Rag1 Deficiency Impairs Arteriogenesis in Mice
Abstract
:1. Introduction
2. Results
2.1. Impaired Perfusion Recovery and Arterial Growth in Rag1 KO Mice
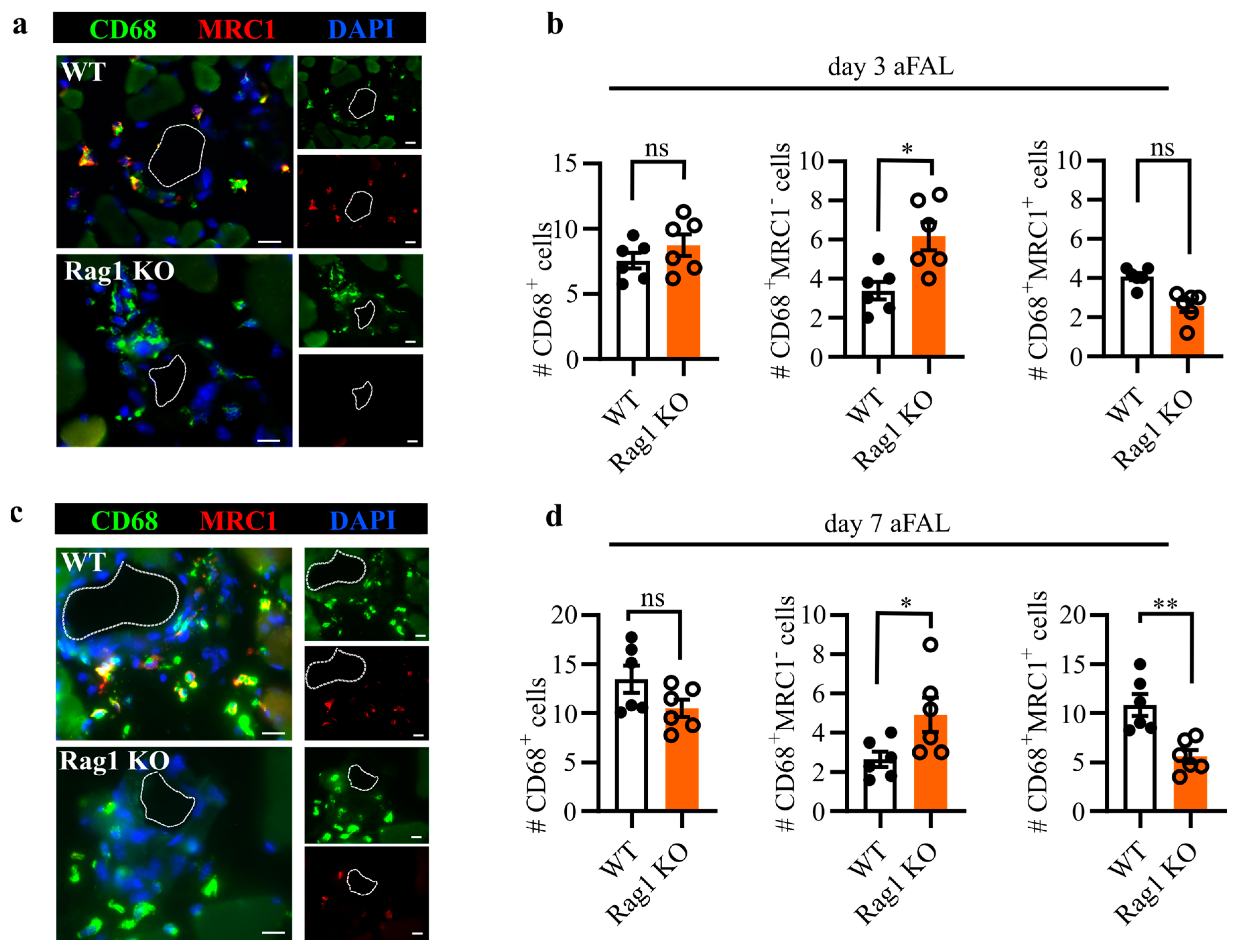
2.2. Altered Perivascular Macrophage Polarization in Rag1 KO Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Femoral Artery Ligation and Laser Doppler Imaging
4.3. BrdU Treatment
4.4. Perfusion and Tissue Harvesting
4.5. Immunohistology
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Faber, J.E.; Chilian, W.M.; Deindl, E.; Van Royen, N.; Simons, M. A brief etymology of the collateral circulation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Deindl, E.; Schaper, W. The art of arteriogenesis. Cell Biochem. Biophys. 2005, 43, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lasch, M.; Kleinert, E.C.; Meister, S.; Kumaraswami, K.; Buchheim, J.-I.; Grantzow, T.; Lautz, T.; Salpisti, S.; Fischer, S.; Troidl, K.; et al. Extracellular RNA released due to shear stress controls natural bypass growth by mediating mechanotransduction in mice. Blood 2019, 134, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Schaper, W.; Scholz, D. Factors Regulating Arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef]
- Kim, C.; Williams, M.A. Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology 2010, 131, 310–317. [Google Scholar] [CrossRef]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Sîrbulescu, R.F.; Mamidi, A.; Chan, S.-Y.C.; Jin, G.; Boukhali, M.; Sobell, D.; Ilieş, I.; Chung, J.Y.; Haas, W.; Whalen, M.J.; et al. B cells support the repair of injured tissues by adopting MyD88-dependent regulatory functions and phenotype. FASEB J. 2021, 35, e22019. [Google Scholar] [CrossRef]
- Weirather, J.; Hofmann, U.D.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3+CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Wu, L.; Dalal, R.; Cao, C.D.; Postoak, J.L.; Yang, G.; Zhang, Q.; Wang, Z.; Lal, H.; Van Kaer, L. IL-10–producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 2019, 116, 21673–21684. [Google Scholar] [CrossRef]
- Adamo, L.; Staloch, L.J.; Rocha-Resende, C.; Matkovich, S.J.; Jiang, W.; Bajpai, G.; Weinheimer, C.J.; Kovacs, A.; Schilling, J.D.; Barger, P.M.; et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight 2018, 3, e120137. [Google Scholar] [CrossRef] [PubMed]
- Zouggari, Y.; Ait-Oufella, H.; Waeckel, L.; Vilar, J.; Loinard, C.; Cochain, C.; Récalde, A.; Duriez, M.; Levy, B.I.; Lutgens, E.; et al. Regulatory T cells modulate postischemic neovascularization. Circulation 2009, 120, 1415–1425. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Grundmann, S.; van Royen, N.; Voskuil, M.; Schirmer, S.H.; Ulusans, S.; Bode, C.; Buschmann, I.R.; Piek, J.J. Leukocyte subpopulations and arteriogenesis: Specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 2005, 181, 285–293. [Google Scholar] [CrossRef]
- Chillo, O.; Kleinert, E.C.; Lautz, T.; Lasch, M.; Pagel, J.-I.; Heun, Y.; Troidl, K.; Fischer, S.; Caballero-Martinez, A.; Mauer, A.; et al. Perivascular Mast Cells Govern Shear Stress-Induced Arteriogenesis by Orchestrating Leukocyte Function. Cell Rep. 2016, 16, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Mombaerts, P.; Iacomini, J.; Johnson, R.S.; Herrup, K.; Tonegawa, S.; Papaioannou, V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68, 869–877. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Troidl, C.; Jung, G.; Troidl, K.; Hoffmann, J.; Mollmann, H.; Nef, H.; Schaper, W.; Hamm, C.; Schmitz-Rixen, T. The Temporal and Spatial Distribution of Macrophage Subpopulations During Arteriogenesis. Curr. Vasc. Pharmacol. 2013, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mantsounga, C.S.; Lee, C.; Neverson, J.; Sharma, S.; Healy, A.; Berus, J.M.; Parry, C.; Ceneri, N.M.; López-Giráldez, F.; Chun, H.J.; et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022, 38, 110309. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Fung, E.; Helisch, A. Macrophages in collateral arteriogenesis. Front. Physiol. 2012, 3, 353. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswami, K.; Salei, N.; Beck, S.; Rambichler, S.; Kluever, A.-K.; Lasch, M.; Richter, L.; Schraml, B.U.; Deindl, E. A simple and effective flow cytometry-based method for identification and quantification of tissue infiltrated leukocyte subpopulations in a mouse model of peripheral arterial disease. Int. J. Mol. Sci. 2020, 21, 3593. [Google Scholar] [CrossRef] [PubMed]
- Bassing, C.H.; Swat, W.; Alt, F.W. The Mechanism and Regulation of Chromosomal V(D)J Recombination. Cell 2002, 109, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Dywicki, J.; Buitrago-Molina, L.E.; Noyan, F.; Davalos-Misslitz, A.C.; Hupa-Breier, K.L.; Lieber, M.; Hapke, M.; Schlue, J.; Falk, C.S.; Raha, S.; et al. The Detrimental Role of Regulatory T Cells in Nonalcoholic Steatohepatitis. Hepatol. Commun. 2022, 6, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef]
- Capell, W.H.; Bonaca, M.P.; Nehler, M.R.; Chen, E.; Kittelson, J.M.; Anand, S.S.; Berkowitz, S.D.; Debus, E.S.; Fanelli, F.; Haskell, L.; et al. Rationale and design for the Vascular Outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am. Heart J. 2018, 199, 83–91. [Google Scholar] [CrossRef]
- Meier, P.; Hemingway, H.; Lansky, A.J.; Knapp, G.; Pitt, B.; Seiler, C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur. Heart J. 2012, 33, 614–621. [Google Scholar] [CrossRef]
- Rentrop, K.; Feit, F.; Sherman, W.; Stecy, P.; Hosat, S.; Cohen, M.; Rey, M.; Ambrose, J.; Nachamie, M.; Schwartz, W.; et al. Late thrombolytic therapy preserves left ventricular function in patients with collateralized total coronary occlusion: Primary end point findings of the second mount sinai-new york university reperfusion trial. J. Am. Coll. Cardiol. 1989, 14, 58–64. [Google Scholar] [CrossRef]
- Wagner, M.; Mahlmann, A.; Deindl, E.; Zuschratter, W.; Riek-Burchardt, M.; Kostin, S.; Luani, B.; Baer, C.; Youssef, A.; Herold, J. Clinical improvement and enhanced collateral vessel growth after xenogenic monocyte transplantation. Am. J. Transl. Res. 2019, 11, 4063–4076. [Google Scholar] [PubMed]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Arnholdt, C.; Kumaraswami, K.; Götz, P.; Kübler, M.; Lasch, M.; Deindl, E. Depletion of γδ T Cells Leads to Reduced Angiogenesis and Increased Infiltration of Inflammatory M1-like Macrophages in Ischemic Muscle Tissue. Cells 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaraswami, K.; Arnholdt, C.; Deindl, E.; Lasch, M. Rag1 Deficiency Impairs Arteriogenesis in Mice. Int. J. Mol. Sci. 2023, 24, 12839. https://doi.org/10.3390/ijms241612839
Kumaraswami K, Arnholdt C, Deindl E, Lasch M. Rag1 Deficiency Impairs Arteriogenesis in Mice. International Journal of Molecular Sciences. 2023; 24(16):12839. https://doi.org/10.3390/ijms241612839
Chicago/Turabian StyleKumaraswami, Konda, Christoph Arnholdt, Elisabeth Deindl, and Manuel Lasch. 2023. "Rag1 Deficiency Impairs Arteriogenesis in Mice" International Journal of Molecular Sciences 24, no. 16: 12839. https://doi.org/10.3390/ijms241612839







