The Effect of Sex-Specific Differences on IL-10−/− Mouse Colitis Phenotype and Microbiota
Abstract
:1. Introduction
2. Results
2.1. Female IL-10−/− Mice Develop a More Severe Colitis Phenotype Than Males
2.2. IL-10−/− Mice Colitis Undergoes Sex-Specific Microbiota Changes
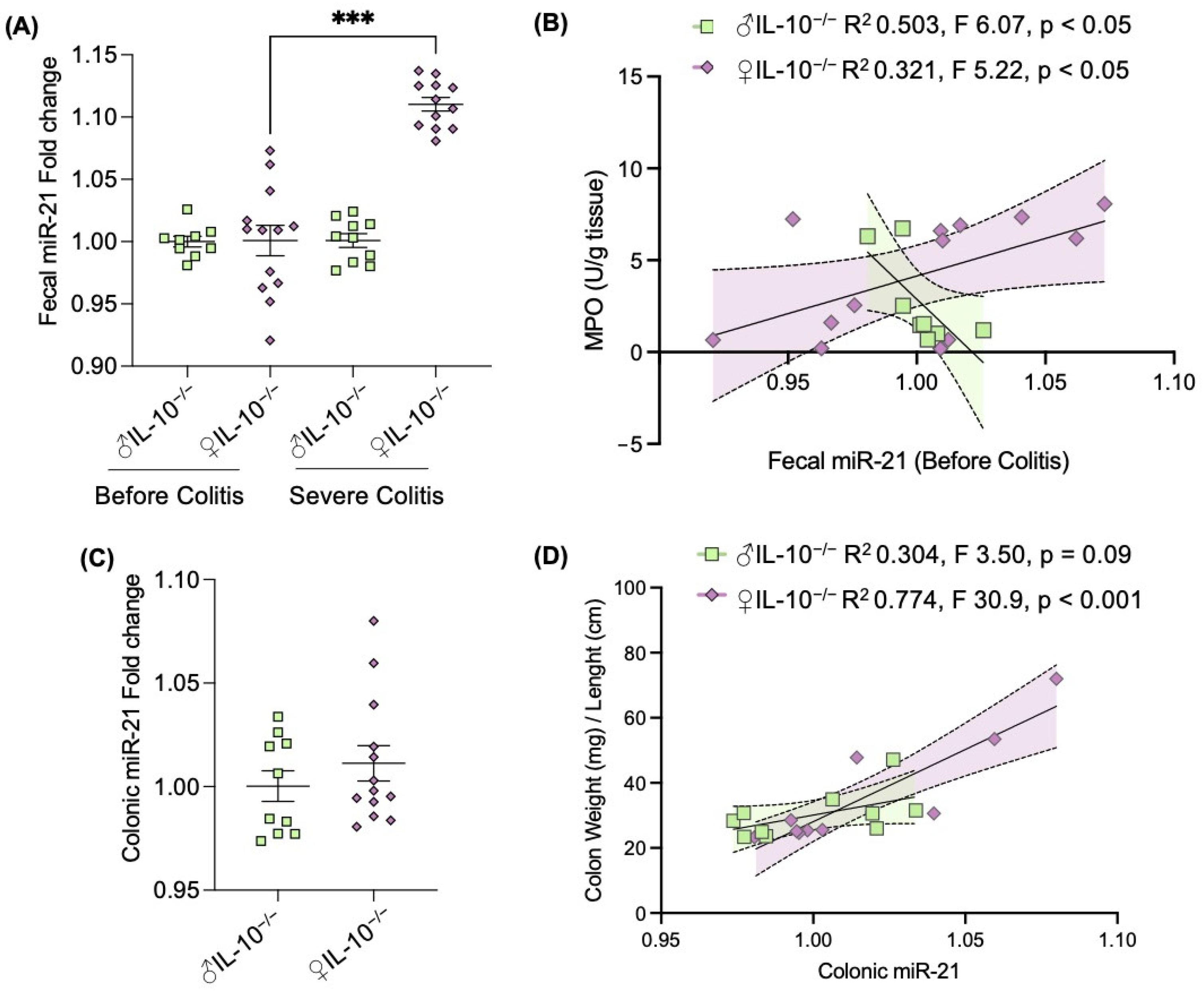
2.3. Expression of Fecal miR-21 Is Influenced by Sex
3. Discussion
4. Material and Methods
4.1. Mice Experiment and Housing
4.2. Sample Collection and Preparation
4.3. Preparation of Fecal Supernatant
4.4. Quantification of Fecal Lipocalin-2 by ELISA
4.5. Colonic Myeloperoxidase Assay
4.6. Colonic mRNA Isolation and Quantitative Real-Time qPCR Assays
4.7. Fecal and Colonic miRNA Extraction and Quantification
4.8. Quantification of Fecal LPS and Flagellin Load
4.9. Microbiota Analysis by 16S rRNA Gene Sequencing Using Illumina Technology
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westergaard, D.; Moseley, P.; Sørup, F.K.H.; Baldi, P.; Brunak, S. Population-wide analysis of differences in disease progression patterns in men and women. Nat. Commun. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Asai, K.; Hiki, N.; Mimura, Y.; Ogawa, T.; Unou, K.; Kaminishi, M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: Role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 2001, 16, 340–343. [Google Scholar] [CrossRef]
- Islam, H.; Jackson, G.S.; Yoon, J.S.J.; Cabral-Santos, C.; Lira, F.S.; Mui, A.L.; Little, J.P. Sex differences in IL-10′s anti-inflammatory function: Greater STAT3 activation and stronger inhibition of TNF-α production in male blood leukocytes ex vivo. Am. J. Physiol.—Cell Physiol. 2022, 322, C1095–C1104. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Mao, R.; Magro, F. Changing paradigms in management of inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1044–1046. [Google Scholar] [CrossRef]
- Van Linschoten, R.C.A.; Visser, E.; Niehot, C.D.; van der Woude, C.J.; Hazelzet, J.A.; van Noord, D.; West, R.L. Systematic review: Societal cost of illness of inflammatory bowel disease is increasing due to biologics and varies between continents. Aliment. Pharmacol. Ther. 2021, 54, 234–248. [Google Scholar] [CrossRef]
- Liu, L.Y.; Schaub, M.A.; Sirota, M.; Butte, A.J. Transmission distortion in Crohn’s disease risk gene ATG16L1 leads to sex difference in disease association. Inflamm. Bowel Dis. 2012, 18, 312–322. [Google Scholar] [CrossRef]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L. Gender Differences in Inflammatory Bowel Disease. Digestion 2020, 101, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tedde, A.; Laura Putignano, A.; Bagnoli, S.; Congregati, C.; Milla, M.; Sorbi, S.; Genuardi, M.; Papi, L. Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand. J. Gastroenterol. 2008, 43, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Onizawa, M.; Fujiwara, T.; Gunji, N.; Imamura, H.; Katakura, K.; Ohira, H. Evaluation of the relationship between the spleen volume and the disease activity in ulcerative colitis and Crohn disease. Medicine 2022, 101, e28515. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Chassaing, B.; Tahsin, A.; Pujada, A.; Wang, L.; Gewirtz, A.T.; Merlin, D. Host-derived fecal microRNAs can indicate gut microbiota healthiness and ability to induce inflammation. Theranostics 2019, 9, 4542–4557. [Google Scholar] [CrossRef]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Chassaing, B.; Ley, R.E.; Gewirtz, A.T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014, 147, 1363–1377.e17. [Google Scholar] [CrossRef]
- Casado-Bedmar, M.; Viennois, E. MicroRNA and Gut Microbiota: Tiny but Mighty-Novel Insights into Their Cross-talk in Inflammatory Bowel Disease Pathogenesis and Therapeutics. J. Crohns Colitis 2022, 16, 992–1005. [Google Scholar] [CrossRef]
- Viennois, E.; Zhao, Y.; Han, M.K.; Xiao, B.; Zhang, M.; Prasad, M.; Wang, L.; Merlin, D. Serum miRNA signature diagnoses and discriminates murine colitis subtypes and predicts ulcerative colitis in humans. Sci. Rep. 2017, 7, 2520. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Polsinelli, M.; Placidi, G.; Di Silvestre, D.; Ginaldi, L. Gender Differences in Osteoporosis: A Single-Center Observational Study. World J. Mens. Health 2021, 39, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001, 2, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ge, L.; Muthupalani, S.; Feng, Y.; Fox, J.G. Male-Dependent Promotion of Colitis in 129 Rag2(-/-) Mice Co-Infected with Helicobacter pylori and Helicobacter hepaticus. Int. J. Mol. Sci. 2020, 21, 8886. [Google Scholar] [CrossRef]
- Prata, M.M.; Havt, A.; Bolick, D.T.; Pinkerton, R.; Lima, A.; Guerrant, R.L. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J. Transl. Sci. 2016, 2, 134–139. [Google Scholar] [CrossRef]
- Gunasekera, D.C.; Ma, J.; Vacharathit, V.; Shah, P.; Ramakrishnan, A.; Uprety, P.; Shen, Z.; Sheh, A.; Brayton, C.F.; Whary, M.T.; et al. The development of colitis in Il10(-/-) mice is dependent on IL-22. Mucosal Immunol. 2020, 13, 493–506. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Stülb, H.; Bachmann, M.; Gonther, S.; Mühl, H. Acetaminophen-Induced Liver Injury Exposes Murine IL-22 as Sex-Related Gene Product. Int. J. Mol. Sci. 2021, 22, 10623. [Google Scholar] [CrossRef]
- Abdelnabi, M.N.; Flores Molina, M.; Soucy, G.; Quoc-Huy Trinh, V.; Bédard, N.; Mazouz, S.; Jouvet, N.; Dion, J.; Tran, S.; Bilodeau, M.; et al. Sex-Dependent Hepatoprotective Role of IL-22 Receptor Signaling in Non-Alcoholic Fatty Liver Disease-Related Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 1269–1294. [Google Scholar] [CrossRef]
- Goodman, W.A.; Havran, H.L.; Quereshy, H.A.; Kuang, S.; De Salvo, C.; Pizarro, T.T. Estrogen Receptor α Loss-of-Function Protects Female Mice From DSS-Induced Experimental Colitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 630–633.e1. [Google Scholar] [CrossRef]
- Pierdominici, M.; Maselli, A.; Varano, B.; Barbati, C.; Cesaro, P.; Spada, C.; Zullo, A.; Lorenzetti, R.; Rosati, M.; Rainaldi, G.; et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 2015, 6, 40443–40451. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Su, J.; Liu, R.; Zhao, S.; Li, W.; Xu, X.; Li, D.; Shi, J.; Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12, 7080. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Kim, N.; Song, C.-H.; Nam, R.H.; Choi, S.I.; Kim, J.S.; Lee, D.H. Sex-related Alterations of Gut Microbiota in the C57BL/6 Mouse Model of Inflammatory Bowel Disease. J. Cancer Prev. 2019, 24, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yang, J.-Y.; Yan, Z.-H.; Hu, S.; Li, J.-Q.; Xu, Z.-X.; Jian, Y.-P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef]
- Gobert, A.P.; Sagrestani, G.; Delmas, E.; Wilson, K.T.; Verriere, T.G.; Dapoigny, M.; Del’homme, C.; Bernalier-Donadille, A. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci. Rep. 2016, 6, 39399. [Google Scholar] [CrossRef]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10−/− Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N.; Nam, R.H.; Sohn, S.H.; Lee, S.M.; Choi, D.; Yoon, H.; Kim, Y.S.; Lee, H.S.; Lee, D.H. Probiotics reduce repeated water avoidance stress-induced colonic microinflammation in Wistar rats in a sex-specific manner. PLoS ONE 2017, 12, e0188992. [Google Scholar] [CrossRef]
- Karunasena, E.; McMahon, K.W.; Chang, D.; Brashears, M.M. Host responses to the pathogen Mycobacterium avium subsp. paratuberculosis and beneficial microbes exhibit host sex specificity. Appl. Environ. Microbiol. 2014, 80, 4481–4490. [Google Scholar] [CrossRef]
- Pigrau, M.; Rodiño-Janeiro, B.K.; Casado-Bedmar, M.; Lobo, B.; Vicario, M.; Santos, J.; Alonso-Cotoner, C. The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: Implications for irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- Ebada, M.A.; Mostafa, A.; Gadallah, A.A.; Alkanj, S.; Alghamdi, B.S.; Ashraf, G.M.; Abuzenadah, A.M.; Alserihi, R.F.; Wadaa-Allah, A.; Salama, M. Potential Regulation of miRNA-29 and miRNA-9 by Estrogens in Neurodegenerative Disorders: An Insightful Perspective. Brain Sci. 2023, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.H.; Potenza, N. What microRNAs could tell us about the human X chromosome. Cell. Mol. Life Sci. 2020, 77, 4069–4080. [Google Scholar] [CrossRef]
- Tomeva, E.; Krammer, U.D.B.; Switzeny, O.J.; Haslberger, A.G.; Hippe, B. Sex-Specific miRNA Differences in Liquid Biopsies from Subjects with Solid Tumors and Healthy Controls. Epigenomes 2023, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Tsai, S.F.; Hsu, C.T.; Huang, S.T.; Chuang, Y.W.; Yu, T.M.; Wu, M.J.; Chen, C.H. Gender Differences in microRNA Expressions as Related to Long-Term Graft Function in Kidney Transplant Patients. Int. J. Mol. Sci. 2022, 23, 12832. [Google Scholar] [CrossRef]
- Francavilla, A.; Gagliardi, A.; Piaggeschi, G.; Tarallo, S.; Cordero, F.; Pensa, R.G.; Impeduglia, A.; Caviglia, G.P.; Ribaldone, D.G.; Gallo, G.; et al. Faecal miRNA profiles associated with age, sex, BMI, and lifestyle habits in healthy individuals. Sci. Rep. 2021, 11, 20645. [Google Scholar] [CrossRef]
- Viennois, E.; Xiao, B.; Ayyadurai, S.; Wang, L.; Wang, P.G.; Zhang, Q.; Chen, Y.; Merlin, D. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab. Investig. 2014, 94, 950–965. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Bedmar, M.; Roy, M.; Viennois, E. The Effect of Sex-Specific Differences on IL-10−/− Mouse Colitis Phenotype and Microbiota. Int. J. Mol. Sci. 2023, 24, 10364. https://doi.org/10.3390/ijms241210364
Casado-Bedmar M, Roy M, Viennois E. The Effect of Sex-Specific Differences on IL-10−/− Mouse Colitis Phenotype and Microbiota. International Journal of Molecular Sciences. 2023; 24(12):10364. https://doi.org/10.3390/ijms241210364
Chicago/Turabian StyleCasado-Bedmar, Maite, Maryline Roy, and Emilie Viennois. 2023. "The Effect of Sex-Specific Differences on IL-10−/− Mouse Colitis Phenotype and Microbiota" International Journal of Molecular Sciences 24, no. 12: 10364. https://doi.org/10.3390/ijms241210364




