Streptomycetes as Microbial Cell Factories for the Biotechnological Production of Melanin
Abstract
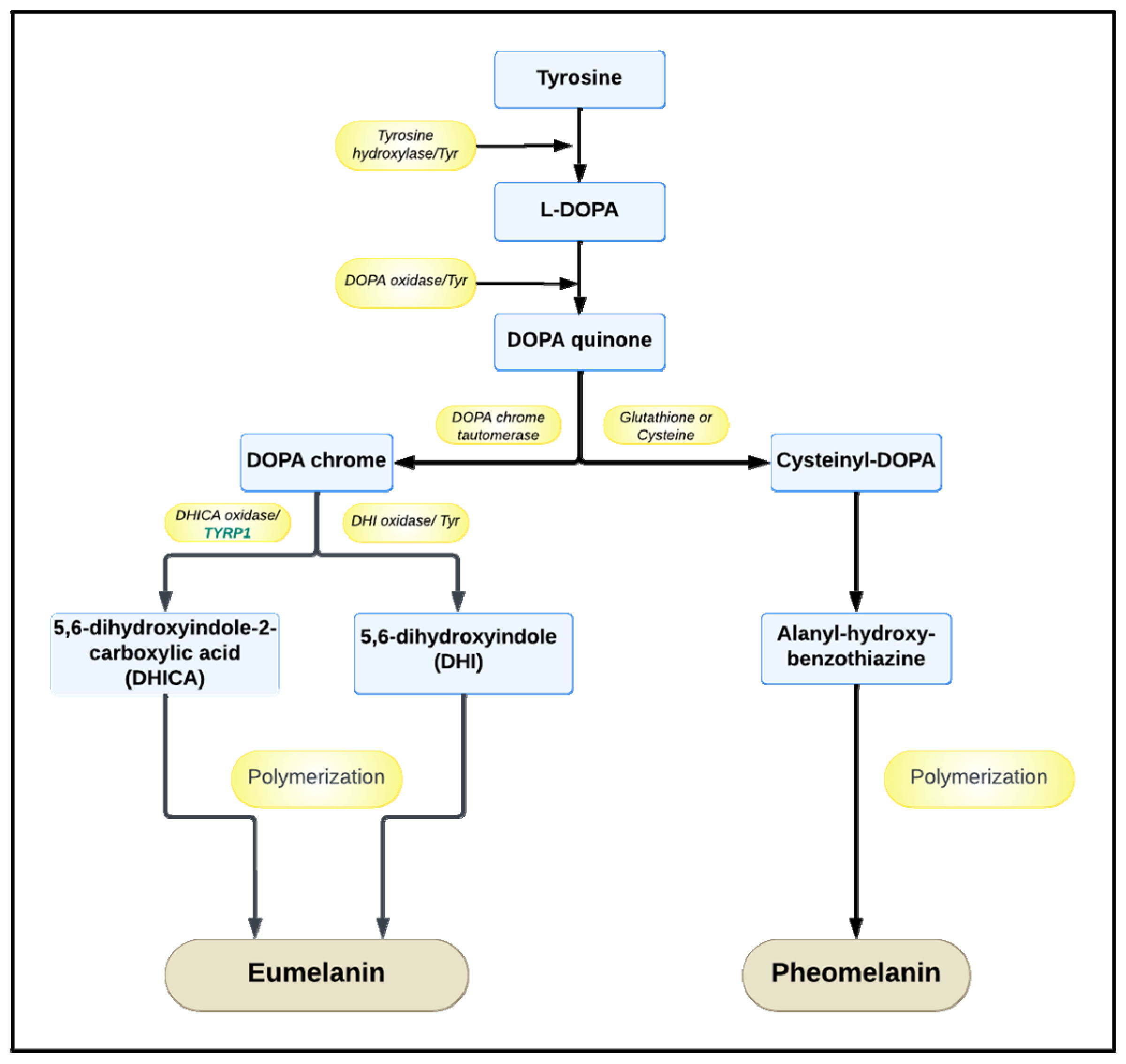
:1. The Role and Biological Functions of Melanin
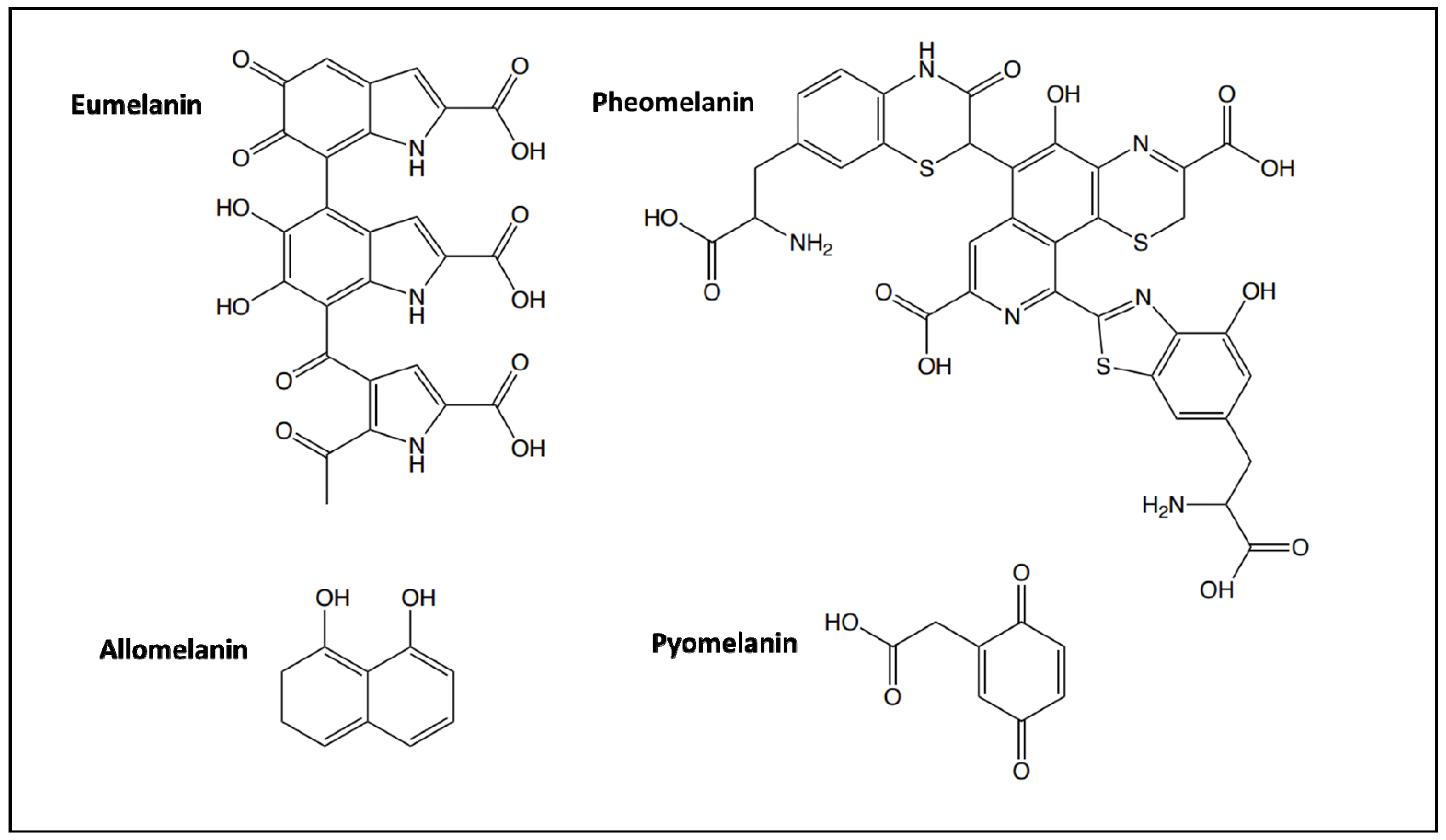
2. The Properties and Manufacturing of Melanin
3. Streptomyces Strains as Melanin Producers
4. Biotechnological Strategies to Improve Melanin Production by Streptomyces Strains
5. Purification Processes for the Melanin Produced by Streptomyces Strains
6. Analytical Methods for Melanin Characterization
6.1. Ultraviolet–Visible Spectroscopy
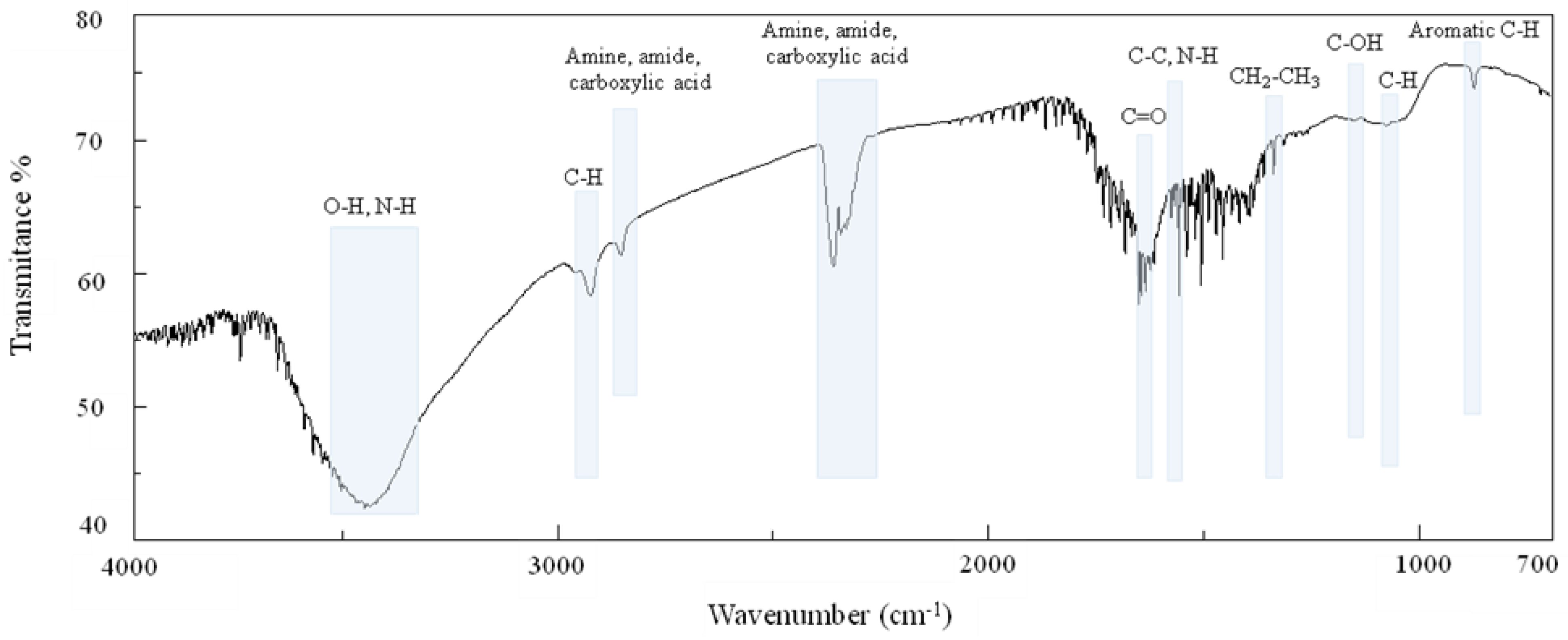
6.2. Fourier-Transform Infrared Spectroscopy
6.3. Nuclear Magnetic Resonance Spectroscopy
6.4. Elemental Analysis
6.5. Scanning Electron Microscopy
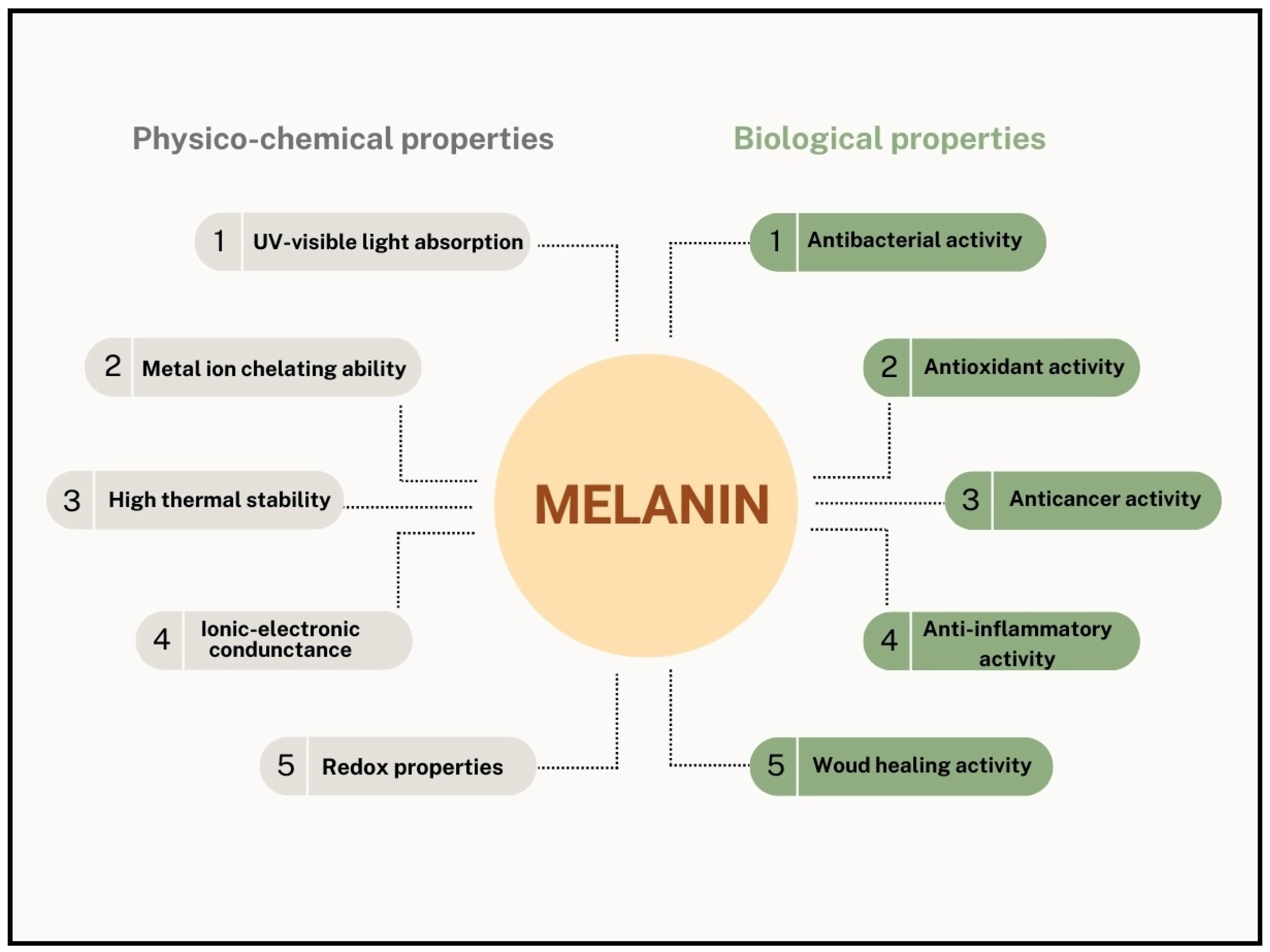
7. Properties of Melanin Produced by Streptomyces Strains
7.1. Physicochemical Properties
7.2. Biological Activities
8. Applications of Melanin Produced by Streptomyces Strains
9. Conclusions
Author Contributions
Funding

Conflicts of Interest
References
- Caldas, M.; Paiva-Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V. Melanin nanoparticles as a promising tool for biomedical applications—A review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Coelho, S.G.; Choi, W.; Brenner, M.; Miyamura, Y.; Yamaguchi, Y.; Wolber, R.; Smuda, C.; Batzer, J.; Kolbe, L.; Ito, S.; et al. Short- and long-term effects of UV radiation on the pigmentation of human skin. J. Investig. Dermatol. Symp. Proc. 2009, 14, 32–35. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.J. An update on the honesty of melanin-based color signals in birds. Pigment Cell Melanoma Res. 2008, 21, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Clusella Trullas, S.; van Wyk, J.H.; Spotila, J.R. Thermal melanism in ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]
- Palumbo, A. Melanogenesis in the Ink Gland of Sepia officinalis. Photochem. Photobiol. 2003, 16, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Vij, R.; Casadevall, A. Microbial melanins for radioprotection and bioremediation. Environ. Microbiol. Rep. 2017, 10, 1186–1190. [Google Scholar] [CrossRef]
- Piñero, S.; Rivera, J.; Romero, D.; Cevallos, M.A.; Martínez, A.; Bolívar, F.; Gosset, G. Tyrosinase from Rhizobium etli is involved in nodulation efficiency and symbiosis-associated stress resistance. J. Mol. Microbiol. Biotechnol. 2007, 13, 35–44. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Hill, H.Z. The function of melanin or six blind people examine an elephant. BioEssays 1992, 14, 49–56. [Google Scholar] [CrossRef]
- Liu, X.H.; Chen, S.M.; Gao, H.M.; Ning, G.A.; Shi, H.B.; Wang, Y.; Dong, B.; Qi, Y.Y.; Zhang, D.M.; Lu, G.D.; et al. The small GTPase MoYpt7 is required for membrane fusion in autophagy and pathogenicity of Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 4495–4510. [Google Scholar] [CrossRef]
- Bothma, J.P.; De Boor, J.; Divakar, U.; Schwenn, P.E.; Meredith, P. Device-quality electrically conducting melanin thin films. Adv. Mater. 2008, 20, 3539–3542. [Google Scholar] [CrossRef]
- Kim, Y.J.; Wu, W.; Chun, S.-E.; Bettinger, C.J. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc. Natl. Acad. Sci. USA 2013, 110, 20912–20917. [Google Scholar] [CrossRef] [PubMed]
- Ambrico, M.; Ambrico, P.F.; Cardone, A.; Cicco, S.R.; Palumbo, F.; Ligonzo, T.; Di Mundo, R.; Petta, V.; Augelli, V.; Favia, P.; et al. Melanin-like polymer layered on a nanotextured silicon surface for a hybrid biomimetic interface. J. Mater. Chem. C 2014, 2, 573–582. [Google Scholar] [CrossRef]
- Apte, M.; Girme, G.; Nair, R.; Bankar, A.; Ravi Kumar, A.; Zinjarde, S. Melanin-mediated synthesis of gold nanoparticles by Yarrowia lipolytica. Mater. Lett. 2013, 95, 149–152. [Google Scholar] [CrossRef]
- Patil, S.; Sistla, S.; Bapat, V.; Jadhav, J. Melanin-mediated synthesis of silver nanoparticles and their affinity towards tyrosinase. Appl. Biochem. Microbiol. 2018, 54, 163–172. [Google Scholar] [CrossRef]
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 8 February 2024).
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural pigments production and their application in food, health, and other industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.; Saber, W. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Scognamiglio, M.; Mirpoor, S.F.; Cammarota, M.; Ventriglia, R.; Giosafatto, C.V.L.; Fiorentino, A.; Porta, R.; Schiraldi, C. Enhanced Streptomyces roseochromogenes melanin production by using the marine renewable source Posidonia oceanica egagropili. Appl. Microbiol. Biotechnol. 2022, 106, 7265–7283. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Molina, F.; Munoz, J.L.; Varon, R.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F.; Tudela, J. A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J. Agric. Food Chem. 2007, 55, 9739–9749. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of melanins. In The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Nordlund, J.J., Boissy, R.E., Hearing, V.J., King, R.A., Oetting, W.S., Ortonne, J.-P., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 282–310. [Google Scholar]
- Endo, K.; Kamo, K.; Hosono, K.; Beppu, T.; Ueda, K. Characterization of mutants defective in melanogenesis and a gene for tyrosinase of Streptomyces griseus. J. Antibiot. 2001, 54, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Decker, H. Bacterial Tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kazi, Z.; Hungund, B.S.; Yaradoddi, J.S.; Banapurmath, R.; Yusuf, A.A.; Kishore, K.L.; Soudagar, M.E.M.; Khan, T.M.Y.; Elfasakhany, A.; Buyondo, K.A. Production, Characterization, and Antimicrobial Activity of Pigment from Streptomyces Species. J. Biomater. Appl. 2022, 2022, 3962301. [Google Scholar] [CrossRef]
- Srinivasan, M.; Merlyn Keziah, S.; Hemalatha, M.; Subathra Devi, C. Pigment from Streptomyces bellus MSA1 isolated from marine sediments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022049. [Google Scholar] [CrossRef]
- Bayram, S.; Dengiz, C.; Gerçek, Y.C.; Cetin, I.; Topcul, M.R. Bioproduction, structure elucidation, and in vitro antiproliferative effect of eumelanin pigment from Streptomyces parvus BSB49. Arch. Microbiol. 2020, 202, 2401–2409. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, Y. Metal Ions Driven Production, Characterization, and Bioactivity of Extracellular Melanin from Streptomyces sp. ZL-24. Int. J. Biol. Macromol. 2019, 123, 521–530. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; El-Ghamery, A.; Gobara, M. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J. Clust. Sci. 2017, 28, 1083–1112. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kumar, M.S.; Kumar, R.S.; Almansour, A.I.; Perumal, K.; Nayaka, S. Bioproduction, purification, and physicochemical characterization of melanin from Streptomyces sp. strain MR28. Microbiol. Res. 2022, 263, 127130. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Kenawy, E.-R.; Ahmed, S.; El-Sapagh, S. Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb. Cell Fact. 2024, 23, 23. [Google Scholar] [CrossRef]
- Polapally, R.; Mansani, M.; Rajkumar, K.; Burgula, S.; Hameeda, B.; Alhazmi, A.; Bantun, F.; Almalki, A.H.; Haque, S.; El Enshasy, H.A.; et al. Melanin pigment of Streptomyces puniceus RHPR9 exhibits antibacterial, antioxidant, and anticancer activities. PLoS ONE 2022, 17, e0197709. [Google Scholar] [CrossRef]
- Ghadge, V.; Kumar, P.; Maity, T.K.; Prasad, K.; Shinde, P.B. Facile alternative sustainable process for the selective extraction of microbial melanin. ACS Sustain. Chem. Eng. 2022, 10, 2681–2688. [Google Scholar] [CrossRef]
- Tsouko, E.; Tolia, E.; Sarris, D. Microbial melanin: Renewable feedstock and emerging applications in food-related systems. Sustainability 2023, 15, 7516. [Google Scholar] [CrossRef]
- Kraseasintra, O.; Sensupa, S.; Mahanil, K.; Yoosathaporn, S.; Pekkoh, J.; Srinuanpan, S.; Pathom-Aree, W.; Pumas, C. Optimization of melanin production by Streptomyces antibioticus NRRL B-1701 using Arthrospira (Spirulina) platensis residues hydrolysates as low-cost L-tyrosine supplement. BioTech 2023, 12, 24. [Google Scholar] [CrossRef]
- Dholakiya, R.N.; Kumar, M.A.; Mody, K.H. Production and Characterization of Melanin from Streptomyces Cavourensis Strain RD8 Using Response Surface Optimization. Environ. Pollut. Prot. 2017, 2, 168–178. [Google Scholar] [CrossRef]
- Arivarasu, L.; Arunkumar, P.; Sivaperumal, P. Isolation of melanin pigment producing marine actinobacterium of Streptomyces isolated from marine sediment samples and their antibacterial activity. J. Res. Med. Dent. Sci. 2022, 10, S84. [Google Scholar]
- Bharathi, V.; Lakshminarayanan, R.; Singaram, J. Melanin production from marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224–11234. [Google Scholar] [CrossRef]
- Li, C.; Ji, C.; Tang, B. Purification, Characterization, and Biological Activity of Melanin from Streptomyces sp. FEMS Microbiol. Lett. 2018, 365, fny077. [Google Scholar] [CrossRef] [PubMed]
- Sivaperumal, P.; Kamala, K.; Rajaram, R.; Mishra, S.S. Melanin from marine Streptomyces sp. (MVCS13) with potential effect against ornamental fish pathogens of Carassius auratus (Linnaeus, 1758). Biocatal. Agric. Biotechnol. 2014, 3, 134–141. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Jang, S.; Sudheer, P.D.V.N.; Choi, K.Y. Microbial production of melanin pigments from caffeic acid and L-tyrosine using Streptomyces glaucescens and FCS-ECH-expressing Escherichia coli. Int. J. Mol. Sci. 2021, 22, 2413. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Kellermann, M.Y.; Petersen, L.E.; Alfiansah, Y.R.; Lattyak, C.; Schupp, P.J. Characterization of an insoluble and soluble form of melanin produced by Streptomyces cavourensis SV21, a sea cucumber-associated bacterium. Mar. Drugs 2022, 20, 54. [Google Scholar] [CrossRef]
- Restaino, O.F.; Manini, P.; Kordjazi, T.; Alfieri, M.L.; Rippa, M.; Mariniello, L.; Porta, R. Biotechnological production and characterization of extracellular melanin by Streptomyces nashvillensis. Microorganisms 2024, 12, 297. [Google Scholar] [CrossRef]
- Martínez, L.M.; Martinez, A.; Gosset, G. Production of melanins with recombinant microorganisms. Front. Bioeng. Biotechnol. 2019, 7, 285. [Google Scholar] [CrossRef]
- Restaino, O.F.; Marseglia, M.; De Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; De Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as a platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Finger, M.; Sentek, F.; Hartmann, L.; Palacio-Barrera, A.M.; Schlembach, I.; Rosenbaum, M.A.; Büchs, J. Insights into Streptomyces coelicolor A3(2) growth and pigment formation with high-throughput online monitoring. Eng. Life Sci. 2022, 23, e2100151. [Google Scholar] [CrossRef]
- Madhusudhan, D.N.; Mazhari, B.B.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. BioMed Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef] [PubMed]
- Leu, W.M.; Chen, L.Y.; Liaw, L.L.; Lee, Y.H. Secretion of the Streptomyces tyrosinase is mediated through its trans-activator protein, MelC1. J. Biol. Chem. 1992, 267, 20108–20113. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, M.Y.; Leu, W.M.; Tsai, T.Y.; Lee, Y.H. Mutational study of Streptomyces tyrosinase trans-activator MelC1. MelC1 is likely a chaperone for apotyrosinase. J. Biol. Chem. 1993, 268, 18710–18716. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chen, B.F.; Wu, S.Y.; Leu, W.M.; Lin, J.J.; Chen, C.W.; Lo, S.C. A trans-acting gene is required for the phenotypic expression of a tyrosinase gene in Streptomyces. Gene 1988, 65, 71–81. [Google Scholar] [CrossRef]
- Yousif, A.; Zhang, J.; Mulcahy, F.; Singh, O.V. Bio-economics of melanin biosynthesis using electromagnetic field resistant Streptomyces sp.-EF1 isolated from cave soil. Ann. Microbiol. 2015, 65, 1573–1582. [Google Scholar] [CrossRef]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable Exploitation of Posidonia oceanica Sea Balls (Egagropili): A Review. Int. J. Mol. Sci. 2023, 24, 7301. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y. Bioprocess of Microbial Melanin Production and Isolation. Front. Bioeng. Biotechnol. 2021, 9, 765110. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, S.; Kulkarni, G.; Yaligara, V.; Lee, K.; Karegoudar, T.B. Purification and Physiochemical Characterization of Melanin Pigment from Klebsiella sp. GSK. J. Microbiol. Biotechnol. 2010, 20, 1513–1520. [Google Scholar] [CrossRef]
- Katritzky, A.; Akhmedov, N.; Denisenko, S.; Denisko, O. 1H NMR Spectroscopic Characterization of Solutions of Sepia Melanin, Sepia Melanin Free Acid, and Human Hair Melanin. Pigment Cell Res. 2002, 15, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Nakanishi, Y.; Miyazaki, N.; Kolbe, L.; Ito, S. UVA-induced oxidative degradation of melanins: Fission of indole moiety in eumelanin and conversion to benzothiazole moiety in pheomelanin. Pigment Cell Melanoma Res. 2012, 25, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, F.; Tan, G.; Costanzo, M.; Oughtred, R.; Hirschman, J.; Theesfeld, C.L.; Bansal, P.; Sahni, N.; Yi, S.; et al. An extended set of yeast-based functional assays accurately identifies human disease mutations. Genome Res. 2016, 26, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Howell, R.C.; Jiang, Z.; Bryan, R.A.; Gerfen, G.; Chen, C.-C.; Mah, D.; Cahill, S.; Casadevall, A.; Dadachova, E. Physico-Chemical Evaluation of Rationally Designed Melanins as Novel Nature-Inspired Radioprotectors. PLoS ONE 2009, 4, e7229. [Google Scholar] [CrossRef] [PubMed]
- Latocha, M.; Chodurek, E.; Kurkiewicz, S.; Świątkowska, L.; Wilczok, T. Pyrolytic GC-MS analysis of melanin from black, gray and yellow strains of Drosophila melanogaster. J. Anal. Appl. Pyrolysis 2000, 56, 89–98. [Google Scholar] [CrossRef]


| Streptomyces Strain | Melanin Production (g/L) | Medium Components (g/L) | Production System | Growth Conditions | Ref. |
|---|---|---|---|---|---|
| S. BJZ10 | 3.0 | Soluble starch (10.0), casein (0.30), KNO3 (2.0), MgSO4·7 H2O (0.05), K2HPO4 (2.0), NaCl (2.0), CaCO3 (0.02), FeSO4·7 H2O (0.01) | Shake flask | Room temperature, 120 rpm, 30 days | [28] |
| S. cavourensis RD8 | 0.087 | Casein (1.0), L-tyrosine (0.15), NaNO3 (1.0), agar (2.5) | Agar plate | pH 7.0, 30 °C, 120 rpm, 7 days | [40] |
| S. bellus MSA1 | not reported | Dextrose (0.1), casein (0.1), MgSO4·7 H2O (0.5), FeSO4·7 H2O (0.01), NaCl (0.5), K2HPO4 (2.28) | Shake flask | pH 6.0, 8.0, 35–40 °C, 10 days | [29] |
| S. parvus BSB49 | 0.160–0.240 | Dextrose (4.0), malt extract (10.0), yeast extract (4.0), agar (20.0) Soluble starch (10.0), MgSO4·7 H2O (1.0), NaCl (1.0), (NH4)2SO4 (2.0), CaCO3 (2.0), 1 mL of trace salt solution [FeSO4·7 H2O (0.01), MnCl·4 H2O (0.01) ZnSO4·7 H2O (0.01)], agar (20.0) | Agar plate | 30 °C, 4–5 days | [30] |
| S. glaucescens NEAE-H | 0.35 | Peptone (15.0), proteose peptone (5.0), yeast extract (1.0), ferric ammonium citrate (0.5), K2HPO4 (1.0), Na2S2O3 (0.08) | Shake flask | 30–37 °C, 100–200 rpm, 3–6 days | [22] |
| S. kathirae | 13.7 | Amylodextrine (3.3), yeast extract (37.0), NaCl (5.0), CaCl2 (0.1), CuSO4 (0.69), L-tyrosine (0.00025) | Shake flask | pH 6.0, 28 °C, 200 rpm, 2 days | [31] |
| S. DMZ-3 | 0.264 | Gelatin (5.0), L-tyrosine (5.0), beef extract (3.0), agar (20.0) | Agar plate | pH 8.5, 50 °C, 5 days | [52] |
| S. roseochromogenes ATCC 13400 | 3.94 (shake flask), 9.20 (batch fermentation) | Glucose (12.0), yeast extract (6.0), malt extract (30.0), KH2PO4 (42.9), K2HPO4 (17.4), egagropili powder (2.5) | Shake flask, batch fermentation (1.8 L) | pH 6.0, 26 °C, 250 rpm, 5 days (shake flasks), 4 days (batch) | [23] |
| S. ZL-24 | 0.189 (insoluble melanin), 4.24 (soluble melanin) | Soy peptone (20.3), NiCl2 (0.39), FeSO4 (1.33) | Shake flask | pH 7.0, 30 °C, 5 days | [32] |
| S. antibioticus NRRL B-1701 | 0.244 | Soluble starch (4.92), yeast extract (5.16), CuSO4 (0.019), L-tyrosine from Arthrospira platensis (Spirulina), hydrolysate (17.11), NaCl (5.0), CaCl2 (0.1) | Shake flask | pH 6.0, 30 °C, 150 rpm, 7 days | [39] |
| S. MVCS 13 | 0.147 | Glycerol (10.0), L-tyrosine (0.5), L-asparagine (1.0), K2HPO4 (0.5), MgSO4·7 H2O (0.5), NaCl (0.5), FeSO4·7 H2O (0.01), trace salt solution | Shake flask | pH 7.0, 28 °C, 200 rpm, 7 days | [44] |
| S. cavourensis SV 21 | 0.116 | Peptone (5.0), yeast extract (1.0), C6H5FeO7 (0.1), NaCl (19.45) MgCl2 (5.9), MgSO4 (3.24), CaCl2 (1.8), KCl (0.55), NaHCO3 (0.16), KBr (0.08), SrCl2 (0.034), H3BO3 (0.022), Na2SiO3 (0.004), NaF (0.0024), NH4NO3 (0.0016), Na2HPO4 (0.008) | Shake flask | 30 °C, 14 days | [46] |
| S. cyaneus | 9.9 (with 2.0% fava bean seed peel supplementation) 11.1 (with gamma irradiation) | Soluble starch (20.0), NaNO3 (0.5), MgSO4·7 H2O (0.5), KCl (0.5), K2HPO4 (1.0), CaCO3 (2.0) | Shake flask | 37 °C, 200 rpm, 7 days | [33] |
| S. sp.-EF1 | 0.082 | Starch (50), NaNO3 (2.0), K2HPO4 (1.0), MgSO4·7 H2O (0.5), KCl (0.5), FeSO4·7 H2O (0.01) | Shake flask | pH 7.2, 22 °C, 120 rpm | [56] |
| S. puniceus RHPR9 | 0.386 | Peptone (15.0), proteose peptone (5.0), yeast extract (1.0), ferric ammonium citrate (0.5), K2HPO4 (1.0), Na2S2O3 (0.08) | Shake flask | pH 6.7, 30 °C, 150 rpm, 7 days | [36] |
| S. 7VPTS-SR | 5.54 | Peptone (15.0), proteose peptone (5.0), yeast extract (1.0), ferric ammonium citrate (0.5), K2HPO4 (1.0), Na2S2O3 (0.08) | Shake flask | pH 6.7, 28 °C, 7–10 days | [37] |
| S. nashvillensis | 0.74 | Glucose (12.0), yeast extract (6.0), malt extract (30.0), NaH2PO4·H2O (5.8), Na2HPO4 (8.2) | Shake flask | pH 7.0, 28 °C, 250 rpm | [47] |
| S. djakartensis NSS-3 | 11.8 | Peptone (15.0), proteose peptone (5.0), yeast extract (1.0), ferric ammonium citrate (0.5), K2HPO4 (1.0), Na2S2O3 (0.08), L-tyrosine (2.0) | Shake flask | 30 °C, 160 rpm, 7 days | [35] |
| S. MR28 | 0.6 | Glycerol (15.0), L-tyrosine (0.5), L-asparagine (1.0), MgSO4·7 H2O (0.5), K2HPO4 (0.5), NaCl (0.5), with 1 mL of trace salt solution (FeSO4.7 H2O (0.00136), CuCl2·2 H2O (0.000027), CoCl2·6 H2O (0.00004), Na2MoO4·6 H2O (0.000025), H3BO3 (0.0028), ZnCl2 (0.00029), C4H4Na2O6 (0.0018), MnCl2·4 H2O (0.0018) | Shake flask | pH 6.0–8.0, 20–40 °C, 10 days | [34] |
| Streptomyces Strain | Isolation and Purification | Ref. |
|---|---|---|
| S. BJZ10 |
| [28] |
| S. cavourensis RD8 |
| [40] |
| S. roseochromogenes |
| [23] |
| S. parvus BSB49 S. glaucescens NEAE-H S. kathirae S. ZL-24 S. glaucescens S. puniceus RHPR9 |
| [22,30,31,32,36,45] |
| S. lusitanus DMZ-3 | Intracellular melanin:
| [52] |
| Strain | Maximum UV Wavelength (nm) | Reference |
|---|---|---|
| S. BJZ10 | 247 | [28] |
| S. parvus BSB49 | 250 | [30] |
| S. glaucescens NEAE-H | 250 | [22] |
| S. puniceus RHPR9 | 250 | [36] |
| S. ZL-24 | Insoluble: 207, soluble: 213 | [32] |
| S. lusitanus DMZ-3 | Insoluble and soluble 230 | [52] |
| S. kathirae SC-1 | 220 | [31] |
| S. roseochromogenes ATTC | 222, 254 | [23] |
| S. MVCS13 | 300 | [44] |
| S. cavourensis SV 21 | 260 | [46] |
| S. nashvillensis DSM 40314 | 220 | [47] |
| Strain | FT/IR Signals (cm−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O-H N-H | Aliphatic C-H | Amine, Amide, Carboxylic Acid | C=O | Aromatic C=C | N-H | CH2-CH3 | S=O | Aromatic Esters | C-OH | Aliphatic C-N | Aliphatic C-H | Aromatic C-H | C-S | Type of Melanin | Ref. | |
| Streptomyces BJZ10 | 3362 | 2902 | 1388 | 1310–1250 | n.d. | [28] | ||||||||||
| Streptomyces cavourensis RD8 | 3346 | 2943 | 1654 | 1112, 1029 | Pheomelanin | [40] | ||||||||||
| Streptomyces bellus MSA1 | 3346 | 2943 | 1654 | 1112, 1029 | n.d. | [29] | ||||||||||
| Streptomyces parvus BSB49 | 3265 | 2923 | 2500 | 1632, 1528 | 1450 | 1210 | Eumelanin | [30] | ||||||||
| Streptomyces sp. | 3362 | 1645 | 1645, 1532 | 1451 | 662 | Pheomelanin | [43] | |||||||||
| Streptomyces glaucescens NEAE-H | 3421 | 2947 | 2800 | 1647 | 1647, 1539 | 1423 | 1305–1243 | 1240 | 1058 | 864 | Eumelanin | [22] | ||||
| Streptomyces kathirae SC-1 | 3200–3400 | 2919 | 1631 | n.d. | [31] | |||||||||||
| Streptomyces lusitanus DMZ-3 | 3305 (insoluble), 3386 (soluble) | 1651 (insoluble), 1644 (soluble) | n.d. | [52] | ||||||||||||
| Streptomyces sp. ZL-24 | 3375 (insoluble), 3282 (soluble) | 2962 (insoluble), 2929 (soluble) | 1631 (insoluble), 1627 (soluble) | 1515 (insoluble and soluble) | 1452 (insoluble), 1450 (soluble) | 1259 (insoluble and soluble) | 1095 (insoluble), 1078 (soluble) | 802 (insoluble and soluble) | Eumelanin | [32] | ||||||
| Streptomyces F1, F2, F3 | 3381 | 2925 | 1645 | 1633 | Eumelanin | [42] | ||||||||||
| Streptomyces sp. MVCS13 | 3422 | 2959, 2924 | 2343, 2361 | 1625 | 1096, 1035 | n.d. | [44] | |||||||||
| Streptomyces glaucescens | 3420 | 2920, 2850 | 1713 | 1623 | n.d. | [45] | ||||||||||
| Streptomyces parvus BSB49 | 3265 | 2923 | 2500 | 1632, 1528 | 1450 | 1210 | Eumelanin | [30] | ||||||||
| Streptomyces puniceus RHPR9 | 3443, 3421 | 2956 | 2800 | 1650 | 1629 | 1246 | 1051 | 835 | Eumelanin | [36] | ||||||
| Streptomyces nashvillensis DSM 40314 | 3464 | 2920, 2851 | 2363, 2338 | 1725 | 1647 | 1647 | 1405 | 1233-1153 | 1075 | 872 | Eumelanin | [37] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordjazi, T.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R.; Restaino, O.F. Streptomycetes as Microbial Cell Factories for the Biotechnological Production of Melanin. Int. J. Mol. Sci. 2024, 25, 3013. https://doi.org/10.3390/ijms25053013
Kordjazi T, Mariniello L, Giosafatto CVL, Porta R, Restaino OF. Streptomycetes as Microbial Cell Factories for the Biotechnological Production of Melanin. International Journal of Molecular Sciences. 2024; 25(5):3013. https://doi.org/10.3390/ijms25053013
Chicago/Turabian StyleKordjazi, Talayeh, Loredana Mariniello, Concetta Valeria Lucia Giosafatto, Raffaele Porta, and Odile Francesca Restaino. 2024. "Streptomycetes as Microbial Cell Factories for the Biotechnological Production of Melanin" International Journal of Molecular Sciences 25, no. 5: 3013. https://doi.org/10.3390/ijms25053013







