Human Cord Blood Endothelial Progenitor Cells and Pregnancy Complications (Preeclampsia, Gestational Diabetes Mellitus, and Fetal Growth Restriction)
Abstract
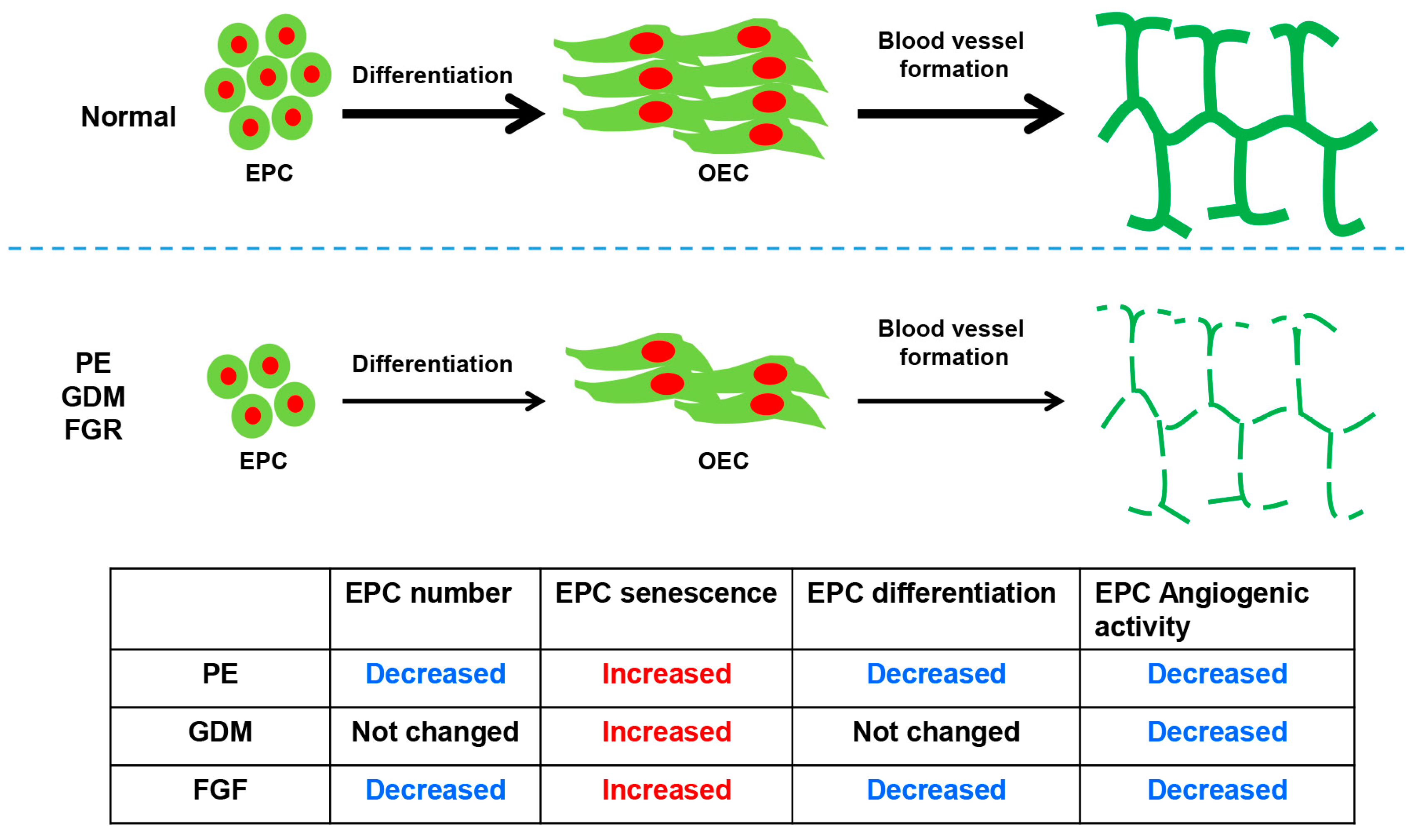
:1. Introduction
2. Preeclampsia
2.1. Number of EPC in Preeclampsia
2.2. Senescence of EPC in Preeclampsia
2.3. Differentiation Activity and Angiogenic Function of EPC in Preeclampsia
3. Gestational Diabetes Mellitus (GDM)
3.1. Number of EPC in GDM
3.2. Senescence of EPC in GDM
3.3. Differentiation and Angiogenic Function of EPC in GDM
4. Number and Senescence of EPC in FGR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Chong, M.S.; Ng, W.K.; Chan, J.K. Concise Review: Endothelial Progenitor Cells in Regenerative Medicine: Applications and Challenges. Stem Cells Transl. Med. 2016, 5, 530–538. [Google Scholar] [CrossRef]
- Ng, C.Y.; Cheung, C. Origins and functional differences of blood endothelial cells. Semin. Cell Dev. Biol. 2024, 155 Pt C, 23–29. [Google Scholar] [CrossRef]
- Yang, J.X.; Pan, Y.Y.; Wang, X.X.; Qiu, Y.G.; Mao, W. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant. 2018, 27, 786–795. [Google Scholar] [CrossRef]
- Jing, J.; Ma, B.; Zhao, X. Fetal endothelial colony-forming cells: Possible targets for prevention of the fetal origins of adult diseases. Placenta 2024, 145, 80–88. [Google Scholar] [CrossRef]
- Emontzpohl, C.; Simons, D.; Kraemer, S.; Goetzenich, A.; Marx, G.; Bernhagen, J.; Stoppe, C. Isolation of Endothelial Progenitor Cells from Healthy Volunteers and Their Migratory Potential Influenced by Serum Samples after Cardiac Surgery. J. Vis. Exp. 2017, 120, 55192. [Google Scholar]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef]
- Haider, K.H.; Aziz, S.; Al-Reshidi, M.A. Endothelial progenitor cells for cellular angiogenesis and repair: Lessons learned from experimental animal models. Regen. Med. 2017, 12, 969–982. [Google Scholar] [CrossRef]
- Liew, A.; Barry, F.; O’Brien, T. Endothelial progenitor cells: Diagnostic and therapeutic considerations. Bioessays 2006, 28, 261–270. [Google Scholar] [CrossRef]
- Yan, F.; Li, J.; Zhang, W. Transplantation of Endothelial Progenitor Cells: Summary and prospect. Acta Histochem. 2023, 125, 151990. [Google Scholar] [CrossRef]
- Li, Y.F.; Ren, L.N.; Guo, G.; Cannella, L.A.; Chernaya, V.; Samuel, S.; Liu, S.X.; Wang, H.; Yang, X.F. Endothelial progenitor cells in ischemic stroke: An exploration from hypothesis to therapy. J. Hematol. Oncol. 2015, 8, 33. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Choi, H.J.; Kwon, J.Y.; Park, Y.W.; Choi, K.S.; Min, J.K.; Kim, Y.H.; Suh, P.G.; Kang, K.S.; Won, M.H.; et al. Endothelial progenitor cell homing: Prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood 2009, 113, 233–243. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Li, J.H.; Li, Y.; Huang, D.; Yao, M. Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration. Tissue Eng. Regen. Med. 2021, 18, 747–758. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef]
- Benitez-Camacho, J.; Ballesteros, A.; Beltran-Camacho, L.; Rojas-Torres, M.; Rosal-Vela, A.; Jimenez-Palomares, M.; Sanchez-Gomar, I.; Duran-Ruiz, M.C. Endothelial progenitor cells as biomarkers of diabetes-related cardiovascular complications. Stem Cell Res. Ther. 2023, 14, 324. [Google Scholar] [CrossRef]
- Jain, R.; Awal, H.; Sen, S. Using adult stem cells to monitor endothelial dysfunction in diabetes mellitus. J. Diabetes Complicat. 2020, 34, 107588. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Rossig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kamper, U.; Dimmeler, S.; Zeiher, A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef]
- Assmus, B.; Urbich, C.; Aicher, A.; Hofmann, W.K.; Haendeler, J.; Rossig, L.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ. Res. 2003, 92, 1049–1055. [Google Scholar] [CrossRef]
- Imanishi, T.; Hano, T.; Sawamura, T.; Nishio, I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin. Exp. Pharmacol. Physiol. 2004, 31, 407–413. [Google Scholar] [CrossRef]
- Shimizu, S.; Iba, T.; Naito, H.; Rahmawati, F.N.; Konishi, H.; Jia, W.; Muramatsu, F.; Takakura, N. Aging impairs the ability of vascular endothelial stem cells to generate endothelial cells in mice. Angiogenesis 2023, 26, 567–580. [Google Scholar] [CrossRef]
- Park, Y.; Lee, H.J.; Jung, Y.J.; Kwon, H.Y.; Kim, H.; Lee, J.; Kim, Y.H.; Kim, H.O.; Maeng, Y.S.; Kwon, J.Y. CD133+/C-kit+Lin(-) endothelial progenitor cells in fetal circulation demonstrate impaired differentiation potency in severe preeclampsia. Pregnancy Hypertens. 2019, 15, 146–153. [Google Scholar] [CrossRef]
- Monga, R.; Buck, S.; Sharma, P.; Thomas, R.; Chouthai, N.S. Effect of preeclampsia and intrauterine growth restriction on endothelial progenitor cells in human umbilical cord blood. J. Matern. Fetal Neonatal Med. 2012, 25, 2385–2389. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, G.; Li, Z.; Liu, X.; Zhao, Y.; Zou, L.; Liu, W. Endothelial progenitor cells in pregnancy-related diseases. Clin Sci. 2023, 137, 1699–1719. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Wijesekara, J.; Baker, P.N.; Ong, S.S. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta 2004, 25, 829–833. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Maeng, Y.S.; Kwon, Y.G.; Kim, Y.H.; Kang, M.H.; Park, Y.W. Decreased endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol. Obstet. Investig. 2007, 64, 103–108. [Google Scholar] [CrossRef]
- Blue, E.K.; Sheehan, B.M.; Nuss, Z.V.; Boyle, F.A.; Hocutt, C.M.; Gohn, C.R.; Varberg, K.M.; McClintick, J.N.; Haneline, L.S. Epigenetic Regulation of Placenta-Specific 8 Contributes to Altered Function of Endothelial Colony-Forming Cells Exposed to Intrauterine Gestational Diabetes Mellitus. Diabetes 2015, 64, 2664–2675. [Google Scholar] [CrossRef]
- Ingram, D.A.; Lien, I.Z.; Mead, L.E.; Estes, M.; Prater, D.N.; Derr-Yellin, E.; DiMeglio, L.A.; Haneline, L.S. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes 2008, 57, 724–731. [Google Scholar] [CrossRef]
- Buemi, M.; Allegra, A.; D’Anna, R.; Coppolino, G.; Crasci, E.; Giordano, D.; Loddo, S.; Cucinotta, M.; Musolino, C.; Teti, D. Concentration of circulating endothelial progenitor cells (EPC) in normal pregnancy and in pregnant women with diabetes and hypertension. Am. J. Obstet. Gynecol. 2007, 196, 68.e1–68.e6. [Google Scholar] [CrossRef]
- Schreiber, V.; Hurst, C.; da Silva Costa, F.; Stoke, R.; Turner, J.; Kumar, S. Definitions matter: Detection rates and perinatal outcome for infants classified prenatally as having late fetal growth restriction using SMFM biometric vs ISUOG/Delphi consensus criteria. Ultrasound Obstet. Gynecol. 2023, 61, 377–385. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kwon, Y.G.; Kwon, J.Y.; Won Park, Y.; Maeng, Y.S.; Kim, Y.H. Senescence of fetal endothelial progenitor cell in pregnancy with idiopathic fetal growth restriction. J. Matern. Fetal Neonatal Med. 2012, 25, 1769–1773. [Google Scholar] [CrossRef]
- Singh, A.; Jaiswar, S.P.; Priyadarshini, A.; Deo, S. Reduced Endothelial Progenitor Cells: A Possible Biomarker for Idiopathic Fetal Growth Restriction in Human Pregnancies. J. Mother. Child. 2023, 27, 182–189. [Google Scholar]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Bianco, M.E.; Josefson, J.L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Curr. Diab. Rep. 2019, 19, 143. [Google Scholar] [CrossRef]
- Low, J.A.; Handley-Derry, M.H.; Burke, S.O.; Peters, R.D.; Pater, E.A.; Killen, H.L.; Derrick, E.J. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am. J. Obstet. Gynecol. 1992, 167, 1499–1505. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. S5), 1344s–1352s. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 1990, 163 Pt 1, 1691–1712. [CrossRef]
- Shen, X.; Wang, C.; Yue, X.; Wang, Q.; Xie, L.; Huang, Z.; Huang, X.; Li, J.; Xu, Y.; Chen, L.; et al. Preeclampsia associated changes in volume density of fetoplacental vessels in Chinese women and mouse model of preeclampsia. Placenta 2022, 121, 116–125. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Charnock-Jones, D.S.; Kaufmann, P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta 2004, 25, 127–139. [Google Scholar] [CrossRef]
- Resta, L.; Capobianco, C.; Marzullo, A.; Piscitelli, D.; Sanguedolce, F.; Schena, F.P.; Gesualdo, L. Confocal laser scanning microscope study of terminal villi vessels in normal term and pre-eclamptic placentas. Placenta 2006, 27, 735–739. [Google Scholar] [CrossRef]
- Fadini, G.P.; Schiavon, M.; Cantini, M.; Baesso, I.; Facco, M.; Miorin, M.; Tassinato, M.; de Kreutzenberg, S.V.; Avogaro, A.; Agostini, C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006, 24, 1806–1813. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Stamm, C.; Westphal, B.; Kleine, H.D.; Petzsch, M.; Kittner, C.; Klinge, H.; Schümichen, C.; Nienaber, C.A.; Freund, M.; Steinhoff, G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003, 361, 45–46. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Hwang, H.S.; Maeng, Y.S.; Park, Y.W.; Koos, B.J.; Kwon, Y.G.; Kim, Y.H. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am. J. Obstet. Gynecol. 2008, 199, 259.e1–259.e7. [Google Scholar] [CrossRef]
- Brodowski, L.; Schroder-Heurich, B.; von Hardenberg, S.; Richter, K.; von Kaisenberg, C.S.; Dittrich-Breiholz, O.; Meyer, N.; Dork, T.; von Versen-Hoynck, F. MicroRNA Profiles of Maternal and Neonatal Endothelial Progenitor Cells in Preeclampsia. Int. J. Mol. Sci. 2021, 22, 5320. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, X.P.; Zhu, J.H.; Xie, X.D.; Zhang, H.; Wang, X.X.; Chen, J.Z.; Jian, S. Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre-eclampsia. J. Obstet. Gynaecol. Res. 2007, 33, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Melk, A.; Schmidt, B.M.; Vongwiwatana, A.; Rayner, D.C.; Halloran, P.F. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am. J. Transplant. 2005, 5, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.; Parish, W.E.; Strongitharm, B.H.; van Heemst, D.; Slagboom, P.E.; de Craen, A.J.; Sedivy, J.M.; Westendorp, R.G.; Gunn, D.A.; Maier, A.B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Komuro, I. Vascular cell senescence: Contribution to atherosclerosis. Circ. Res. 2007, 100, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Hilgers, K.F.; Steinbach, M.P.; Hartner, A.; Klanke, B.; Amann, K.; Melk, A. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 2008, 52, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Lain, K.Y. Recent Insights into the pathogenesis of pre-eclampsia. Placenta 2002, 23, 359–372. [Google Scholar] [CrossRef]
- Frusca, T.; Morassi, L.; Pecorelli, S.; Grigolato, P.; Gastaldi, A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br. J. Obstet. Gynaecol. 1989, 96, 835–839. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015, 89 Pt B, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Li, Y. Endothelial cell senescence and age-related vascular diseases. J. Genet. Genom. 2014, 41, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Regina, C.; Panatta, E.; Candi, E.; Melino, G.; Amelio, I.; Balistreri, C.R.; Annicchiarico-Petruzzelli, M.; Di Daniele, N.; Ruvolo, G. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016, 159, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Mitsui-Saito, M.; Hayashi, C.; Hoshiai, T.; Senoo, M.; Chisaka, H.; Yaegashi, N.; Okamura, K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 5329–5332. [Google Scholar] [CrossRef] [PubMed]
- Gumina, D.L.; Su, E.J. Endothelial Progenitor Cells of the Human Placenta and Fetoplacental Circulation: A Potential Link to Fetal, Neonatal, and Long-term Health. Front. Pediatr. 2017, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Osmond, C.; Eriksson, J.G. Gestational hypertension is associated with increased risk of type 2 diabetes in adult offspring: The Helsinki Birth Cohort Study. Am. J. Obstet. Gynecol. 2017, 216, 281.e1–281.e7. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Eriksson, J.G.; Osmond, C.; Thornburg, K.; Barker, D.J. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The Helsinki birth cohort study. Stroke 2009, 40, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Warshafsky, C.; Pudwell, J.; Walker, M.; Wen, S.W.; Smith, G.N. Prospective assessment of neurodevelopment in children following a pregnancy complicated by severe pre-eclampsia. BMJ Open 2016, 6, e010884. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.; Brown, S.; Pennell, C.; Lye, S.; Torpy, D.J. Evidence for central hypercortisolism and elevated blood pressure in adolescent offspring of mothers with pre-eclampsia. Clin. Endocrinol. 2016, 85, 583–589. [Google Scholar] [CrossRef]
- Eguchi, M.; Masuda, H.; Asahara, T. Endothelial progenitor cells for postnatal vasculogenesis. Clin. Exp. Nephrol. 2007, 11, 18–25. [Google Scholar] [CrossRef]
- Kunz, G.A.; Liang, G.; Cuculi, F.; Gregg, D.; Vata, K.C.; Shaw, L.K.; Goldschmidt-Clermont, P.J.; Dong, C.; Taylor, D.A.; Peterson, E.D. Circulating endothelial progenitor cells predict coronary artery disease severity. Am. Heart J. 2006, 152, 190–195. [Google Scholar] [CrossRef]
- Fadini, G.P.; Sartore, S.; Albiero, M.; Baesso, I.; Murphy, E.; Menegolo, M.; Grego, F.; Vigili de Kreutzenberg, S.; Tiengo, A.; Agostini, C.; et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2140–2146. [Google Scholar] [CrossRef]
- Fadini, G.P.; Coracina, A.; Baesso, I.; Agostini, C.; Tiengo, A.; Avogaro, A.; de Kreutzenberg, S.V. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 2006, 37, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.; Picard, F.; Richter, J.; Darrelmann, E.; Schneider, M.; Strauer, B.E.; Brehm, M. Accumulation of VEGFR-2+/CD133+ cells and decreased number and impaired functionality of CD34+/VEGFR-2+ cells in patients with SLE. Rheumatology 2010, 49, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S.; Singh, U.; Huet, B.A. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: Implications for increased cardiovascular risk. Atherosclerosis 2010, 211, 297–302. [Google Scholar] [CrossRef]
- Beasley, K.M.; Lovering, A.T.; Gilbert, J.S. Decreased endothelial progenitor cells in preeclampsia and consequences for developmental programming. Hypertension 2014, 64, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.; Kwon, J.Y.; Maeng, Y.S. Human Cord Blood-Derived CD133(+)/C-Kit(+)/Lin(-) Cells Have Bipotential Ability to Differentiate into Mesenchymal Stem Cells and Outgrowth Endothelial Cells. Stem Cells Int. 2016, 2016, 7162160. [Google Scholar] [CrossRef]
- Muñoz-Hernandez, R.; Miranda, M.L.; Stiefel, P.; Lin, R.Z.; Praena-Fernández, J.M.; Dominguez-Simeon, M.J.; Villar, J.; Moreno-Luna, R.; Melero-Martin, J.M. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension 2014, 64, 165–171. [Google Scholar] [CrossRef]
- Gumina, D.L.; Black, C.P.; Balasubramaniam, V.; Winn, V.D.; Baker, C.D. Umbilical Cord Blood Circulating Progenitor Cells and Endothelial Colony-Forming Cells Are Decreased in Preeclampsia. Reprod. Sci. 2017, 24, 1088–1096. [Google Scholar] [CrossRef]
- Voigt, P.; Tee, W.W.; Reinberg, D. A double take on bivalent promoters. Genes. Dev. 2013, 27, 1318–1338. [Google Scholar] [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30 (Suppl. S2), S251–S260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Pucci, L.; Lucchesi, D.; Lencioni, C.; Iorio, M.C.; Vanacore, R.; Storti, E.; Resi, V.; Di Cianni, G.; Del Prato, S. Circulating endothelial progenitor cells in women with gestational alterations of glucose tolerance. Diabetes Vasc. Dis. Res. 2011, 8, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Altabas, V. Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye on? Int. J. Endocrinol. 2015, 2015, 848272. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Avogaro, A.; Fadini, G.P. Levels of Circulating Progenitor Cells, Cardiovascular Outcomes and Death: A Meta-Analysis of Prospective Observational Studies. Circ. Res. 2016, 118, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Bittante, C.; Albiero, M.; Avogaro, A.; Fadini, G.P. Circulating Progenitor Cell Count Predicts Microvascular Outcomes in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2015, 100, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Mordwinkin, N.M.; Ouzounian, J.G.; Yedigarova, L.; Montoro, M.N.; Louie, S.G.; Rodgers, K.E. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. J. Matern. Fetal Neonatal Med. 2013, 26, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Rohrbach, A.; Borns, K.; Hillemanns, P.; Feng, L.; Hubel, C.A.; von Versen-Hoynck, F. Vitamin D rescues dysfunction of fetal endothelial colony forming cells from individuals with gestational diabetes. Placenta 2015, 36, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Blue, E.K.; DiGiuseppe, R.; Derr-Yellin, E.; Acosta, J.C.; Pay, S.L.; Hanenberg, H.; Schellinger, M.M.; Quinney, S.K.; Mund, J.A.; Case, J.; et al. Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells. Pediatr. Res. 2014, 75, 266–272. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, C.; Li, B.; Liu, C.; He, Z.; Li, X.E.; Wang, A.; Ma, G.; Yao, Y. Bradykinin Protects Human Endothelial Progenitor Cells from High-Glucose-Induced Senescence through B2 Receptor-Mediated Activation of the Akt/eNOS Signalling Pathway. J. Diabetes Res. 2021, 2021, 6626627. [Google Scholar] [CrossRef]
- Sahin, E.; Depinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.G.; Lavery, R.; Caporali, F.; Fondelli, C.; Laghi-Pasini, F.; Dotta, F.; Sorrentino, V. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia 2008, 51, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Loomans, C.J.; de Koning, E.J.; Staal, F.J.; Rookmaaker, M.B.; Verseyden, C.; de Boer, H.C.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; van Zonneveld, A.J. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Pucci, L.; Vanacore, R.; Baesso, I.; Penno, G.; Balbarini, A.; Di Stefano, R.; Miccoli, R.; de Kreutzenberg, S.; Coracina, A.; et al. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia 2007, 50, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Bunt, J.C.; Tataranni, P.A.; Salbe, A.D. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J. Clin. Endocrinol. Metab. 2005, 90, 3225–3229. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Silverman, B.L.; Rizzo, T.A.; Metzger, B.E. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J. Pediatr. 2000, 136, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Varberg, K.M.; Garretson, R.O.; Blue, E.K.; Chu, C.; Gohn, C.R.; Tu, W.; Haneline, L.S. Transgelin induces dysfunction of fetal endothelial colony-forming cells from gestational diabetic pregnancies. Am. J. Physiol. Cell Physiol. 2018, 315, C502–C515. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Potthast, A.; Rohrbach, A.; Borns, K.; Das, A.M.; von Versen-Hoynck, F. Gestational diabetes induces alterations of sirtuins in fetal endothelial cells. Pediatr. Res. 2016, 79, 788–798. [Google Scholar] [CrossRef]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells functional characterization. Trends Cardiovasc. Med. 2004, 14, 318–322. [Google Scholar] [CrossRef]
- Robb, A.O.; Mills, N.L.; Newby, D.E.; Denison, F.C. Endothelial progenitor cells in pregnancy. Reproduction 2007, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Houde, A.A.; Guay, S.P.; Desgagne, V.; Hivert, M.F.; Baillargeon, J.P.; St-Pierre, J.; Perron, P.; Gaudet, D.; Brisson, D.; Bouchard, L. Adaptations of placental and cord blood ABCA1 DNA methylation profile to maternal metabolic status. Epigenetics 2013, 8, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Dilli, D.; Dogan, N.N.; Ipek, M.S.; Cavus, Y.; Ceylaner, S.; Dogan, H.; Dursun, A.; Kucukozkan, T.; Zenciroglu, A. MaFOS-GDM trial: Maternal fish oil supplementation in women with gestational diabetes and cord blood DNA methylation at insulin like growth factor-1 (IGF-1) gene. Clin. Nutr. ESPEN 2018, 23, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin. Epigenetics 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Ott, R.; Melchior, K.; Stupin, J.H.; Ziska, T.; Schellong, K.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Reduced Insulin Receptor Expression and Altered DNA Methylation in Fat Tissues and Blood of Women With GDM and Offspring. J. Clin. Endocrinol. Metab. 2019, 104, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Awamleh, Z.; Butcher, D.T.; Hanley, A.; Retnakaran, R.; Haertle, L.; Haaf, T.; Hamilton, J.; Weksberg, R. Exposure to Gestational Diabetes Mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Res. Clin. Pract. 2021, 174, 108690. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Jung, Y.J.; Lee, Y.; Son, G.H.; Kim, H.O.; Maeng, Y.S.; Kwon, J.Y. Impaired Angiogenic Function of Fetal Endothelial Progenitor Cells via PCDH10 in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 6082. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Illanes, S.; Soothill, P. Management of fetal growth restriction. Semin. Fetal Neonatal Med. 2004, 9, 395–401. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Resnik, R. Intrauterine growth restriction. Obstet. Gynecol. 2002, 99, 490–496. [Google Scholar] [PubMed]
- Rosso, I.M.; Cannon, T.D.; Huttunen, T.; Huttunen, M.O.; Lönnqvist, J.; Gasperoni, T.L. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am. J. Psychiatry 2000, 157, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Martyn, C.N. Birth weight and later risk of depression in a national birth cohort. Br. J. Psychiatry 2004, 184, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Frisk, V.; Amsel, R.; Whyte, H.E. The importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children. Dev. Neuropsychol. 2002, 22, 565–593. [Google Scholar] [CrossRef]
- Amarilyo, G.; Oren, A.; Mimouni, F.B.; Ochshorn, Y.; Deutsch, V.; Mandel, D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J. Perinatol. 2011, 31, 30–32. [Google Scholar] [CrossRef]
- Wallner, W.; Sengenberger, R.; Strick, R.; Strissel, P.L.; Meurer, B.; Beckmann, M.W.; Schlembach, D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin. Sci. 2007, 112, 51–57. [Google Scholar] [CrossRef]
| Normal Pregnancy (n = 30) | Preeclampsia (n = 30) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 33.7 ± 5.3 | 32.75 ± 3.1 | 0.549 |
| Gestational age at delivery (weeks) | 38.65 ± 0.8 | 36.35 ± 2.1 | 0.386 |
| Gravida | 2.5 ± 1.4 | 1.55 ± 1.0 | 0.05 |
| Pre-pregnancy body mass index (BMI) (kg/m2) | 21.75 ± 4.7 | 22.1 ± 3.8 | 0.387 |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 115.1 ±7 | 151.05 ±15 | <0.015 |
| Diastolic blood pressure | 74.25 ±15 | 95.7 ±18 | <0.015 |
| Birth weight (g) | 3301.0 ± 365 | 2303.1 ± 536 | <0.001 |
| Small gestational age (n) | 0 | 21 | 0.03 |
| Free VEGF, mean (SD) | 22.66 (12.88) | 12.84 (1.56) | 0.04 |
| sVEGFR-1, median (range) | 1.11 (1.66) | 1.25 (27.81) | 0.24 |
| EPC count (×106/50 mL) | 8.61 | 2.65 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-Y.; Maeng, Y.-S. Human Cord Blood Endothelial Progenitor Cells and Pregnancy Complications (Preeclampsia, Gestational Diabetes Mellitus, and Fetal Growth Restriction). Int. J. Mol. Sci. 2024, 25, 4444. https://doi.org/10.3390/ijms25084444
Kwon J-Y, Maeng Y-S. Human Cord Blood Endothelial Progenitor Cells and Pregnancy Complications (Preeclampsia, Gestational Diabetes Mellitus, and Fetal Growth Restriction). International Journal of Molecular Sciences. 2024; 25(8):4444. https://doi.org/10.3390/ijms25084444
Chicago/Turabian StyleKwon, Ja-Young, and Yong-Sun Maeng. 2024. "Human Cord Blood Endothelial Progenitor Cells and Pregnancy Complications (Preeclampsia, Gestational Diabetes Mellitus, and Fetal Growth Restriction)" International Journal of Molecular Sciences 25, no. 8: 4444. https://doi.org/10.3390/ijms25084444





