van Biezen, A.; Finke, J.; Bacigalupo, A.; Beelen, D.; Devergie, A.; Alessandrino, E.; Willemze, R.; et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Huang, Y.-H.; Chen, J.-L. Understanding and targeting cancer stem cells: Therapeutic implications. Acta Pharmacol. Sin 2013, 34, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Yan, L.; Zhou, M. Targeted selection of CAR T cell therapy in accordance with the TME for solid cancers. Am. J. Cancer Res. 2019, 9, 228–241. [Google Scholar] [PubMed]
- di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Le-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015, 41, 1–115. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Lowenberg, B.; Naoe, T.; Lengfelder, E.; Dohner, H.; Burnet, A.K.; Chen, S.J.; et al. Management of acute promyelocytic leukema: Updated recommendations from an expert panel of the European Leukemia. Net. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.H.; Wasik, M.A.; Finan, J.; Rodriquez, R.; Moore, J.; Kamoun, M.; Rennert, H.; Bird, J.; Novell, P.C.; Salhany, K.E. Evidence for early progenitor cell involvement in promyelocytic leukemia. Am. J. Clin. Pathol. 1999, 112, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Huynh, T.T.; Sultan, M.; Vidovic, D.; Dean, C.A.; Cruichshank, B.M.; Lee, K.; Loung, C.Y.; Holloway, R.W.; Hoskin, D.W.; Waisman, D.M.; et al. Retinoic acid and arsenic trioxide induce lasting differentiation and demethylation of target genes in APL cells. Sci. Rep. 2019, 9, 9414. [Google Scholar] [CrossRef]
- Purton, L.E.; Dworkin, S.; Olsen, G.M.; Walkley, C.; Fabb, S.A.; Collins, S.J.; Chambon, P. RAR© is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006, 203, 1283–1293. [Google Scholar] [CrossRef]
- Purton, L.E.; Bernstein, I.D.; Collins, S.J. All-trans retinoic acid delays the differentiation of primitive hematopoietic precursors (lin−c-kit+Sca-1(+)) while enhancing the terminal maturation of committed granulocyte/monocyte progenitors. Blood 1999, 94, 483–495. [Google Scholar] [CrossRef]
- Wai, H.A.; Kawakami, K.; Wada, H.; Muller, F.; Vernalis, A.B.; Brown, G.; Johnson, W.E.B. The development and growth of tissues derived from cranial neural crest and primitive mesoderm is dependent on the ligation status of retinoic acid receptor ©: Evidence that retinoic acid receptor γ functions to maintain stem/progenitor cells in the absence of retinoic acid. Stem. Cells Dev. 2015, 24, 507–519. [Google Scholar]
- Kashyap, V.; Laursen, K.B.; Brenet, F.; Viale, A.J.; Scandura, J.M.; Gudas, L.J. RAR© is essential for retinoic acid induced chromatin remodelling and transcriptional activation in embryonic stem cells. J. Cell Sci. 2012, 126, 999–1008. [Google Scholar]
- Chatagon, A.; Veber, P.; Morin, V.; Bedo, J.; Triqueneaux, G.; Semon, M.; Laudet, V.; d’Alche-Buc, F.; Benoit, G. RAR/RXR binding dynamics distinguish pluripotency from differentiation associated cis-regulatory elements. Nucl. Acids Res. 2015, 43, 4833–4854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Such, E.; Cervera, J.; Valencia, A. A novel NUP98/RARG gene fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood 2011, 117, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Z.; Huang, X.J.; Zhu, H.H. Identification of a novel CPSF6-RARG fusion transcript in acute myeloid leukemia resembling acute promyelocytic leukemia. Leukemia 2018, 32, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Conserva, M.R.; Redavid, I.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. RARG gene dysregulation in acute myeloid leukemia. Front. Mol. Biosci. 2019, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Liu, X.; Xu, Y.; Zhao, C.; Zhao, H.; Feng, X.; Zhang, S.; Yang, S.; Yang, J.; Shi, X.; et al. Novel reciprocal fusion genes involving HNRPC1 and RARG in acute promyelocytic leukemia lacking RARA rearrangement. Haematologica 2020, 105, e376. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.M.; Perez, A.; Pereira, L.; Fan, Y.S.; Brown, G.; Vega, F.; Petrie, K.; Swords, R.T.; Zelent, A.A. A case of AML characterised by a novel t(4;15)(q31;q22) translocation that confers a growth-stimulatory response to retinoid-based therapy. Int. J. Mol. Sci. 2017, 18, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.L.; Song, W.; Zhou, P.; Fu, Q.R.; Lin, C.L.; Chen, Q.X.; Shen, D.Y. Oncogenic retinoic acid receptor gamma knockdown reverses multi-drug resistance of human colorectal cancer via Wnt/beta-catenin pathway. Cell Cycle 2017, 16, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.L.; Luo, Q.; Rui, G.; Zhang, W.; Zhang, Q.Y.; Chen, Q.X.; Shen, D.Y. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol. Cell. Biol. 2013, 33, 3416–3425. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.D.; Wu, H.; Zhang, H.P.; Lu, N.; Ye, P.; Yu, F.H.; Zhou, H.; Li, W.G.; Cao, X.; Lin, Y.Y.; et al. Oncogenic potential of retinoic acid receptor-gamma in hepatocellular carcinoma. Cancer Res. 2010, 70, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, A.V.; Nyushko, K.M.; Zaretsky, A.R.; Shagin, D.A.; Kaprin, A.D.; Alekseev, B.Y.; Snezhkina, A.V. Upregulation of Rarb, Rarg, and Rorc Genes in Clear Cell Renal Cell Carcinoma. Biomed. Pharmacol. J. 2016, 9, 967–975. [Google Scholar] [CrossRef]
- Pasquali, D.; Thaller, C.; Eichele, G. Abnormal level of retinoic acid in prostate cancer tissues. J. Clin. Endocrinol. Metab. 1996, 81, 2186–2191. [Google Scholar] [PubMed]
- Farboud, B.; Hauksdotter, H.; Wu, Y.; Privalsky, M.L. Isotype-restricted corepressor recruitment: A constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol. Cell. Biol. 2003, 23, 2844–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.; Marchwicka, A.; Cunningham, A.; Toellner, K.-M.; Marcinkowska, E. Antagonizing retinoic acid receptors increases myeloid cell production by cultured human hematopoietic stem cells. Arch. Immunol. Ther. Exp. 2017, 65, 69–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryzlak, M.T.; Ambroziak, W.; Schaffer, C.P. Humam prostatic aldehyde dehydrogenase of healthy controls and diseased prostates. Biochim. Biophys. Acta 1992, 1139, 287–294. [Google Scholar] [CrossRef]
- Trasino, S.E.; Harrison, E.H.; Wang, T.T. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp. Biol. Med. 2007, 232, 762–771. [Google Scholar]
- Chen, Y.; Clarke, O.B.; Kim, J.; Stowe, S.; Kim, Y.-K.; Assur, Z.; Cavalier, M.; Goday-Ruiz, R.; von Alpen, D.C.; Manzini, C.; et al. Structure of the STRA6 receptor for retinol uptake. Science 2016, 353, aad8266. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Gudas, L.J. Retinoic acid receptors and GATA transcription factors activate the transcription of the human lecithin: Retinol acyltransferase gene. Int. J. Biochem. Cell Biol. 2009, 41, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.T.; Wang, L.; Standeven, A.M.; Escobar, M.; Chandraratna, R.A. Synthesis and biological activity of high-affinity retinoic acid receptor antagonists. Bioorg. Med. Chem. 1999, 7, 1321–1338. [Google Scholar] [CrossRef]
- Heyman, R.; Mangelsdorf, D.; Dyck, J.; Stein, R.; Eichele, G.; Evans, R.; Thaller, C. 9-cis-retinoic acid is a high affinity ligand for the retinoid-X-receptor. Cell 1992, 68, 392–406. [Google Scholar] [CrossRef]
- Hughes, P.J.; Zhao, Y.; Chandraratna, R.A.; Brown, G. Retinoid-mediated stimulation of steroid sulfatase activity in myeloid leukemic cells requires RARα and RXR and involves the phosphoinositide 3-kinase and ERK-MAP pathways. J. Cell. Biochem. 2006, 97, 327–350. [Google Scholar] [CrossRef]
- Berges, R.R.; Vukanovic, J.; Epstein, J.L.; Carmichel, M.; Cisek, L.; Johnson, D.E.; Veltri, R.W.; Walsh, P.C.; Isaaccs, J.T. Implication of cell kinetic changes during the progression of human prostate cancer. Clin. Cancer Res. 1995, 1, 473–480. [Google Scholar]
- Knudsen, K.E.; Penning, T.M. Partners in crime: Deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 2010, 21, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebello, R.J.; Ding, C.; Knudsen, K.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Quan, H.; Loblaw, D.A. Androgen deprivation for prostate cancer–review of indications in 2010. Curr. Oncol. 2010, 17, S38–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attard, G.; Reid, A.H.; Olmos, D.; de Bono, J.S. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009, 69, 4937–4940. [Google Scholar] [CrossRef] [Green Version]
- Namekawa, T.; Ikeda, K.; Harie-Inoue, K.; Inoue, S. Application of prostate cancer models for preclinical studies: Advantages and limitations of cell lines, patient derived xenografts, and three-dimensional culture of patient-derived cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Linja, M.J.; Vinsakorpi, T. Alternations of androgen receptor in prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 255–264. [Google Scholar] [CrossRef]
- Culig, Z.; Klocker, K.; Eberle, J.; Kaspar, F.; Habish, A.; Cronauer, M.V.; Bartsch, G. DNA sequence of the androgen receptor in prostate tumor cell lines and tissue specimens assessed by means of the polymerase chain reaction. Prostate 1993, 22, 11–22. [Google Scholar] [CrossRef]
- Tilley, W.D.; Bentel, J.M.; Aspinall, J.O.; Hall, R.E.; Horsfall, D.J. Evidence for a novel mechanism of androgen resistance in the human prostate cancer cell line PC-3. Steroids 1995, 60, 180–186. [Google Scholar] [CrossRef]
- Alimireh, F.; Chen, J.; Basrawala, Z.; Xin, H.; Choubey, D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Lett. 2006, 580, 2294–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, L.A.; Krinks, C.H.V.; Durham, J.; Tomkins, S.E.; Burnett, R.D.; Jones, E.L.; Chandraratna, R.A.S.; Brown, G. Antagonists of retinoic acid receptors (RARs) are potent growth inhibitors of prostate carcinoma cells. Br. J. Cancer 2001, 85, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keedwell, R.G.; Zhao, Y.; Hammond, L.A.; Wen, K.; Qin, S.; Atangan, L.I.; Shurland, D.-L.; Wallace, D.M.A.; Bird, R.; Reitmair, A.; et al. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br. J. Cancer 2004, 91, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, K.; Urban, Z.; Sbirkov, Y.; Graham, A.; Hamann, A.; Brown, G. Retinoic acid receptor-© is a therapeutically targetable driver of growth and survival in prostate cancer. Cancer Rep. 2020, 3, e1284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hashomoto, Y.; Agadir, A.; Kagechika, H.; Zhang, X.K. Identification of a novel class of retinoic acid receptor beta-selective retinol antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in huam breast cancer cells. J. Biol. Chem. 1999, 274, 15360–15366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, G.; Balaguer, P.; Michel, S.; Rahmani, R. Activation of retinoic acid receptor-dependent transcription by organochlorine pesticides. Toxicol. Appl. Pharmacol. 2005, 207, 38–49. [Google Scholar] [CrossRef]
- Lu, Y.; Bertan, S.; Samuels, T.A.; Mira-y-Lopez, R.; Farias, E.F. Mechanism of inhibition of MMTV-neu and MMTV-wnt induced mammary oncogenesis by RARα agonist Am580. Oncogene 2010, 29, 3554–3576. [Google Scholar] [CrossRef] [Green Version]
- Bosch, A.; Bertran, S.P.; Lu, Y.; Garcia, A.; Jones, A.M.; Dawson, M.I.; Farias, E.F. Reversal by RARα agonist Am580 of c-Myc-induced imbalance in RARα/RAR© expression during MMTV-Myc tumorigenesis. Breast Cancer Res. 2012, 14, R121. [Google Scholar] [CrossRef] [Green Version]
- Beaver, M.; Ahmed, A.; Masters, J.R. Clonogenic holoclones and merclones contain stem cells. PLoS ONE 2014, 9, e89834. [Google Scholar] [CrossRef]
- Chiu, H.J.; Fishman, D.A.; Hammerling, U. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprevation. FASEB J. 2008, 22, 3878–3887. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, S.L.; Ullrich, N.L. Pediatric brain tumors. Neurol. Clin. 2003, 4, 897–913. [Google Scholar]
- Kleihues, P.; Loius, D.; Scheithauer, B.W.; Rourke, L.R.; Reifenberger, G.; Burges, P.C.; Cavanee, W.K. The WHO classification of tumors of the nervous stsyem. Neurpathol. Exp. Neurol. 2002, 61, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Role and distribution of retonic acid during CNS development. Int. Rev. Cytol. 2001, 209, 1–77. [Google Scholar] [PubMed]
- Mattay, K.K.; Maris, J.M.; Schleirmacher, G.; Nagagawara, A.; Mackell, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Des. Prim. 2016, 2, 16078. [Google Scholar] [CrossRef]
- Rosolen, A.; Favaretto, G.; Masarotto, G.; Cavazzana, A.; Zanesco, L.; Franscella, E. Effect of all-trans retinoic acid and interferon α in peripheral neuroectodermal tumour cell cultures and xenografts. Int. J. Oncol. 1998, 13, 943–949. [Google Scholar]
- Biswas, A.K.; Han, S.; Tai, Y.; Ma, W.; Coker, C.; Quinn, S.A.; Shakri, A.R.; Zhong, T.J.; Scholze, H.; Lagos, G.G.; et al. Targeting S100A9-ALDH1AI-retinoic acid signaling to suppress brain relapse in EGFR-mutant lung cancer. Cancer Discov. 2022, 12, 1002–1021. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yu, J.; Zhang, L. Necroptosis: An alternative cell death program defending against cancer. Biochim. Biophys. Acta 2016, 1865, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Parra, M.A.; Walia, M.; Sankar, M.; Gronemeyer, H. Dissecting the retinoid-induced differentiation of F9 embryonal stem cells by integrative genomics. Mol. Syst. Biol. 2011, 7, 538. [Google Scholar] [CrossRef]
- Xu, Q.; Jitkaew, S.; Choksi, S.; Kadigamuwa, C.; Choe, M.; Jang, J.; Liu, C.; Liu, Z.-G. The cytoplasmic nuclear RAR© controls RIPI initiated cell death when cIAP activity is inhibited. Nat. Commun. 2017, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Kadigamuwa, C.; Choksi, S.; Xu, Q.; Cataisson, C.; Greenbaum, S.S.; Yuspa, S.H.; Liu, Z.-G. Role of retinoic acid receptor-© in DNA dmaage-induced necroptosis. IScience 2019, 17, 74–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
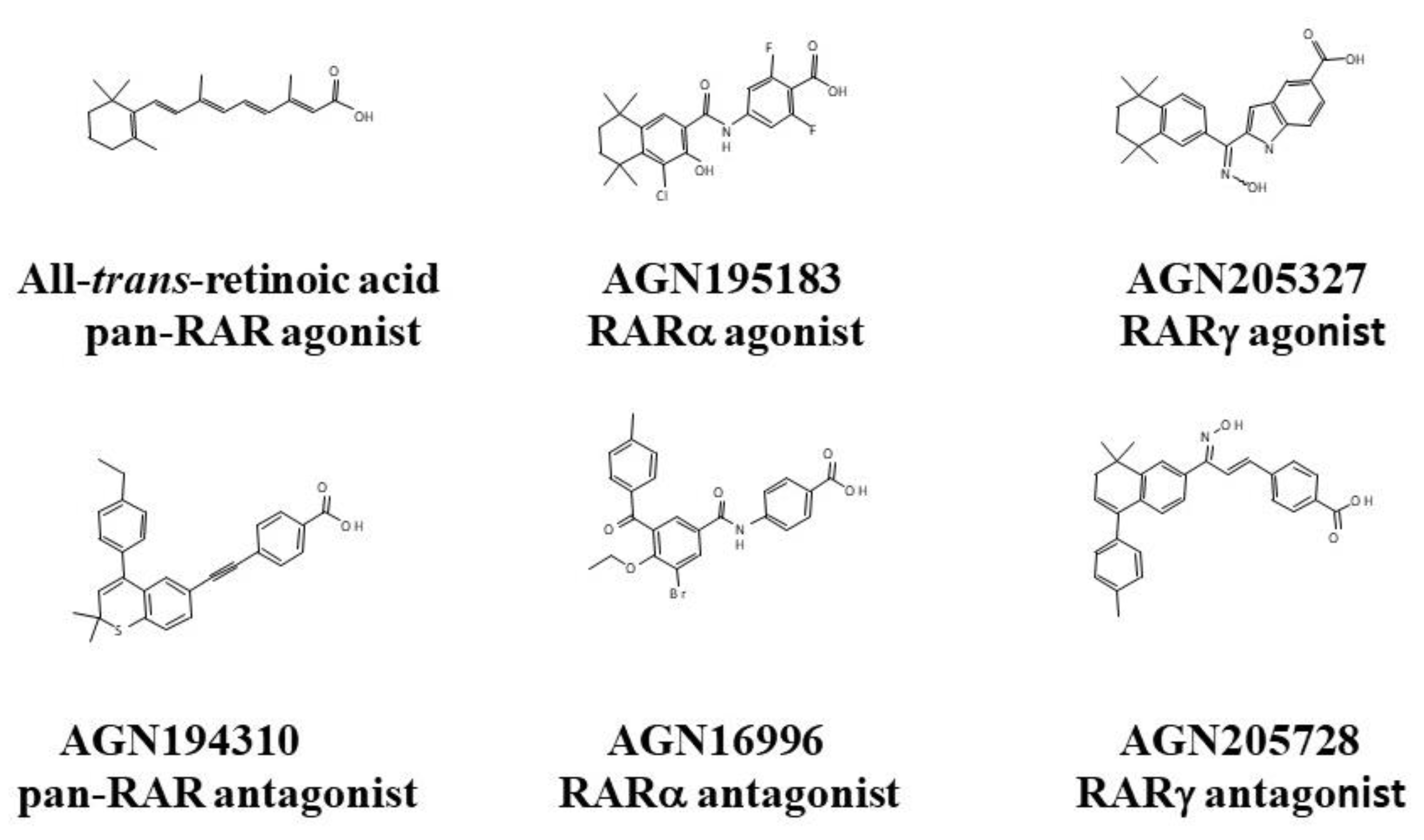

| Retinoids | RARα | RARβ | RARγ | Classification |
|---|---|---|---|---|
| RAR Agonists—Equilibrium Binding Affinities in nM | ||||
| ATRA | ND | ND | ND | RARαβγ |
| AGN195183 | 20.1 | >5000 | >5000 | RARα |
| AGN190168 | >1000 | 14.2 | 135 | RARβγ |
| AGN205327 | 3700 | 734 | 32 | RARγ |
| RAR Antagonists—Equilibrium Binding Affinities in nM | ||||
| AGN194310 | 4.3 | 5 | 2 | RARαβγ |
| AGN196996 | 3.9 | 4036 | >10,000 | RARα |
| AGN194431 | 300 | 6 | 20 | RARβγ |
| AGN205728 | 2400 | 4248 | 3 | RARγ |
| Cells | AGN194310 pan-RAR Antagonist IC50 Values | AGN193109 pan-RAR Antagonist IC50 Values | AGN193776 pan-RAR Antagonist IC50 Values | LG100815 pan-RAR Antagonist IC50 Values | AGN205728 RARγ Antagonist IC50 Values |
|---|---|---|---|---|---|
| PCa cells | |||||
| DU-145 | 5. 0 × 10−7 M | 1.8 × 10−6 M | 6.0 × 10−7 M | ||
| LNCaP | 4.0 × 10−7 M | 4.2 × 10−7 M | 3.9 × 10−7 M | 5.2 × 10−7 M | 4.5 × 10−7 M |
| PC-3 | 3.5 × 10−7 M | 6.8 × 10−7 M | 5.7 × 10−7 M | 1.0 × 10−6 M | 4.7 × 10−7 M |
| Patients’ cells | 4.7 ± 2.1 × 10−7 M * | 3.0 × 10−7 M | |||
| Non-malignant prostate cells | |||||
| Prostate epithelial | 1.0 × 10−6 M | 1.4 × 10−6 M | 1.1 × 10−6 M | >1 × 10−5 M | 7.2 × 10−7 M |
| RWPE-1 | 2.3 × 10−6 M | ||||
| PCa Lines | AGN194310 pan-RAR Antagonist IC50 Values | AGN205728 RARγ Antagonist IC50 Values | AGN196996 RARα Antagonist IC50 Values | ATRA pan-RAR Agonist IC50 Values |
|---|---|---|---|---|
| DU-145 | 34 × 10−9 M | 60 × 10−9 M | >1 × 10−5 M | 4.0 × 10−7 M |
| LNCaP | 16 × 10−9 M | 55 × 10−9 M | >1 × 10−5 M | 3.2 × 10−7 M |
| PC-3 | 18 × 10−9 M | 50 × 10−9 M | >1 × 10−5 M | 4.2 × 10−7 M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, G. Antagonizing RARγ Drives Necroptosis of Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 4814. https://doi.org/10.3390/ijms23094814
Brown G. Antagonizing RARγ Drives Necroptosis of Cancer Stem Cells. International Journal of Molecular Sciences. 2022; 23(9):4814. https://doi.org/10.3390/ijms23094814
Chicago/Turabian StyleBrown, Geoffrey. 2022. "Antagonizing RARγ Drives Necroptosis of Cancer Stem Cells" International Journal of Molecular Sciences 23, no. 9: 4814. https://doi.org/10.3390/ijms23094814






