Association of Blood Metabolomics Biomarkers with Brain Metabolites and Patient-Reported Outcomes as a New Approach in Individualized Diagnosis of Schizophrenia
Abstract
:1. Introduction
2. Results
2.1. Distributions of Clinical Parameters by Groups
2.2. Correlation Analysis of Lactates, Glutamic Acid and Cortisol Concentrations and Questionnaire Results in the Test Group
2.3. Correlation Analysis of Lactates, Glutamic acid and Cortisol Concentrations and Selected Clinical and Brain Parameters
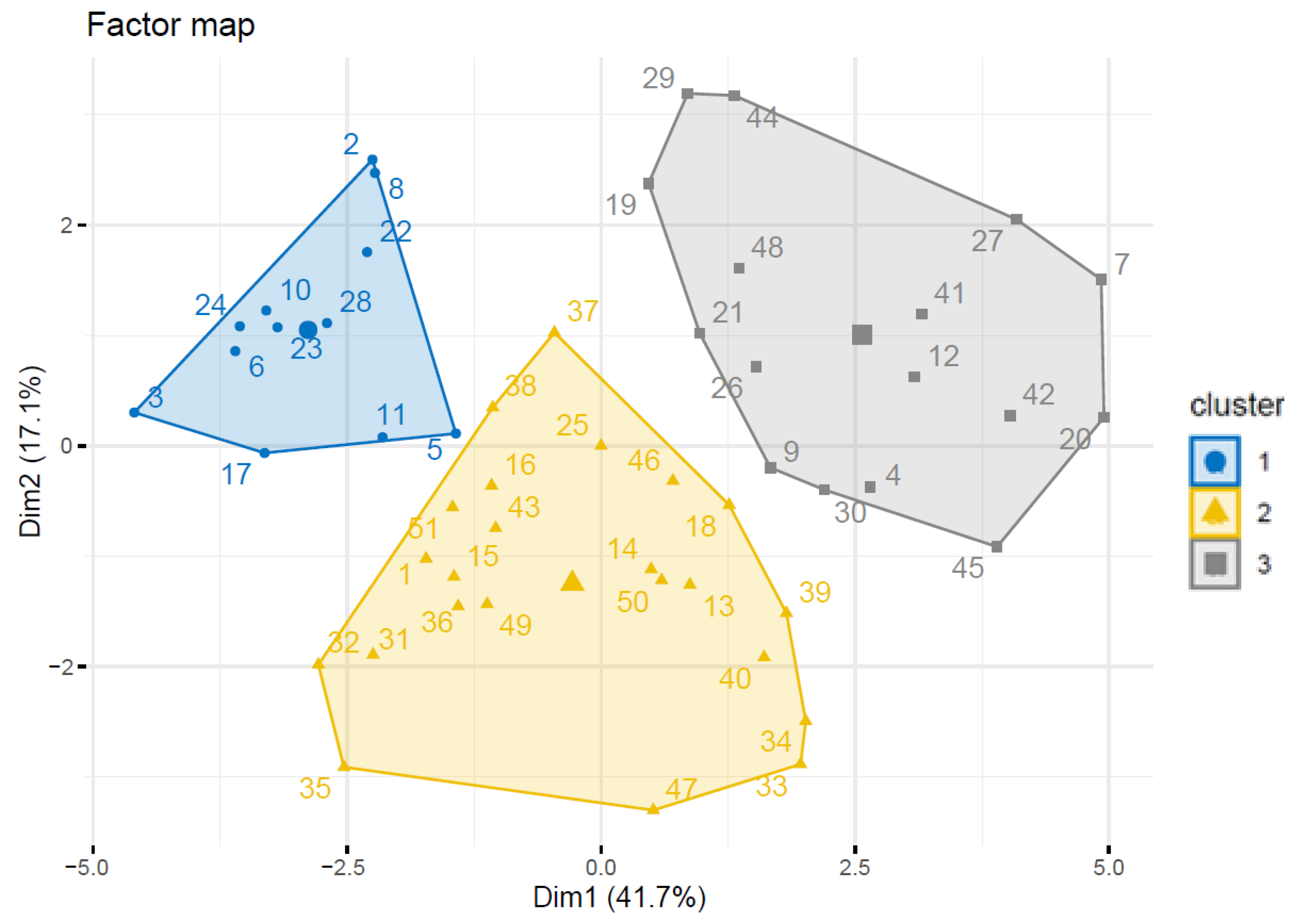
2.4. The Cluster Analysis of the Test Results in the Test Group
3. Discussion
3.1. Distributions of Clinical Parameters by Groups
3.2. Correlation Analysis of Lactates, Glutamic Acid and Cortisol Concentrations and Questionnaire Results in the Studied Group
3.3. Correlation Analysis of Lactates, Glutamic Acid and Cortisol Concentrations and Selected Clinical and Brain Parameters
3.4. The Cluster Analysis of the Test Results in the Studied Group
3.5. Limitations
4. Materials and Methods
4.1. Participants
4.2. Material
Characteristics of the Sample
4.3. Methods
4.3.1. Laboratory Routine Tests
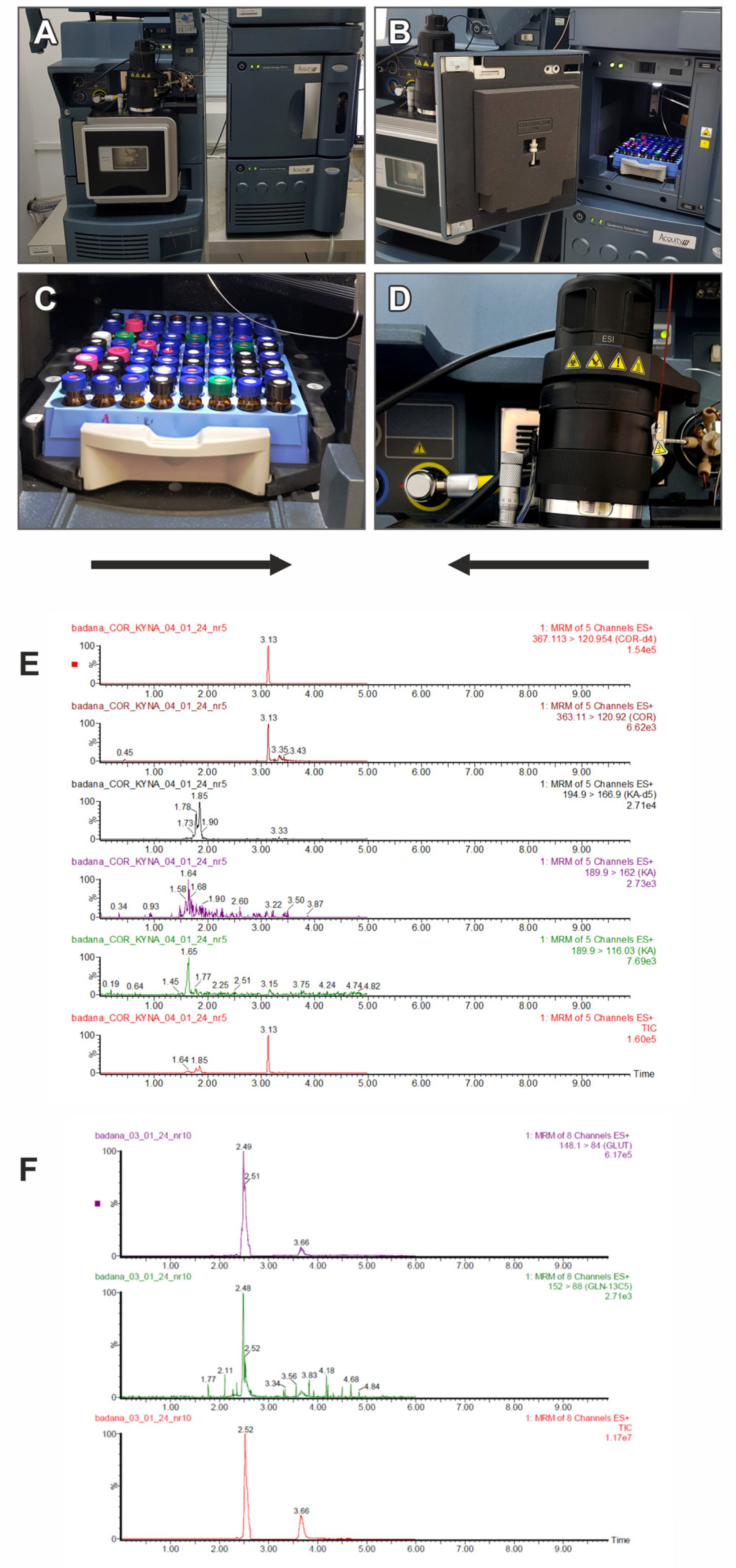
4.3.2. Determination of Metabolites in Peripheral Blood
4.3.3. Magnetic Resonance Techniques
4.3.4. Statistical Methods
Clustering Analysis
Statistical Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadakia, A.; Catillon, M.; Fan, Q.; Williams, G.R.; Marden, J.R.; Anderson, A.; Kirson, N.; Dembek, C. The Economic Burden of Schizophrenia in the United States. J. Clin. Psychiatry 2022, 83, 5377. [Google Scholar] [CrossRef]
- Evers, S.M.A.A.; Ament, A.J.H.A. Costs of Schizophrenia in The Netherlands. Schizophr. Bull. 1995, 21, 141–153. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- International Schizophrenia Consortium. Common Polygenic Variation Contributes to Risk of Schizophrenia That with Bipolar Disorder. Nature 2009, 460, 748. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, K.M.; Harrington, P.D.B. Electrospray Ionization Ion Mobility Mass Spectrometry. Crit. Rev. Anal. Chem. 2023, 53, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bruce, J.E.; Hill, H.H. Characterizing Electrospray Ionization Using Atmospheric Pressure Ion Mobility Spectrometry. Anal. Chem. 2006, 78, 7751–7760. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.X.; Hernly, E.; Hu, H.; Hilger, R.T.; Neuweger, H.; Yang, M.; Laskin, J. Nanospray Desorption Electrospray Ionization (Nano-DESI) Mass Spectrometry Imaging with High Ion Mobility Resolution. J. Am. Soc. Mass. Spectrom. 2023, 34, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Uetrecht, C.; Rose, R.J.; van Duijn, E.; Lorenzen, K.; Heck, A.J.R. Ion Mobility Mass Spectrometry of Proteins and Proteinassemblies. Chem. Soc. Rev. 2010, 39, 1633–1655. [Google Scholar] [CrossRef] [PubMed]
- Dibner, B. Ten Founding Fathers of the Electrical Science: VIII. Michael Faraday. Electr. Eng. 2013, 73, 966–967. [Google Scholar] [CrossRef]
- Thomson, J.J.; Rutherford, E. On the Passage of Electricity through Gases Exposed to Röntgen Rays. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 42, 392–407. [Google Scholar] [CrossRef]
- Schaefer, C.; Schlottmann, F.; Kirk, A.T.; Zimmermann, S. Influence of Sample Gas Humidity on Product Ion Formation in High Kinetic Energy Ion Mobility Spectrometry (HiKE-IMS). J. Am. Soc. Mass. Spectrom. 2022, 33, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Dempster, A.J. On The Mobility of Ions in Air at High Pressures. Phys. Rev. 1912, 34, 53. [Google Scholar] [CrossRef]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass. Spectrom. 2019, 30, 2185. [Google Scholar] [CrossRef] [PubMed]
- May, J.C.; McLean, J.A. Ion Mobility-Mass Spectrometry: Time-Dispersive Instrumentation. Anal. Chem. 2015, 87, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.J.; Oranzi, N.R.; Ahmadireskety, A.; Kemperman, R.H.J.; Wei, M.S.; Yost, R.A. Recent Progress in Metabolomics Using Ion Mobility-Mass Spectrometry. TrAC Trends Anal. Chem. 2019, 116, 274–281. [Google Scholar] [CrossRef]
- Delvaux, A.; Rathahao-Paris, E.; Alves, S. Different Ion Mobility-Mass Spectrometry Coupling Techniques to Promote Metabolomics. Mass. Spectrom. Rev. 2022, 41, 695–721. [Google Scholar] [CrossRef]
- Wang, F.; Guo, L.; Zhang, T.; Cui, Z.; Wang, J.; Zhang, C.; Xue, F.; Zhou, C.; Li, B.; Tan, Q.; et al. Alterations in Plasma Lipidomic Profiles in Adult Patients with Schizophrenia and Major Depressive Disorder. Medicina 2022, 58, 1509. [Google Scholar] [CrossRef]
- Campeau, A.; Mills, R.H.; Stevens, T.; Rossitto, L.A.; Meehan, M.; Dorrestein, P.; Daly, R.; Nguyen, T.T.; Gonzalez, D.J.; Jeste, D.V.; et al. Multi-Omics of Human Plasma Reveals Molecular Features of Dysregulated Inflammation and Accelerated Aging in Schizophrenia. Mol. Psychiatry 2022, 27, 1217. [Google Scholar] [CrossRef]
- Okamoto, N.; Ikenouchi, A.; Watanabe, K.; Igata, R.; Fujii, R.; Yoshimura, R. A Metabolomics Study of Serum in Hospitalized Patients With Chronic Schizophrenia. Front. Psychiatry 2021, 12, 763547. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Targeted Lipidomics and Metabolomics Evaluations of Cortical Neuronal Stress in Schizophrenia. Schizophr. Res. 2019, 212, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Wang, P.; Cui, G.; Zhang, J.; Liang, K.; Xia, Z.; Wang, P.; He, L.; Jia, W. Targeted Metabolomics Reveals Aberrant Profiles of Serum Bile Acids in Patients with Schizophrenia. Schizophrenia 2022, 8, 65. [Google Scholar] [CrossRef]
- Amdanee, N.; Shao, M.; Hu, X.; Fang, X.; Zhou, C.; Chen, J.; Ridwan Chattun, M.; Wen, L.; Pan, X.; Zhang, X.; et al. Serum Metabolic Profile in Schizophrenia Patients With Antipsychotic-Induced Constipation and Its Relationship with Gut Microbiome. Schizophr. Bull. 2023, 49, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Simić, K.; Todorović, N.; Trifunović, S.; Miladinović, Z.; Gavrilović, A.; Jovanović, S.; Avramović, N.; Gođevac, D.; Vujisić, L.; Tešević, V.; et al. NMR Metabolomics in Serum Fingerprinting of Schizophrenia Patients in a Serbian Cohort. Metabolites 2022, 12, 707. [Google Scholar] [CrossRef]
- World Health Organization. Comprehensive Mental Health Action Plan 2013–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bryll, A.; Krzyściak, W.; Karcz, P.; Pilecki, M.; Śmierciak, N.; Szwajca, M.; Skalniak, A.; Popiela, T.J. Determinants of Schizophrenia Endophenotypes Based on Neuroimaging and Biochemical Parameters. Biomedicines 2021, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Tobolska, D.; Maria Wilczyyski, K.; Lorek, M.; Mazgaj, E.; Krysta, K.; Gawlik, A.; Krzystanek, M. Evaluation of the Cortisol Concentrations in Patients with Schizophrenia. Psychiatr. Danub. 2016, 28, 162–164. [Google Scholar]
- Streit, F.; Memic, A.; Hasandedić, L.; Rietschel, L.; Frank, J.; Lang, M.; Witt, S.H.; Forstner, A.J.; Degenhardt, F.; Wüst, S.; et al. Perceived Stress and Hair Cortisol: Differences in Bipolar Disorder and Schizophrenia. Psychoneuroendocrinology 2016, 69, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Jezova, D.; Makatsori, A.; Duncko, R.; Moncek, F.; Jakubek, M. High Trait Anxiety in Healthy Subjects Is Associated with Low Neuroendocrine Activity during Psychosocial Stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, L.L.; Smit, A.M.; Stalder, T.; Kirschbaum, C.; Seedat, S.; Emsley, R. Hair Cortisol Levels in Schizophrenia and Metabolic Syndrome. Early Interv. Psychiatry 2022, 16, 902–911. [Google Scholar] [CrossRef]
- Seitz, R.; Vracotas, N.; Bechard-Evans, L.; King, S.; Abadi, S.; Joober, R.; Shah, J.L.; Malla, A.K.; Pruessner, M. The Trier Social Stress Test in First Episode Psychosis Patients: Impact of Perceived Stress, Protective Factors and Childhood Trauma. Psychoneuroendocrinology 2019, 105, 155–163. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, D.F.; Cao, L.Y.; Wu, G.Y.; Shen, Y.C. Cortisol and Cytokines in Chronic and Treatment-Resistant Patients with Schizophrenia: Association with Psychopathology and Response to Antipsychotics. Neuropsychopharmacology 2005, 30, 1532–1538. [Google Scholar] [CrossRef]
- Woldesenbet, Y.M.; Alenko, A.; Bukata, I.T.; Gedefaw, L.; Fikru, C. The Status of Serum Cortisol before and after Treatment of Schizophrenia and Its Correlation to Disease Severity and Improvement: A Longitudinal Study. SAGE Open Med. 2021, 9. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate Uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Kim, J.S.; Kornhuber, H.H.; Schmid-Burgk, W.; Holzmüller, B. Low Cerebrospinal Fluid Glutamate in Schizophrenic Patients and a New Hypothesis on Schizophrenia. Neurosci. Lett. 1980, 20, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Coyle, J.T. The Emerging Role of Glutamate in the Pathophysiology and Treatment of Schizophrenia. Am. J. Psychiatry 2001, 158, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Palomino, A.; González-Pinto, A.; Aldama, A.; González-Gómez, C.; Mosquera, F.; González-García, G.; Matute, C. Decreased Levels of Plasma Glutamate in Patients with First-Episode Schizophrenia and Bipolar Disorder. Schizophr. Res. 2007, 95, 174–178. [Google Scholar] [CrossRef]
- Marsman, A.; Van Den Heuvel, M.P.; Klomp, D.W.J.; Kahn, R.S.; Luijten, P.R.; Hulshoff Pol, H.E. Glutamate in Schizophrenia: A Focused Review and Meta-Analysis of 1H-MRS Studies. Schizophr. Bull. 2013, 39, 120–129. [Google Scholar] [CrossRef]
- Kuang, H.; Duong, A.; Jeong, H.; Zachos, K.; Andreazza, A.C. Lactate in Bipolar Disorder: A Systematic Review and Meta-Analysis. Psychiatry Clin. Neurosci. 2018, 72, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Leweke, F.M.; Tsang, T.M.; Koethe, D.; Kranaster, L.; Gerth, C.W.; Gross, S.; Schreiber, D.; Ruhrmann, S.; Schutlze-Lutter, F.; et al. CSF Metabolic and Proteomic Profiles in Patients Prodromal for Psychosis. PLoS ONE 2007, 2, e756. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K.; McGuire, P.K.; Egerton, A.; Aleman, A.; Block, W.; Bloemen, O.J.N.; Borgan, F.; Bustillo, J.R.; Capizzano, A.A.; Coughlin, J.M.; et al. Association of Age, Antipsychotic Medication, and Symptom Severity in Schizophrenia With Proton Magnetic Resonance Spectroscopy Brain Glutamate Level: A Mega-Analysis of Individual Participant-Level Data. JAMA Psychiatry 2021, 78, 667–681. [Google Scholar] [CrossRef]
- Onozato, M.; Umino, M.; Shoji, A.; Ichiba, H.; Tsujino, N.; Funatogawa, T.; Tagata, H.; Nemoto, T.; Mizuno, M.; Fukushima, T. Serum d- and l-Lactate, Pyruvate and Glucose Levels in Individuals with at-Risk Mental State and Correlations with Clinical Symptoms. Early Interv. Psychiatry 2020, 14, 410–417. [Google Scholar] [CrossRef]
- Krzyściak, W.; Karcz, P.; Bystrowska, B.; Szwajca, M.; Bryll, A.; Śmierciak, N.; Ligęzka, A.; Turek, A.; Kozicz, T.; Skalniak, A.E.; et al. The Association of the Oral Microbiota with the Effects of Acid Stress Induced by an Increase of Brain Lactate in Schizophrenia Patients. Biomedicines 2023, 11, 240. [Google Scholar] [CrossRef]
- Averill, L.A.; Purohit, P.; Averill, C.L.; Boesl, M.A.; Krystal, J.H.; Abdallah, C.G. Glutamate Dysregulation and Glutamatergic Therapeutics for PTSD: Evidence from Human Studies. Neurosci. Lett. 2017, 649, 147–155. [Google Scholar] [CrossRef]
- Riaza Bermudo-Soriano, C.; Perez-Rodriguez, M.M.; Vaquero-Lorenzo, C.; Baca-Garcia, E. New Perspectives in Glutamate and Anxiety. Pharmacol. Biochem. Behav. 2012, 100, 752–774. [Google Scholar] [CrossRef]
- Bergink, V.; Van Megen, H.J.G.M.; Westenberg, H.G.M. Glutamate and Anxiety. Eur. Neuropsychopharmacol. 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Nordman, J.C. Anger Management: Mechanisms of Glutamate Receptor-Mediated Synaptic Plasticity Underlying Animal Aggression. Int. J. Biochem. Cell Biol. 2022, 142, 106120. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Papadakis, K.; Csernansky, J.; Litman, R.; Volavka, J.; Jia, X.D.; Gage, A. A Randomized, Placebo-Controlled Study of Memantine as Adjunctive Treatment in Patients with Schizophrenia. Neuropsychopharmacology 2008, 34, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Gruenbaum, B.F.; Shelef, I.; Zvenigorodsky, V.; Severynovska, O.; Fleidervish, I.; Knyazer, B.; Frenkel, A.; Zlotnik, A.; Kofman, O.; et al. Blood Glutamate Scavenging as a Novel Glutamate-Based Therapeutic Approach for Post-Traumatic Brain Injury Anxiety and Social Impairment. Transl. Psychiatry 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Szwajca, M.; Śmierciak, N.; Chrzan, R.; Turek, A.; Karcz, P.; Bryll, A.; Pilecki, M.; Morava, E.; Kozicz, T.; et al. From Periphery Immunity to Central Domain Through Clinical Interview as a New Insight on Schizophrenia. Sci. Rep. 2024; 60930144-0c5a-4776-9ac3-2a1d7f1b2d88./v.2.1, Manuscript Awaiting Reviewers Report. [Google Scholar]
- Michael, N.; Erfurth, A.; Ohrmann, P.; Gössling, M.; Arolt, V.; Heindel, W.; Pfleiderer, B. Acute Mania Is Accompanied by Elevated Glutamate/Glutamine Levels within the Left Dorsolateral Prefrontal Cortex. Psychopharmacology 2003, 168, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Shevelkin, A.V.; Terrillion, C.E.; Hasegawa, Y.; Mychko, O.A.; Jouroukhin, Y.; Sawa, A.; Kamiya, A.; Pletnikov, M.V. Astrocyte DISC1 Contributes to Cognitive Function in a Brain Region-Dependent Manner. Hum. Mol. Genet. 2020, 29, 2936–2950. [Google Scholar] [CrossRef] [PubMed]
- Śmierciak, N.; Szwajca, M.; Popiela, T.J.; Bryll, A.; Karcz, P.; Donicz, P.; Turek, A.; Krzyściak, W.; Pilecki, M. Redefining the Cut-off Ranges for TSH Based on the Clinical Picture, Results of Neuroimaging and Laboratory Tests in Unsupervised Cluster Analysis as Individualized Diagnosis of Early Schizophrenia. J. Pers. Med. 2022, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the Glutamatergic System to Treat Major Depressive Disorder. Drugs 2012, 72, 1313–1333. [Google Scholar] [CrossRef] [PubMed]
- Küçükibrahimoğlu, E.; Saygın, M.Z.; Çalışkan, M.; Kaplan, O.K.; Ünsal, C.; Gören, M.Z. The Change in Plasma GABA, Glutamine and Glutamate Levels in Fluoxetine- or S-Citalopram-Treated Female Patients with Major Depression. Eur. J. Clin. Pharmacol. 2009, 65, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H.Y. Plasma Concentrations of Excitatory Amino Acids, Serine, Glycine, Taurine and Histidine in Major Depression. Eur. Neuropsychopharmacol. 1995, 5, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Shirayama, Y.; Yamada, T.; Maeda, K.; Ashby, C.R.; Kawahara, R. Correlation between Plasma Levels of Glutamate, Alanine and Serine with Severity of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A. The Possible Place for Psychedelics in Pharmacotherapy of Mental Disorders. Pharmacol. Rep. 2023, 75, 1313–1325. [Google Scholar] [CrossRef]
- Aghajani, R.; Naderi, N.; Sadeghi, N.; Ani, M.; Ani, S.; Nasr-Esfahani, M.H. Distribution of Plasma One-Carbon Metabolism Factors and Amino Acids Profile in Depression State Treated with Paroxetine: A Model Study. Cell J. 2023, 25, 165. [Google Scholar] [CrossRef]
- Zlotnik, A.; Klin, Y.; Kotz, R.; Dubilet, M.; Boyko, M.; Ohayon, S.; Shapira, Y.; Teichberg, V.I. Regulation of Blood L-Glutamate Levels by Stress as a Possible Brain Defense Mechanism. Exp. Neurol. 2010, 224, 465–471. [Google Scholar] [CrossRef]
- Goldberg, D. Use of the General Health Questionnaire in Clinical Work. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1188–1189. [Google Scholar] [CrossRef]
- Goldberg, D.P.; Hillier, V.F. A Scaled Version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef]
- Mattson, M.P. Glutamate and Neurotrophic Factors in Neuronal Plasticity and Disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Gruenbaum, B.F.; Oleshko, A.; Merzlikin, I.; Zlotnik, A. Diet’s Impact on Post-Traumatic Brain Injury Depression: Exploring Neurodegeneration, Chronic Blood–Brain Barrier Destruction, and Glutamate Neurotoxicity Mechanisms. Nutrients 2023, 15, 4681. [Google Scholar] [CrossRef] [PubMed]
- Luykx, J.J.; Laban, K.G.; van den Heuvel, M.P.; Boks, M.P.M.; Mandl, R.C.W.; Kahn, R.S.; Bakker, S.C. Region and State Specific Glutamate Downregulation in Major Depressive Disorder: A Meta-Analysis of 1H-MRS Findings. Neurosci. Biobehav. Rev. 2012, 36, 198–205. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.M.; Schwarz, M.J. The Impact of Neuroimmune Dysregulation on Neuroprotection and Neurotoxicity in Psychiatric Disorders—Relation to Drug Treatment. Dialogues Clin. Neurosci. 2009, 11, 319–332. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and Psychiatric Illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- De Bie, T.H.; Witkamp, R.F.; Jongsma, M.A.; Balvers, M.G.J. Development and Validation of a UPLC-MS/MS Method for the Simultaneous Determination of Gamma-Aminobutyric Acid and Glutamic Acid in Human Plasma. J. Chromatogr. B 2021, 1164, 122519. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, H.H. Glutamate Receptor Genes: Susceptibility Factors in Schizophrenia and Depressive Disorders? Mol. Neurobiol. 2002, 25, 191–212. [Google Scholar] [CrossRef]
- Shan, B.; Ai, Z.; Zeng, S.; Song, Y.; Song, J.; Zeng, Q.; Liao, Z.; Wang, T.; Huang, C.; Su, D. Gut Microbiome-Derived Lactate Promotes to Anxiety-like Behaviors through GPR81 Receptor-Mediated Lipid Metabolism Pathway. Psychoneuroendocrinology 2020, 117, 104699. [Google Scholar] [CrossRef]
- Kowalski, K.; Szponar, B.; Bochen, P.; Żebrowska-Różańska, P.; Łaczmański, Ł.; Samochowiec, J.; Misiak, B. Altered Levels of Fecal Short-Chain Fatty Acids Are Associated with Subclinical Inflammation and Worse Cognitive Performance in Patients with Schizophrenia. J. Psychiatr. Res. 2023, 165, 298–304. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of Acetate, Propionate, and Butyrate Formation by the Human Fecal Microbial Flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Miller, B.J. Inflammation and Schizophrenia. Schizophr. Bull. 2013, 39, 1174–1179. [Google Scholar] [CrossRef]
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.T.J.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial Dysfunction in Schizophrenia: Evidence for Compromised Brain Metabolism and Oxidative Stress. Mol. Psychiatry 2004, 9, 684–697. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Brooks, G.A.; Schoeller, D.A.; Budinger, T.F. Disposal of Blood [1-13C]Lactate in Humans during Rest and Exercise. J. Appl. Physiol. 1986, 60, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Shahda, M.; Mahmoud, E.H.M.; Rakha, S.A.; Shoaib, M. Blood Lactate Levels as a Biomarker of Antipsychotic Side Effects in Patients with Schizophrenia. J. Psychopharmacol. 2016, 30, 63–68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Diseases ICD-11; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Unterberg, A.W.; Stover, J.; Kress, B.; Kiening, K.L. Edema and Brain Trauma. Neuroscience 2004, 129, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P. Umbilical Cord PH, Blood Gases, and Lactate at Birth: Normal Values, Interpretation, and Clinical Utility. Am. J. Obstet. Gynecol. 2023, 228, S1222–S1240. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, S.; Lustenberger, T.; Relja, B.; Lehnert, M.; Marzi, I. Pathophysiologie Des Polytraumas. Der Chir. 2013, 84, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Gruenbaum, B.F.; Frank, D.; Natanel, D.; Negev, S.; Azab, A.N.; Barsky, G.; Knyazer, B.; Kofman, O.; Zlotnik, A. The Integrity of the Blood–Brain Barrier as a Critical Factor for Regulating Glutamate Levels in Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 5897. [Google Scholar] [CrossRef] [PubMed]
- Ousdal, O.T.; Milde, A.M.; Craven, A.R.; Ersland, L.; Endestad, T.; Melinder, A.; Huys, Q.J.; Hugdahl, K. Prefrontal Glutamate Levels Predict Altered Amygdala–Prefrontal Connectivity in Traumatized Youths. Psychol. Med. 2019, 49, 1822–1830. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The Role of Childhood Trauma in the Neurobiology of Mood and Anxiety Disorders: Preclinical and Clinical Studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Flory, J.D.; Yehuda, R.; Grossman, R.; New, A.S.; Mitropoulou, V.; Siever, L.J. Childhood Trauma and Basal Cortisol in People with Personality Disorders. Compr. Psychiatry 2009, 50, 34. [Google Scholar] [CrossRef]
- Mazer, A.K.; Cleare, A.J.; Young, A.H.; Juruena, M.F. Bipolar Affective Disorder and Borderline Personality Disorder: Differentiation Based on the History of Early Life Stress and Psychoneuroendocrine Measures. Behav. Brain Res. 2019, 357–358, 48–56. [Google Scholar] [CrossRef]
- Bauer, M.E.; Wieck, A.; Lopes, R.P.; Teixeira, A.L.; Grassi-Oliveira, R. Interplay between Neuroimmunoendocrine Systems during Post-Traumatic Stress Disorder: A Minireview. Neuroimmunomodulation 2010, 17, 192–195. [Google Scholar] [CrossRef]
- Tendolkar, I.; Mårtensson, J.; Kühn, S.; Klumpers, F.; Fernández, G. Physical Neglect during Childhood Alters White Matter Connectivity in Healthy Young Males. Hum. Brain Mapp. 2018, 39, 1283–1290. [Google Scholar] [CrossRef]
- Lai, J.C.L.; Lee, D.Y.H.; Leung, M.O.Y. Childhood Adversities and Salivary Cortisol Responses to the Trier Social Stress Test: A Systematic Review of Studies Using the Children Trauma Questionnaire (CTQ). Int. J. Environ. Res. Public. Health 2020, 18, 29. [Google Scholar] [CrossRef]
- Chen, L.; Schär, M.; Chan, K.W.Y.; Huang, J.; Wei, Z.; Lu, H.; Qin, Q.; Weiss, R.G.; van Zijl, P.C.M.; Xu, J. In Vivo Imaging of Phosphocreatine with Artificial Neural Networks. Nat. Commun. 2020, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, Y.; Wang, H.; Zhang, T.; Duan, F.; Wu, K.; Yang, S.; Xu, K.; Jiang, X.; Sun, X. Lactate and Lactylation in the Brain: Current Progress and Perspectives. Cell Mol. Neurobiol. 2023, 43, 2541–2555. [Google Scholar] [CrossRef]
- Sharma, N.K.; Pal, J.K. Metabolic Ink Lactate Modulates Epigenomic Landscape: A Concerted Role of Pro-Tumor Microenvironment and Macroenvironment During Carcinogenesis. Curr. Mol. Med. 2021, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cai, M.; Shang, Q.; Li, Z.; Feng, Y.; Liu, B.; Xue, X.; Lou, S. Elevated Lactate by High-Intensity Interval Training Regulates the Hippocampal BDNF Expression and the Mitochondrial Quality Control System. Front. Physiol. 2021, 12, 629914. [Google Scholar] [CrossRef] [PubMed]
- Golaghaei, A.; Hassanian-Moghaddam, H.; Shadnia, S.; Zamani, N.; Amraei, F. Potential Prognostic Roles of Serum Lactate and Creatine Kinase Levels in Poisoned Patients. BMC Emerg. Med. 2020, 20, 32. [Google Scholar] [CrossRef]
- Mächler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; Von Faber-Castell, A.; Kaelin, V.; Zuend, M.; San Martín, A.; Romero-Gómez, I.; et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef]
- Cai, M.; Wang, H.; Song, H.; Yang, R.; Wang, L.; Xue, X.; Sun, W.; Hu, J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front. Nutr. 2022, 9, 800901. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Sheibani, V.; Esmaeili-Mahani, S. Endurance Training Increases Brain Lactate Uptake during Hypoglycemia by up Regulation of Brain Lactate Transporters. Mol. Cell Endocrinol. 2014, 394, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Hosoya, K.I.; Ohtsuki, S.; Terasaki, T. A Novel Relationship between Creatine Transport at the Blood-Brain and Blood-Retinal Barriers, Creatine Biosynthesis, and Its Use for Brain and Retinal Energy Homeostasis. Subcell. Biochem. 2007, 46, 83–98. [Google Scholar] [PubMed]
- Sundqvist, N.; Sten, S.; Thompson, P.; Andersson, B.J.; Engström, M.; Cedersund, G. Mechanistic Model for Human Brain Metabolism and Its Connection to the Neurovascular Coupling. PLoS Comput. Biol. 2022, 18, e1010798. [Google Scholar] [CrossRef] [PubMed]
- Šagud, M.; Madžarac, Z.; Nedic Erjavec, G.; Šimunović Filipčić, I.; Mikulić, F.L.; Rogić, D.; Bradaš, Z.; Bajs Janović, M.; Pivac, N. The Associations of Neutrophil-Lymphocyte, Platelet-Lymphocyte, Monocyte-Lymphocyte Ratios and Immune-Inflammation Index with Negative Symptoms in Patients with Schizophrenia. Biomolecules 2023, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Śmierciak, N.; Krzyściak, W.; Szwajca, M.; Karcz, P.; Bryll, A.; Popiela, T.J.; Donicz, P.; Turek, A.; Aleksandrovych, V.; Pilecki, M. Benefits and Meaning of Lipids Profile in Relation to Oxidative Balance and Brain Morphology in Schizophrenia. Int. J. Mol. Sci. 2023, 24, 11375. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H. Inflammation and Negative Symptoms of Schizophrenia: Implications for Reward Processing and Motivational Deficits. Front. Psychiatry 2020, 11, 46. [Google Scholar] [CrossRef]
- Skalniak, A.; Krzyściak, W.; Śmierciak, N.; Szwajca, M.; Donicz, P.; Kozicz, T.; Pilecki, M. Immunological Routine Laboratory Parameters at Admission Influence the Improvement of Positive Symptoms in Schizophrenia Patients after Pharmacological Treatment. Front. Psychiatry 2023, 14, 1082135. [Google Scholar] [CrossRef]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood Maltreatment Predicts Adult Inflammation in a Life-Course Study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Schizophrenia: The New Etiological Synthesis. Neurosci. Biobehav. Rev. 2022, 142, 104894. [Google Scholar] [CrossRef] [PubMed]
- Rǎdulescu, A.R.; Mujica-Parodi, L.R. A Systems Approach to Prefrontal-Limbic Dysregulation in Schizophrenia. Neuropsychobiology 2008, 57, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder-Translating Findings From Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef]
- Bonnekoh, L.M.; Seidenbecher, S.; Knigge, K.; Hünecke, A.K.; Metzger, C.D.; Tempelmann, C.; Kanowski, M.; Kaufmann, J.; Meyer-Lotz, G.; Schlaaff, K.; et al. Long-Term Cortisol Stress Response in Depression and Comorbid Anxiety Is Linked with Reduced N-Acetylaspartate in the Anterior Cingulate Cortex. World J. Biol. Psychiatry 2023, 24, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Neylan, T.C.; Schuff, N.; Lenoci, M.; Yehuda, R.; Weiner, M.W.; Marmar, C.R. Cortisol Levels Are Positively Correlated with Hippocampal N-Acetylaspartate. Biol. Psychiatry 2003, 54, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Schuff, N.; Neylan, T.C.; Lenoci, M.A.; Du, A.T.; Weiss, D.S.; Marmar, C.R.; Weiner, M.W. Decreased Hippocampal N-Acetylaspartate in the Absence of Atrophy in Posttraumatic Stress Disorder. Biol. Psychiatry 2001, 50, 952–959. [Google Scholar] [CrossRef]
- Schubert, M.I.; Kalisch, R.; Sotiropoulos, I.; Catania, C.; Sousa, N.; Almeida, O.F.X.; Auer, D.P. Effects of Altered Corticosteroid Milieu on Rat Hippocampal Neurochemistry and Structure-An in Vivo Magnetic Resonance Spectroscopy and Imaging Study. J. Psychiatr. Res. 2008, 42, 902–912. [Google Scholar] [CrossRef]
- Glynn, H.; Möller, S.P.; Wilding, H.; Apputhurai, P.; Moore, G.; Knowles, S.R. Prevalence and Impact of Post-Traumatic Stress Disorder in Gastrointestinal Conditions: A Systematic Review. Dig. Dis. Sci. 2021, 66, 4109–4119. [Google Scholar] [CrossRef]
- Halverson, T.; Alagiakrishnan, K. Gut Microbes in Neurocognitive and Mental Health Disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Gupta, M.A. Review of Somatic Symptoms in Post-Traumatic Stress Disorder. Int. Rev. Psychiatry 2013, 25, 86–99. [Google Scholar] [CrossRef]
- Gradus, J.L.; Farkas, D.K.; Svensson, E.; Ehrenstein, V.; Lash, T.L.; Sørensen, H.T. Posttraumatic Stress Disorder and Gastrointestinal Disorders in the Danish Population. Epidemiology 2017, 28, 354–360. [Google Scholar] [CrossRef]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. Br. J. Psychiatry 1989, 155, 49–52. [Google Scholar] [CrossRef]
- Andreasen, N.C. Scale for the Assessment of Positive Symptoms (SAPS); University of Iowa: Iowa City, IA, USA, 1984. [Google Scholar]
- Addington, D.; Addington, J.; Schissel, B. A Depression Rating Scale for Schizophrenics. Schizophr. Res. 1990, 3, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Aas, I.M. Global Assessment of Functioning (GAF): Properties and Frontier of Current Knowledge. Ann. Gen. Psychiatry 2010, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Beck, A.; Steer, R.; Brown, G. Manual for the BDI-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Spielberger, C.; Gorsuch, R.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory (Form Y1-Y2), 1st ed.; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. (Eds.) Manual Cuestionario de Ansiedad Estado-Rasgo Adaptación Española Manual 10-th Edicion TEA Madrid 2015, 10th ed. TEA Madrid: Madrid, Spain, 2015; Volume 9.
- Strelau, J.; Jaworowska, A.; Wrześniewski, K.; Szczepaniak, P. CISS Kwestionariusz Radzenia Sobie w Sytuacjach Stresowych. [CISS Coping Inventory for Stressful Situations]; Pracownia Testów Psychologicznych PTP: Warsaw, Poland, 2005. [Google Scholar]
- Cloitre, M.; Shevlin, M.; Brewin, C.R.; Bisson, J.I.; Roberts, N.P.; Maercker, A.; Karatzias, T.; Hyland, P. The International Trauma Questionnaire: Development of a Self-Report Measure of ICD-11 PTSD and Complex PTSD. Acta Psychiatr. Scand. 2018, 138, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, E.; Van der Hart, O.; Kruger, K. The Psychometric Characteristics of the Traumatic Experiences Checklist (TEC): First Findings among Psychiatric Outpatients. Clin. Psychol. Psychother. 2002, 9, 200–210. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and Validation of a Brief Screening Version of the Childhood Trauma Questionnaire. Child. Abuse Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Sterling, M. General Health Questionnaire—28 (GHQ-28). J. Physiother. 2011, 57, 259. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A Clinical Rating Scale for Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome and Peptic Ulcer Disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Újszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and Validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) Questionnaire in Dyspepsia: A Six-Country Study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Fraley, R.C.; Waller, N.G.; Brennan, K.A. An Item Response Theory Analysis of Self-Report Measures of Adult Attachment. J. Pers. Soc. Psychol. 2000, 78, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Lubiewska, K.; Głogowska, K.; Mickiewicz, K.; Wojtynkiewicz, E.; Izdebski, P.; Wiśniewski, C. Skala Experience in Close Relationships-Revised: Struktura, Rzetelność Oraz Skrócona Wersja Skali w Polskiej Próbie. Psychol. Rozw. 2016, 21, 49–63. [Google Scholar] [CrossRef]
- Provencher, S.W. Automatic Quantitation of Localized in Vivo 1H Spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Egerton, A.; Murphy, A.; Donocik, J.; Anton, A.; Barker, G.J.; Collier, T.; Deakin, B.; Drake, R.; Eliasson, E.; Emsley, R.; et al. Dopamine and Glutamate in Antipsychotic-Responsive Compared with Antipsychotic-Nonresponsive Psychosis: A Multicenter Positron Emission Tomography and Magnetic Resonance Spectroscopy Study (STRATA). Schizophr. Bull. 2021, 47, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Size. Trends Sport. Sci. 2014, 1, 19–25. [Google Scholar]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s Rho. Appl. Stat. 1975, 24, 377. [Google Scholar] [CrossRef]
- Thiele, C.; Hirschfeld, G. Cutpointr: Improved Estimation and Validation of Optimal Cutpoints in R. J. Stat. Softw. 2021, 98, 1–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Chan, C.; Chan, G.; Leeper, T.; Becker, J. A Swiss-Army Knife for Data I/O [R Package Rio Version 0.5.29]; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kassambara, A. Pipe-Friendly Framework for Basic Statistical Tests [R Package Rstatix Version 0.7.1]; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses [R Package Factoextra Version 1.0.7]; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption; CRAN: Vienna, Austria, 2023; Available online: https://easystats.github.io/report/ (accessed on 10 October 2023).
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the Summary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Wickham, H. Ggpolt2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files; R Package Version 1.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation; R Package Version 1.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
| Characteristic | N | Group | p 2 | r | |
|---|---|---|---|---|---|
| Control, n = 45 1 | Test, n = 51 1 | ||||
| Serotonin [µg/mL] | 96 | 133.46 (127.63, 138.31) | 130.30 (126.21, 137.87) | 0.301 | 0.11 |
| Alanine [µg/mL] | 96 | 1.52 (1.23, 1.83) | 1.52 (1.22, 1.76) | 0.977 | <0.01 |
| Glutamic acid [µg/mL] | 95 | 2923.96 (2139.69, 3630.11) | 2367.73 (1411.55, 3246.98) | 0.048 | 0.20 |
| missing value | 0 | 1 | |||
| Glutamine [µg/mL] | 96 | 334.20 (286.22, 367.14) | 354.35 (307.89, 382.01) | 0.249 | 0.12 |
| Cortisol [ng/mL] | 96 | 162.34 (131.19, 184.54) | 120.57 (93.37, 174.50) | 0.031 | 0.22 |
| Lactates [µg/mL] | 94 | 156.98 (129.59, 177.59) | 120.92 (88.37, 146.86) | <0.001 | 0.41 |
| missing value | 1 | 1 | |||
| Lactates [mmol/L] | 94 | 1.74 (1.44, 1.97) | 1.34 (0.98, 1.63) | <0.001 | 0.41 |
| missing value | 1 | 1 | |||
| Kinurenic acid [ng/mL] | 95 | 54.80 (40.01, 65.52) | 46.81 (35.55, 60.14) | 0.167 | 0.14 |
| missing value | 0 | 1 | |||
| Acetic acid [nM] | 96 | 9.56 (7.71, 11.15) | 8.86 (6.19, 10.86) | 0.071 | 0.19 |
| Propionic acid [nM] | 96 | 0.54 (0.42, 0.64) | 0.50 (0.36, 0.60) | 0.146 | 0.15 |
| Butyric acid [nM] | 96 | 0.07 (0.03, 0.10) | 0.06 (0.03, 0.10) | 0.675 | 0.04 |
| Isobutyric acid [nM] | 96 | 0.18 (0.14, 0.24) | 0.17 (0.06, 0.24) | 0.215 | 0.13 |
| Valerian acid [nM] | 96 | 0.06 (0.04, 0.13) | 0.05 (0.01, 0.12) | 0.145 | 0.15 |
| Isovaleric acid [nM] | 96 | 0.31 (0.23, 0.48) | 0.35 (0.18, 0.52) | 0.863 | 0.02 |
| Characteristic | Optimal Cutpoint | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| Cortisol [ng/mL] | 131.19 | 0.69 | 0.76 | 0.63 | 0.63 |
| Glutamic acid [µg/mL] | 1671.59 | 0.62 | 0.91 | 0.36 | 0.62 |
| Lactates [µg/mL] | 112.15 | 0.70 | 0.95 | 0.48 | 0.74 |
| Questionnaire | Lactates [µg/mL] | Glutamic Acid [µg/mL] | Cortisol [ng/mL] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| npairs | rho | p | npairs | rho | p | npairs | rho | p | |
| Positive symptoms | 49 | 0.09 | 0.507 | 49 | 0.24 | 0.093 | 50 | 0.14 | 0.322 |
| Negative symptoms | 49 | -0.09 | 0.547 | 49 | 0.13 | 0.360 | 50 | −0.04 | 0.773 |
| Disorganized speech | 49 | 0.10 | 0.500 | 49 | 0.18 | 0.205 | 50 | 0.17 | 0.242 |
| Uncontrolled hostility excitement | 49 | 0.12 | 0.412 | 49 | 0.34 | 0.016 | 50 | 0.07 | 0.628 |
| Anxiety depression | 49 | 0.04 | 0.806 | 49 | 0.15 | 0.290 | 50 | 0.06 | 0.658 |
| P1–P7 | 49 | 0.09 | 0.536 | 49 | 0.23 | 0.100 | 50 | 0.07 | 0.614 |
| N1–N7 | 49 | −0.06 | 0.701 | 49 | 0.09 | 0.511 | 50 | −0.01 | 0.925 |
| G1–G16 | 49 | 0.09 | 0.546 | 49 | 0.30 | 0.030 | 50 | 0.17 | 0.238 |
| Total score | 49 | 0.04 | 0.790 | 49 | 0.25 | 0.075 | 50 | 0.13 | 0.353 |
| Total score | 49 | −0.06 | 0.699 | 49 | 0.18 | 0.198 | 50 | 0.01 | 0.960 |
| Total score | 49 | 0.23 | 0.108 | 49 | 0.26 | 0.062 | 50 | −0.09 | 0.548 |
| Total score | 49 | 0.14 | 0.340 | 49 | 0.22 | 0.128 | 50 | −0.06 | 0.674 |
| Total score | 49 | −0.15 | 0.288 | 49 | −0.23 | 0.106 | 50 | 0.03 | 0.811 |
| Total score | 46 | 0.21 | 0.145 | 46 | 0.17 | 0.224 | 47 | 0.07 | 0.647 |
| Total score | 45 | 0.14 | 0.313 | 45 | 0.17 | 0.229 | 46 | 0.08 | 0.581 |
| Total score | 45 | 0.29 | 0.038 | 45 | 0.26 | 0.068 | 46 | −0.02 | 0.900 |
| SSZ | 38 | 0.07 | 0.609 | 38 | 0.18 | 0.216 | 39 | 0.09 | 0.513 |
| SSE | 38 | 0.08 | 0.596 | 38 | 0.17 | 0.234 | 39 | 0.09 | 0.532 |
| SSU | 38 | −0.15 | 0.294 | 38 | −0.06 | 0.689 | 39 | −0.07 | 0.649 |
| ACZ | 38 | −0.16 | 0.251 | 38 | 0.01 | 0.919 | 39 | −0.13 | 0.349 |
| PKT | 38 | −0.03 | 0.852 | 38 | −0.19 | 0.175 | 39 | −0.02 | 0.909 |
| Total score | 38 | 0.04 | 0.789 | 38 | 0.17 | 0.225 | 39 | 0.03 | 0.852 |
| Total score | 45 | 0.30 | 0.032 | 45 | 0.33 | 0.019 | 46 | 0.09 | 0.552 |
| Reexperiencing trauma | 41 | 0.09 | 0.547 | 41 | 0.34 | 0.014 | 42 | 0.16 | 0.263 |
| Avoidance | 41 | 0.39 | 0.005 | 41 | 0.36 | 0.009 | 42 | 0.13 | 0.347 |
| Threat | 41 | 0.18 | 0.206 | 41 | 0.25 | 0.071 | 42 | 0.17 | 0.231 |
| Affective dysregulation | 41 | −0.23 | 0.099 | 41 | 0.00 | 0.974 | 42 | −0.01 | 0.921 |
| Negative self-concept | 41 | −0.03 | 0.853 | 41 | 0.06 | 0.700 | 42 | 0.06 | 0.683 |
| Disturbance relationships | 41 | 0.04 | 0.779 | 41 | 0.05 | 0.711 | 42 | 0.03 | 0.847 |
| PTSDFI | 41 | 0.07 | 0.606 | 41 | −0.03 | 0.814 | 42 | 0.08 | 0.579 |
| DSOFI | 41 | −0.01 | 0.929 | 41 | −0.05 | 0.722 | 42 | 0.06 | 0.681 |
| PTSD | 41 | 0.26 | 0.069 | 41 | 0.36 | 0.010 | 42 | 0.16 | 0.264 |
| DSO | 41 | −0.05 | 0.735 | 41 | 0.07 | 0.601 | 42 | −0.02 | 0.904 |
| Total score | 41 | 0.10 | 0.470 | 41 | 0.10 | 0.475 | 42 | 0.05 | 0.739 |
| Emotional abuse | 41 | 0.13 | 0.376 | 41 | 0.02 | 0.865 | 42 | 0.01 | 0.924 |
| Physical abuse | 41 | −0.20 | 0.168 | 41 | −0.18 | 0.200 | 42 | −0.07 | 0.650 |
| sexual abuse | 41 | 0.12 | 0.411 | 41 | 0.03 | 0.832 | 42 | −0.22 | 0.129 |
| Emotional neglect | 41 | −0.12 | 0.393 | 41 | −0.09 | 0.525 | 42 | −0.15 | 0.285 |
| Physical neglect | 41 | 0.01 | 0.949 | 41 | 0.16 | 0.270 | 42 | −0.28 | 0.045 |
| Denial | 41 | 0.01 | 0.938 | 41 | 0.00 | 0.998 | 42 | 0.08 | 0.593 |
| Total score | 41 | −0.08 | 0.585 | 41 | −0.05 | 0.731 | 42 | −0.08 | 0.567 |
| Total score | 40 | 0.03 | 0.825 | 40 | 0.01 | 0.940 | 41 | −0.06 | 0.680 |
| Total score | 40 | 0.02 | 0.866 | 40 | −0.08 | 0.575 | 41 | −0.24 | 0.088 |
| Characteristic | Lactates [µg/mL] | Glutamic acid [µg/mL] | Cortisol [ng/mL] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| npairs | rho | p | npairs | rho | p | npairs | rho | p | |
| WBC [×103/µL] | 94 | −0.12 | 0.226 | 95 | −0.07 | 0.522 | 96 | −0.18 | 0.074 |
| NEUT [×103/µL] | 94 | −0.21 | 0.044 | 95 | −0.12 | 0.248 | 96 | −0.16 | 0.120 |
| Re-Lymph [×103/µL] | 94 | −0.35 | <0.001 | 95 | −0.07 | 0.498 | 96 | −0.24 | 0.020 |
| IG [×103/µL] | 94 | −0.11 | 0.284 | 95 | 0.07 | 0.496 | 96 | −0.22 | 0.035 |
| NEUT [%] | 94 | −0.29 | 0.005 | 95 | −0.21 | 0.041 | 96 | −0.12 | 0.225 |
| Lymph [%] | 94 | 0.26 | 0.012 | 95 | 0.15 | 0.133 | 96 | 0.06 | 0.549 |
| Re-Lymph [%] | 94 | −0.33 | 0.001 | 95 | −0.04 | 0.734 | 96 | −0.18 | 0.073 |
| EO [%] | 94 | 0.16 | 0.115 | 95 | 0.32 | 0.001 | 96 | −0.01 | 0.951 |
| BASO [%] | 94 | 0.12 | 0.244 | 95 | 0.11 | 0.273 | 96 | 0.10 | 0.328 |
| RBC [×106/µL] | 94 | −0.06 | 0.585 | 95 | 0.01 | 0.894 | 96 | −0.14 | 0.168 |
| Hgb [g/dl] | 94 | −0.08 | 0.448 | 95 | 0.01 | 0.944 | 96 | −0.12 | 0.253 |
| Hct [%] | 94 | −0.09 | 0.406 | 95 | −0.05 | 0.596 | 96 | −0.17 | 0.092 |
| Macrocytes [%] | 94 | −0.03 | 0.777 | 95 | 0.03 | 0.768 | 96 | −0.08 | 0.435 |
| K+ [mmol/L] | 94 | −0.09 | 0.378 | 95 | 0.00 | 0.983 | 96 | −0.13 | 0.200 |
| Glucose [mmol/L] | 94 | −0.33 | 0.001 | 95 | −0.40 | <0.001 | 96 | −0.01 | 0.924 |
| Uric acid [µmol/L] | 94 | −0.11 | 0.266 | 95 | 0.03 | 0.809 | 96 | −0.11 | 0.271 |
| Cholesterol HDL [µmol/L] | 93 | 0.26 | 0.011 | 94 | 0.01 | 0.903 | 95 | 0.21 | 0.037 |
| Triglycerides [µmol/L] | 93 | −0.03 | 0.796 | 94 | 0.04 | 0.677 | 95 | −0.10 | 0.337 |
| FT4 [pmol/L] | 94 | 0.27 | 0.008 | 95 | 0.11 | 0.295 | 96 | 0.00 | 0.992 |
| DHEA-S [µmol/L] | 94 | −0.16 | 0.131 | 95 | −0.04 | 0.681 | 96 | 0.09 | 0.408 |
| Insulin [µU/mL] | 94 | 0.00 | 0.993 | 95 | 0.04 | 0.709 | 96 | −0.10 | 0.342 |
| HOMA-IR | 92 | −0.04 | 0.674 | 93 | −0.02 | 0.853 | 94 | −0.10 | 0.309 |
| Creatine conc [×106] | 91 | 0.24 | 0.019 | 92 | 0.13 | 0.217 | 93 | 0.19 | 0.062 |
| Glucose Cr+PCr | 91 | −0.10 | 0.329 | 92 | 0.02 | 0.828 | 93 | −0.09 | 0.404 |
| Glucose Cr+PCr | 91 | −0.15 | 0.144 | 92 | 0.05 | 0.630 | 93 | −0.14 | 0.174 |
| Glutamine Cr+PCr | 91 | 0.17 | 0.098 | 92 | 0.15 | 0.152 | 93 | 0.28 | 0.005 |
| Glutamine Cr+PCr | 91 | 0.09 | 0.364 | 92 | 0.20 | 0.049 | 93 | 0.17 | 0.097 |
| Inositol conc [×106] | 91 | 0.06 | 0.586 | 92 | 0.01 | 0.928 | 93 | 0.04 | 0.722 |
| N-Acetylaspartate conc [×106] | 91 | 0.15 | 0.148 | 92 | 0.04 | 0.729 | 93 | 0.23 | 0.026 |
| Cr+PCr conc [×106] | 91 | 0.27 | 0.008 | 92 | 0.05 | 0.604 | 93 | 0.30 | 0.003 |
| Glu+Gln conc [×106] | 91 | 0.33 | 0.001 | 92 | 0.08 | 0.465 | 93 | 0.21 | 0.042 |
| Glu+Gln Cr+PCr | 91 | 0.19 | 0.059 | 92 | 0.10 | 0.334 | 93 | 0.02 | 0.869 |
| Phosphocreatine conc | 91 | 0.19 | 0.067 | 92 | 0.08 | 0.467 | 93 | 0.10 | 0.344 |
| Glutamate conc | 91 | 0.26 | 0.009 | 92 | −0.04 | 0.707 | 93 | 0.13 | 0.193 |
| Glu/Cr+PCr | 91 | 0.20 | 0.048 | 92 | −0.09 | 0.403 | 93 | 0.04 | 0.683 |
| N-Acetylaspartate conc. | 91 | 0.30 | 0.003 | 92 | 0.03 | 0.787 | 93 | 0.07 | 0.496 |
| NAA/Cr+PCr | 91 | 0.21 | 0.039 | 92 | 0.01 | 0.900 | 93 | −0.07 | 0.504 |
| N-Acetyaspartate + N-Acetylspartylglutamate conc | 91 | 0.27 | 0.009 | 92 | −0.03 | 0.785 | 93 | 0.15 | 0.150 |
| NAA+NAAG/Cr+PCr | 91 | 0.19 | 0.069 | 92 | 0.00 | 0.965 | 93 | 0.04 | 0.713 |
| Cr+PCr conc | 91 | 0.23 | 0.026 | 92 | 0.04 | 0.728 | 93 | 0.14 | 0.164 |
| Glu+Gln conc | 91 | 0.23 | 0.025 | 92 | 0.00 | 0.965 | 93 | 0.12 | 0.249 |
| Glu+GlnCr+PCr | 91 | 0.13 | 0.197 | 92 | 0.00 | 0.999 | 93 | 0.06 | 0.537 |
| Phosphocreatine conc [×103] | 91 | −0.01 | 0.960 | 92 | −0.05 | 0.618 | 93 | 0.16 | 0.111 |
| N-Acetylaspartate conc [×106] | 91 | 0.04 | 0.727 | 92 | 0.02 | 0.867 | 93 | 0.09 | 0.371 |
| Taurine conc [×106] | 91 | −0.18 | 0.087 | 92 | −0.02 | 0.873 | 93 | −0.22 | 0.028 |
| Taurine Cr+PCr [×103] | 91 | −0.18 | 0.081 | 92 | −0.01 | 0.896 | 93 | −0.24 | 0.020 |
| Cr+PCr conc [×106] | 91 | 0.12 | 0.242 | 92 | 0.02 | 0.833 | 93 | 0.20 | 0.056 |
| Lip20 conc [×106] | 91 | 0.13 | 0.222 | 92 | 0.13 | 0.205 | 93 | 0.08 | 0.427 |
| Lip20 Cr+PCr [×103] | 91 | 0.11 | 0.265 | 92 | 0.13 | 0.223 | 93 | 0.07 | 0.495 |
| L-alanine conc | 91 | 0.07 | 0.528 | 92 | −0.10 | 0.350 | 93 | 0.21 | 0.045 |
| Creatine Cr conc. | 91 | −0.06 | 0.539 | 92 | −0.07 | 0.475 | 93 | −0.14 | 0.189 |
| Creatine, Cr/(Cr+PCr) | 91 | −0.05 | 0.599 | 92 | −0.10 | 0.354 | 93 | −0.12 | 0.250 |
| Phosphocreatine, PCr | 91 | 0.03 | 0.769 | 92 | 0.04 | 0.694 | 93 | 0.11 | 0.283 |
| Phosphocreatine, PCr/(Cr+PCr) | 91 | 0.05 | 0.599 | 92 | 0.010 | 0.354 | 93 | 0.12 | 0.250 |
| Scylloinositol conc. | 91 | −0.04 | 0.692 | 92 | 0.08 | 0.451 | 93 | −0.10 | 0.321 |
| Scylloinositol/(Cr+PCr) | 91 | −0.04 | 0.703 | 92 | 0.09 | 0.387 | 93 | −0.10 | 0.322 |
| GPC+PCh/Cr+PCr | 91 | −0.12 | 0.244 | 92 | 0.02 | 0.848 | 93 | 0.02 | 0.860 |
| Test | V test | MCluster | Mtest group | SDCluster | SDtest group | p |
|---|---|---|---|---|---|---|
| Cluster 1 (n = 12) | ||||||
| GAF | 4.25 | 68.92 | 52.34 | 10.48 | 15.31 | <0.001 |
| Gastrointestinal symptoms | −2.36 | 9.50 | 18.83 | 4.68 | 15.49 | 0.018 |
| ITQ | −2.44 | 23.75 | 33.83 | 11.35 | 16.19 | 0.015 |
| CTQ | −2.46 | 57.08 | 66.45 | 8.23 | 14.95 | 0.014 |
| GHQ-28 | −2.51 | 22.42 | 31.85 | 7.94 | 14.77 | 0.012 |
| STAI | −2.57 | 81.83 | 95.83 | 11.98 | 21.34 | 0.010 |
| CALGARY | −3.14 | 3.67 | 8.58 | 3.25 | 6.14 | 0.002 |
| BDI II | −3.34 | 7.33 | 18.76 | 5.88 | 13.41 | 0.001 |
| SAPS | −4.69 | 8.50 | 35.28 | 7.31 | 22.38 | <0.001 |
| SANS | −5.06 | 20.25 | 51.28 | 10.10 | 24.05 | <0.001 |
| PANSS | −5.08 | 47.83 | 78.20 | 19.58 | 23.46 | <0.001 |
| Cluster 2 (n = 23) | ||||||
| SANS | 2.88 | 62.10 | 51.28 | 13.78 | 24.05 | 0.004 |
| PANSS | 2.60 | 87.70 | 78.20 | 13.39 | 23.46 | 0.009 |
| SAPS | 2.50 | 44.01 | 35.28 | 22.25 | 22.38 | 0.012 |
| ECR-RS | −2.09 | 47.44 | 52.76 | 12.53 | 16.34 | 0.037 |
| CTQ | −2.21 | 61.29 | 66.45 | 9.68 | 14.95 | 0.027 |
| STAI | −2.34 | 88.05 | 95.83 | 17.32 | 21.34 | 0.020 |
| BDI II | −2.36 | 13.82 | 18.76 | 7.88 | 13.41 | 0.018 |
| ITQ | −3.02 | 26.20 | 33.83 | 9.90 | 16.19 | 0.003 |
| GAF | −3.03 | 45.10 | 52.34 | 11.55 | 15.31 | 0.002 |
| Cluster 3 (n = 16) | ||||||
| BDI II | 5.59 | 34.44 | 18.76 | 8.90 | 13.41 | <0.001 |
| ITQ | 5.47 | 52.36 | 33.83 | 9.75 | 16.19 | <0.001 |
| STAI | 4.86 | 117.50 | 95.83 | 14.45 | 21.34 | <0.001 |
| CTQ | 4.62 | 80.90 | 66.45 | 14.62 | 14.95 | <0.001 |
| GHQ 28 | 4.34 | 45.25 | 31.85 | 13.13 | 14.77 | <0.001 |
| ECR-RS | 3.94 | 66.22 | 52.76 | 15.23 | 16.34 | <0.001 |
| Gastrointestinal symptoms | 3.55 | 30.31 | 18.83 | 20.58 | 15.49 | <0.001 |
| TEC PL | 2.34 | 28.08 | 21.32 | 16.13 | 13.80 | 0.019 |
| CALGARY | 2.23 | 11.44 | 8.58 | 6.22 | 6.14 | 0.026 |
| CISS | 2.00 | 149.05 | 141.87 | 19.63 | 17.15 | 0.046 |
| Characteristic | N | Distribition 1 |
|---|---|---|
| Serotonin [µg/mL] | 96 | 131.32 (126.66, 138.05) |
| Alanine [µg/mL] | 96 | 1.52 (1.23, 1.83) |
| Glutamic acid [µg/mL] | 95 | 2732.44 (1785.60, 3575.55) |
| Glutamic acid [µg/mL] | 96 | 341.84 (289.56, 380.60) |
| Cortisol [ng/mL] | 96 | 138.96 (106.69, 184.22) |
| Lactates [µg/mL] | 94 | 135.33 (111.05, 167.62) |
| Lactates [mmol/L] | 94 | 1.50 (1.23, 1.86) |
| Kinurenic acid [ng/mL] | 95 | 48.76 (37.05, 64.44) |
| Acetic acid [nM] | 96 | 8.96 (6.78, 11.11) |
| Propionic acid [nM] | 96 | 0.53 (0.38, 0.62) |
| Butyric acid [nM] | 96 | 0.07 (0.03, 0.10) |
| Isobutyric acid [nM] | 96 | 0.18 (0.11, 0.24) |
| Valerian acid [nM] | 96 | 0.06 (0.02, 0.13) |
| Isovaleric acid [nM] | 96 | 0.34 (0.21, 0.49) |
| LC-ESI-MS/MS | LCMS-SCFA | LC-ESI-MS/MS Cortisol | |
|---|---|---|---|
| Sample preparation | 4 µL of internal standard (methanolic solution of the tested compounds with a concentration of 500 µg/mL) was added to 100 µL of sample (serum). Protein was precipitated by adding 100 μL of ice-cold acetonitrile. After mixing, the samples were centrifuged (8000 rpm, 5 min, 15 °C). The supernatant was collected and placed in chromatographic vessels. | 60 µL of a mixture of methanol and water (1:1, v/v) was added to 20 µL of serum, the samples were vortexed, then centrifuged (14000 rpm, 10 min, 4 °C), 40 µL of the supernatant was collected. Derivatization: 40 µL of water was added to 40 µL of the supernatant, then 10 µL of 0.1 M BHA and 10 µL of 0.25 M EDC. After mixing, the samples were incubated for 1 h at 25 °C with constant stirring. The derivatization process was completed by adding 100 µL of 50% methanol and 600 µL of dichloromethane. The samples were then mixed and centrifuged (8000 rpm, 10 min). The organic phase was collected and evaporated to dryness under nitrogen and then dissolved in 30 µL of 50% methanol. | 4 µL of internal standard (methanolic solution containing cortisol with a concentration of 500 µg/mL) was added to 100 µL of sample (serum). Protein was precipitated by adding 100 μL of ice-cold acetonitrile. After mixing, the samples were centrifuged (8000 rpm, 5 min, 15 °C). The supernatant was collected and placed in chromatographic vessels. |
| Chromatography column | ZIC®-HILIC (5 µm, 200 Å, 150 × 21.2 mm; Merck, Darmstadt, Germany), thermostated at 40 °C | Kinetex (1.7 µm Biphenyl, 100 Å; 100 × 2.1 mm; Phenomenex Companies Worldwide, Torrance, CA, USA) | Kinetex (2.6 µm Biphenyl 100 Å, 100 × 2.1 mm; Phenomenex Companies Worldwide, Torrance, CA, USA), thermostated at 40 °C |
| Mobile phases | Phase A: 0.1% aqueous HCOOH solution; Phase B: 0.1% HCOOH in acetonitrile | Phase A: 0.1% HCOOH + 10 mM NH4COOH; Phase B: 0.1% HCOOH in a mixture of methanol: isopropanol (9: 1 v/v) | Phase A: 0.1% aqueous HCOOH solution; Phase B: methanol |
| Phase flow rate | 0.6 mL/min | 0.4 mL/min | 0.4 mL/min |
| Separation program | Gradient separation program: 0–0.2 min, isocratic gradient 5.0% of phase A; 0.2–1.5 min, linear gradient 5–55% of phase A; 1.5–3.1 min, isocratic gradient 55% of phase A; 3.1–4.5 min, linear gradient 55.0–5.0% of phase A; 4.5–6 min, isocratic gradient 5.0% of phase A | Gradient separation program: 0–4 min, 68.0–40.0% of phase A, 4–4.8 min, 35% of phase A, 4.8–4.9 min, 2% of phase A; after this time, isocratic conditions were achieved (4.9–5.2 min), and then the initial conditions were returned—68% of phase A (5.2–5.3 min) | Gradient separation program: 0–0.4 min, isocratic gradient 5.0% of phase B; 0.4–0.5 min, linear gradient 5–50% of phase B; 0.5–1.9 min, isocratic gradient 50% of phase B; 1.9–2.0 min, linear gradient 100.0% of phase B; 2.0–2.9 min, isocratic gradient 100.0% of phase B; 2.9–4.0 min, linear gradient 100–5.0% of phase B; 4.0–5.0 min, isocratic gradient 5.0% of phase B |
| Injection volume | 4 µL | 4 µL | 2 µL |
| Time of single sample analysis | 6 min | 5.3 min | 5 min |
| Retention time | ALA, ALA-d4—2.44 min; SER, SER-d4—2.35 min; GLUT, GLUT-C13—2.40 min; LA—0.99 min | acetic acid—1.4 min; propionic acid—1.9 min; isobutyric acid—2.59 min; butyric acid—2.8 min; isovaleric acid—3.2 min; valeric acid—3.5 min | COR, COR-d4—3.39 min |
| Ion source parameters | IS: 5500 V; atomizing gas (gas 1): 30 psi; turbo gas (gas 2): 20 psi; TEM: 550 °C; CUR: 30 psi | IS: 5400 V; atomizing gas (gas 1): 30 psi; turbo gas (gas 2): 20 psi; TEM: 550 °C; CUR: 30 psi | IS: 5500 V; atomizing gas (gas 1): 30 psi; turbo gas (gas 2): 20 psi; TEM) 300 °C; CUR: 30 psi |
| Ion pairs | ALA: m/z = 90.09/44.9; ALA-d4: m/z = 93.93/47.97; GLUT: m/z = 148.0/84.0; GLUT-C13: m/z = 153.0/89.0; SER: m/z = 177.0/119.0; SER-d4: m/z = 182.83/119.76; LA: m/z = 88.84/43.948 | acetic acid: m/z = 166.1/91.1; propionic acid: m/z = 180.1/91.1; butyric and isobutyric acid: 194.1/91.1; valeric and isovaleric acid: m/z = 208.1/124.1 | COR: m/z = 363.11/120.92; COR-d4: m/z = 367.11/120.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyściak, W.; Bystrowska, B.; Karcz, P.; Chrzan, R.; Bryll, A.; Turek, A.; Mazur, P.; Śmierciak, N.; Szwajca, M.; Donicz, P.; et al. Association of Blood Metabolomics Biomarkers with Brain Metabolites and Patient-Reported Outcomes as a New Approach in Individualized Diagnosis of Schizophrenia. Int. J. Mol. Sci. 2024, 25, 2294. https://doi.org/10.3390/ijms25042294
Krzyściak W, Bystrowska B, Karcz P, Chrzan R, Bryll A, Turek A, Mazur P, Śmierciak N, Szwajca M, Donicz P, et al. Association of Blood Metabolomics Biomarkers with Brain Metabolites and Patient-Reported Outcomes as a New Approach in Individualized Diagnosis of Schizophrenia. International Journal of Molecular Sciences. 2024; 25(4):2294. https://doi.org/10.3390/ijms25042294
Chicago/Turabian StyleKrzyściak, Wirginia, Beata Bystrowska, Paulina Karcz, Robert Chrzan, Amira Bryll, Aleksander Turek, Paulina Mazur, Natalia Śmierciak, Marta Szwajca, Paulina Donicz, and et al. 2024. "Association of Blood Metabolomics Biomarkers with Brain Metabolites and Patient-Reported Outcomes as a New Approach in Individualized Diagnosis of Schizophrenia" International Journal of Molecular Sciences 25, no. 4: 2294. https://doi.org/10.3390/ijms25042294





