Exosomes Secreted by Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote the Ability of Cell Proliferation and Migration for Keratinocyte
Abstract
:1. Introduction
2. Results
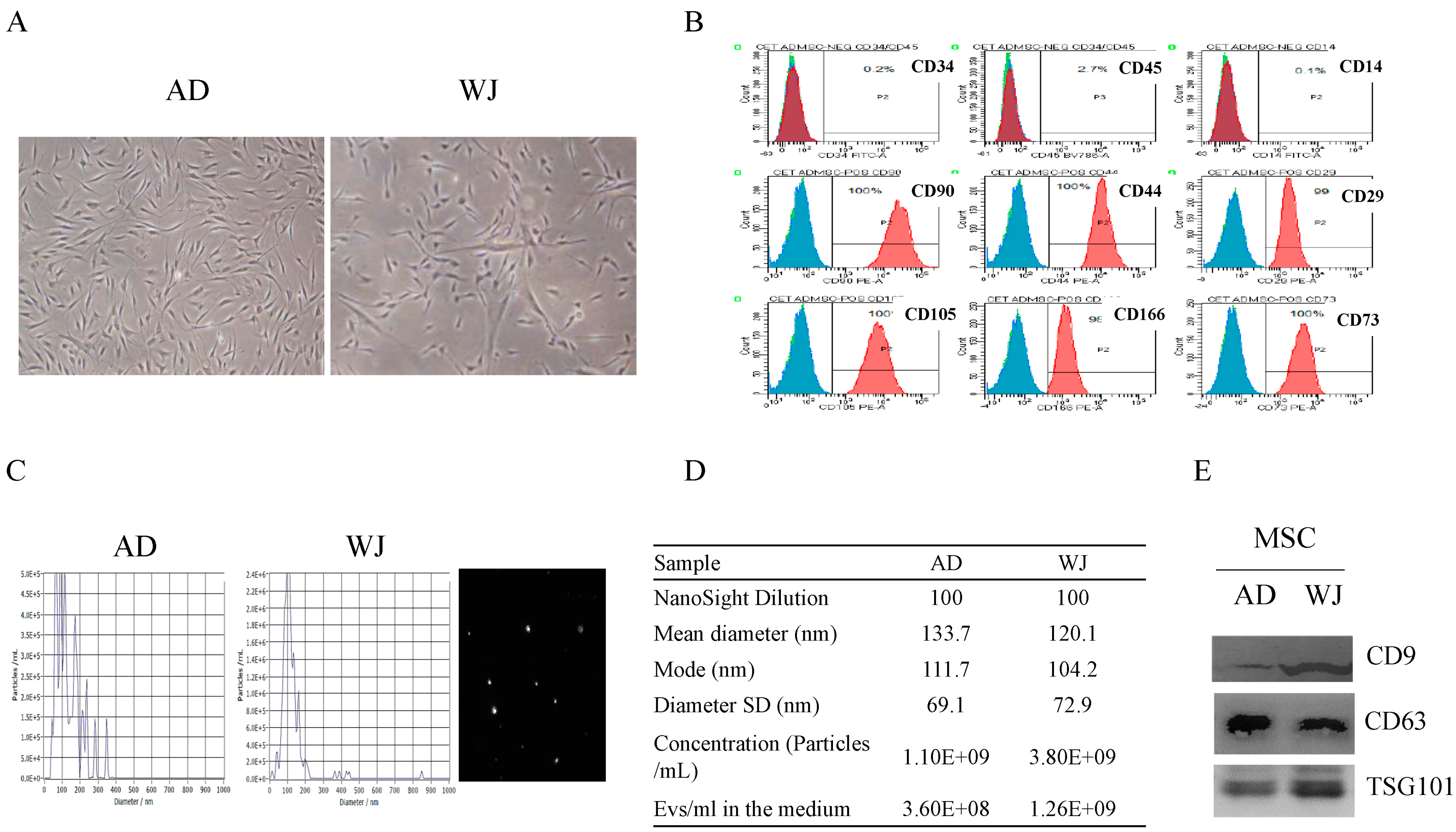
2.1. Discrimination of Exosomes Derived from AD-MSCs and WJ-MSCs
2.2. Protein Abundance Profiles of AD-MSC and WJ-MSC Exosomes
2.3. Enrichment of AD-MSC and WJ-MSC Exosome-Specific Proteins
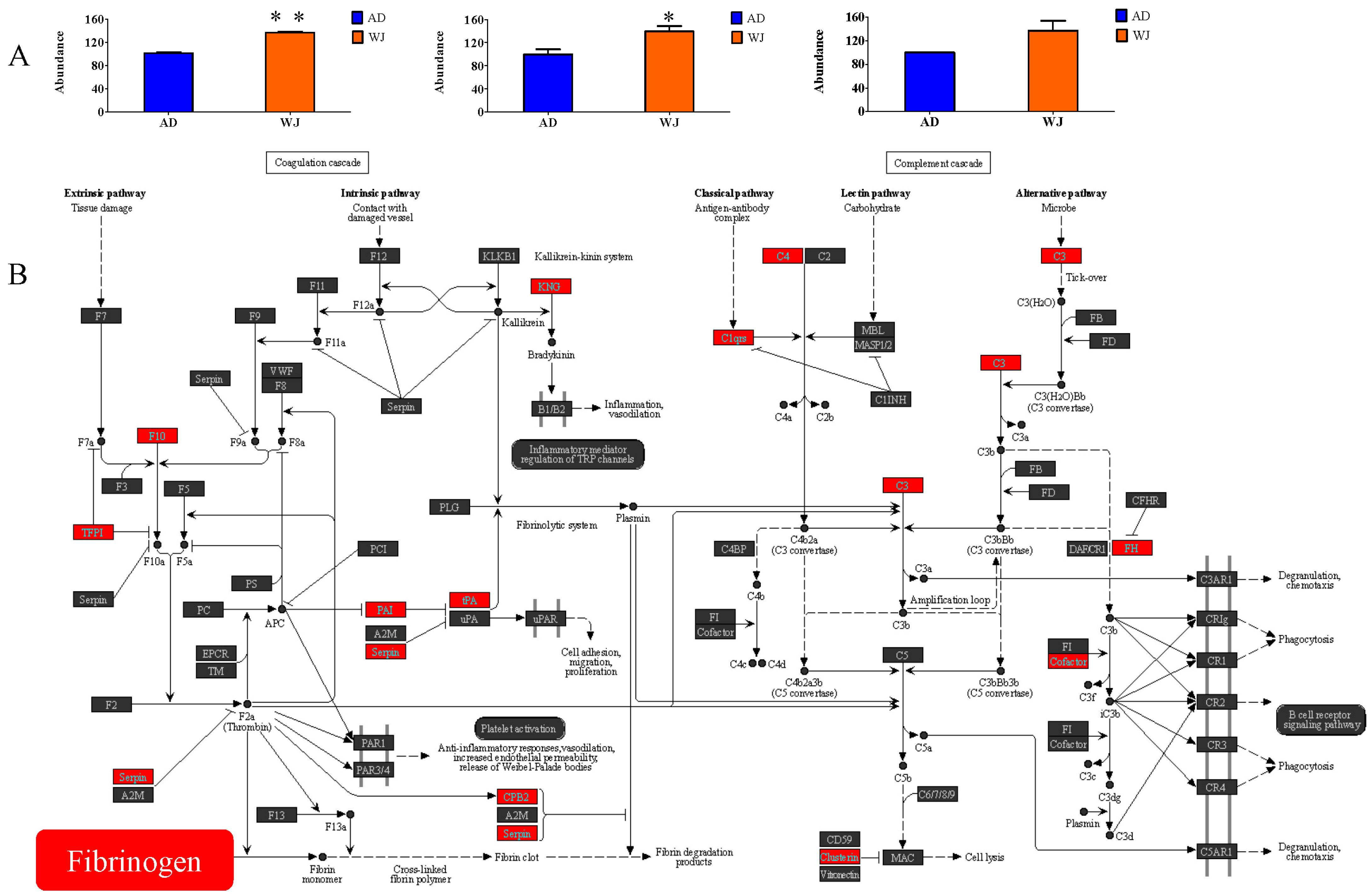
2.4. Abundance of Fibrinogen Proteins in WJ-MSC Exosomes
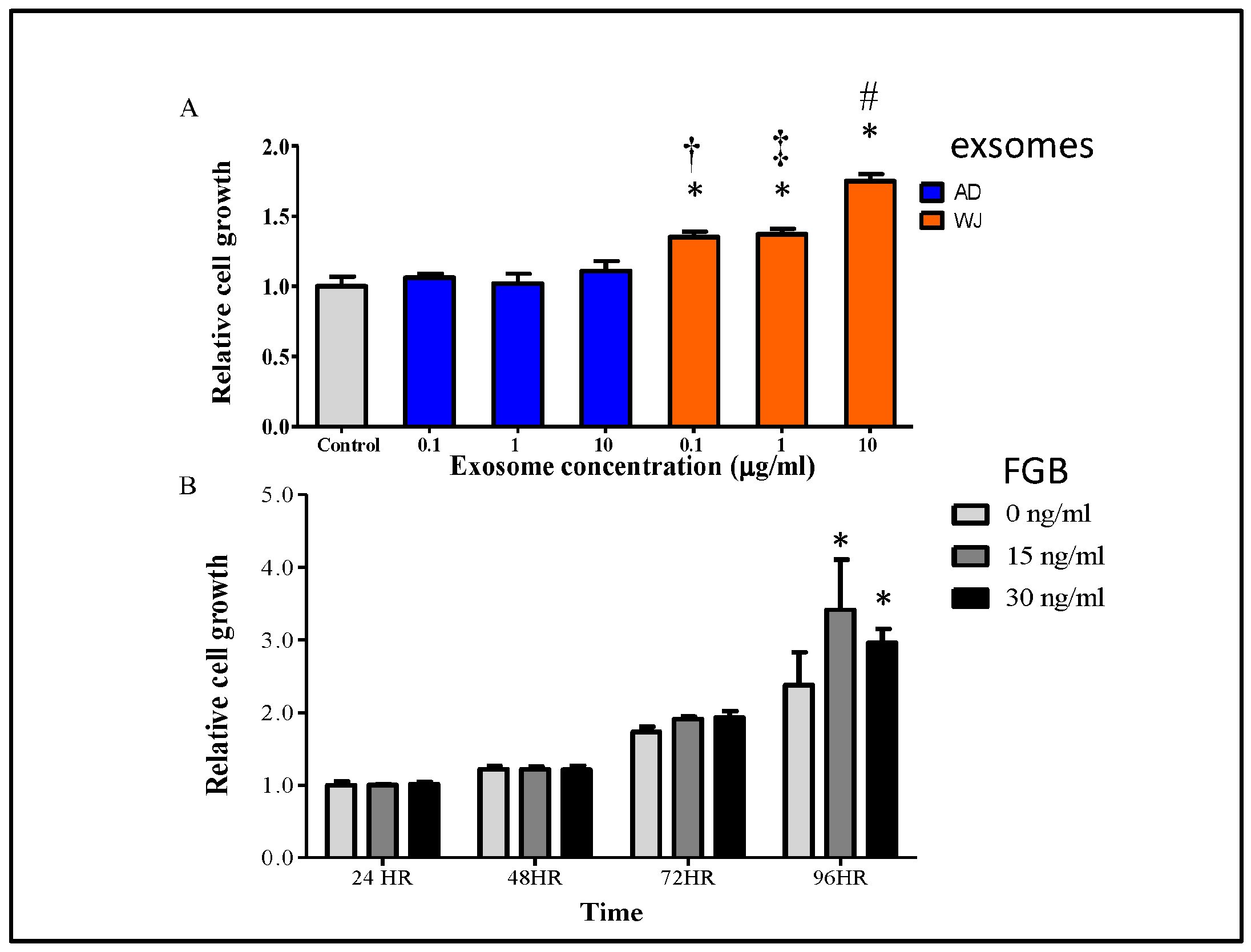
2.5. Effects of WJ-MSC Exosomes on Keratinocyte Growth
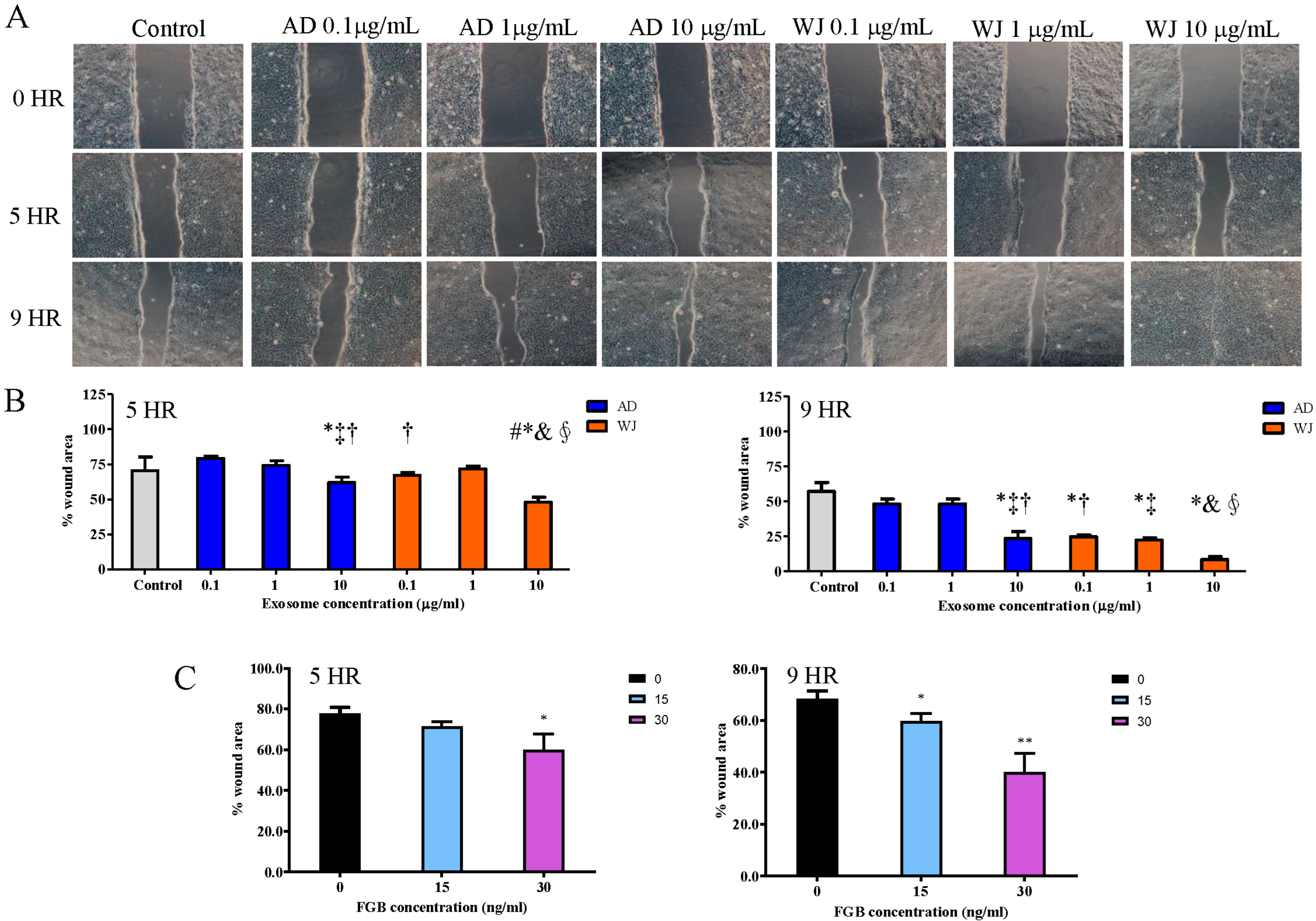
2.6. Effects of WJ-MSC Exosomes on Keratinocyte Migration
3. Discussion
4. Materials and Methods
4.1. Isolation of AD-MSC and WJ-MSC Exosomes
4.2. Western Blotting
4.3. Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Gel-Free Proteomics
4.4. Culture and Growth of Keratinocytes
4.5. Cell Proliferation Assay
4.6. Wound Healing Assay
4.7. Trans-Well Migration Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef]
- Abu Kasim, N.H.; Govindasamy, V.; Gnanasegaran, N.; Musa, S.; Pradeep, P.J.; Srijaya, T.C.; Aziz, Z.A. Unique molecular signatures influencing the biological function and fate of post-natal stem cells isolated from different sources. J. Tissue Eng. Regen. Med. 2015, 9, E252–E266. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, G.; Cao, W.; Li, C.H.; Pan, S.H.; Wang, L.; Liu, Y.; Ma, L.; Cui, D. Proteomics Analyses Reveal Functional Differences between Exosomes of Mesenchymal Stem Cells Derived from The Umbilical Cord and Those Derived from The Adipose Tissue. Cell J. 2021, 23, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Miana, V.V.; Gonzalez, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Wuputra, K.; Ku, C.C.; Wu, D.C.; Lin, Y.C.; Saito, S.; Yokoyama, K.K. Prevention of tumor risk associated with the reprogramming of human pluripotent stem cells. J. Exp. Clin. Cancer Res. CR 2020, 39, 100. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dhar, R.; Jonnalagadda, S.; Gorai, S.; Nag, S.; Kar, R.; Mukerjee, N.; Mukherjee, D.; Vatsa, R.; Arikketh, D.; et al. Exosomal miRNAs and breast cancer: A complex theranostics interlink with clinical significance. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2023, 28, 502–518. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Dhar, R.; Mukherjee, S.; Gorai, S.; Devi, A.; Krishnan, A.; Alexiou, A.; Papadakis, M. Exosome DNA: An untold story of cancer. Clin. Transl. Discov. 2023, 3, e218. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Deng, P.; Li, Z.; Xu, Y.; Li, H.; Su, W.; Qin, J. Advances of Exosomal miRNAs in Breast Cancer Progression and Diagnosis. Diagnostics 2021, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Esser, J.; Gehrmann, U.; D’Alexandri, F.L.; Hidalgo-Estevez, A.M.; Wheelock, C.E.; Scheynius, A.; Gabrielsson, S.; Radmark, O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 2010, 126, 1032–1040, 1040 e1031-1034. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Sastre, B.; Canas, J.A.; Rodrigo-Munoz, J.M.; Del Pozo, V. Novel Modulators of Asthma and Allergy: Exosomes and MicroRNAs. Front. Immunol. 2017, 8, 826. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203. [Google Scholar] [CrossRef]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.H.; Nguyen, T.D.; Nguyen, H.P.; Nguyen, X.H.; Do, P.T.X.; Dang, V.D.; Dam, P.T.M.; Bui, H.T.H.; Trinh, M.Q.; Vu, D.M.; et al. Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition. Front. Mol. Biosci. 2020, 7, 119. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, L.; Zhang, B.; Li, B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J. Cell. Biochem. 2020, 121, 2089–2102. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.B.; Pereira, E.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, J.; Farack, J.; Vater, C.; Johnsen, M.; Gelinsky, M.; Tonn, T.; Kasten, P. Translation of cell therapy into clinical practice: Validation of an application procedure for bone marrow progenitor cells and platelet rich plasma. J. Appl. Biomater. Funct. Mater. 2016, 14, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Sinno, H.; Prakash, S. Complements and the wound healing cascade: An updated review. Plast. Surg. Int. 2013, 2013, 146764. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Bakhtyar, N.; Jeschke, M.G.; Herer, E.; Sheikholeslam, M.; Amini-Nik, S. Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res. Ther. 2018, 9, 193. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.H.; Lee, H.; Sung, J.H.; Bang, O.Y. Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. Int. J. Mol. Sci. 2023, 24, 4273. [Google Scholar] [CrossRef]
- Lv, X.; Wang, L.; Zou, X.; Huang, S. Umbilical Cord Mesenchymal Stem Cell Therapy for Regenerative Treatment of Rheumatoid Arthritis: Opportunities and Challenges. Drug Des. Dev. Ther. 2021, 15, 3927–3936. [Google Scholar] [CrossRef]
- Paganelli, A.; Tarentini, E.; Benassi, L.; Kaleci, S.; Magnoni, C. Mesenchymal stem cells for the treatment of psoriasis: A comprehensive review. Clin. Exp. Dermatol. 2020, 45, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Zhang, Y.; Zhang, M.; Chen, J.; Li, X.; Hu, X.; Jiang, S.; Shi, S.; Sun, L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res. Ther. 2014, 16, R79. [Google Scholar] [CrossRef]
- Escalhao, C.C.M.; Ramos, I.P.; Hochman-Mendez, C.; Brunswick, T.H.K.; Souza, S.A.L.; Gutfilen, B.; Dos Santos Goldenberg, R.C.; Coelho-Sampaio, T. Safety of Allogeneic Canine Adipose Tissue-Derived Mesenchymal Stem Cell Intraspinal Transplantation in Dogs with Chronic Spinal Cord Injury. Stem Cells Int. 2017, 2017, 3053759. [Google Scholar] [CrossRef] [PubMed]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.S.; Santos, B.S.D.; Almeida, B.L.; Hiura, E.; Fiorio, W.A.B.; Valdetaro, G.P.; Goncalves, D.V.; Silva, C.S.; Champion, T.; Campagnol, D. Adipose tissue derived mesenchymal stem cell transplantation in the treatment of ischemia/reperfusion induced acute kidney injury in rats. Application route and therapeutic window1. Acta Cir. Bras. 2018, 33, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, Y.M.; Zhang, H.; Li, J.; Wang, W.; Wei, Y.J.; Hu, S.S. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: Implications for tissue engineering heart valve construction. Artif. Organs 2010, 34, 215–222. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Baharlooi, H.; Azimi, M.; Salehi, Z.; Izad, M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int. J. Stem Cells 2020, 13, 13–23. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Janockova, J.; Slovinska, L.; Harvanova, D.; Spakova, T.; Rosocha, J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021, 28, 39. [Google Scholar] [CrossRef]
- de Moerloose, P.; Casini, A.; Neerman-Arbez, M. Congenital fibrinogen disorders: An update. Semin. Thromb. Hemost. 2013, 39, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. JTH 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/beta-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Vargas, V.; Revello, P.; Bull, D.A. Mesenchymal stem cell population isolated from the subepithelial layer of umbilical cord tissue. Cell Transplant. 2013, 22, 513–519. [Google Scholar] [CrossRef]
- Wang, Z.G.; He, Z.Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511. [Google Scholar] [CrossRef]
- Li, S.C.; Tsai, K.W.; Huang, L.H.; Weng, K.P.; Chien, K.J.; Lin, Y.; Tu, C.Y.; Lin, P.H. Serum proteins may facilitate the identification of Kawasaki disease and promote in vitro neutrophil infiltration. Sci. Rep. 2020, 10, 15645. [Google Scholar] [CrossRef]


| Category | Pathway | p-Value |
|---|---|---|
| WJ | Complement and coagulation cascades | 2.45 × 10−9 |
| WJ | ECM–receptor interaction | 2.11 × 10−7 |
| WJ | Focal adhesion | 1.05 × 10−6 |
| WJ | Amoebiasis | 4.06 × 10−6 |
| WJ | Proteasome | 2.98 × 10−5 |
| WJ | Glycosaminoglycan biosynthesis—chondroitin sulfate/dermatan sulfate | 2.37 × 10−4 |
| WJ | Hypertrophic cardiomyopathy (HCM) | 3.55 × 10−4 |
| WJ | Dilated cardiomyopathy (DCM) | 5.92 × 10−4 |
| WJ | Staphylococcus aureus infection | 1.38 × 10−3 |
| WJ | PI3K-Akt signaling pathway | 1.39 × 10−3 |
| AD | Parkinson’s disease | 5.63 × 10−9 |
| AD | Protein digestion and absorption | 3.02 × 10−8 |
| AD | Cardiac muscle contraction | 3.87 × 10−8 |
| AD | Alzheimer’s disease | 3.95 × 10−8 |
| AD | Oxidative phosphorylation | 2.76 × 10−6 |
| AD | Huntington’s disease | 2.24 × 10−5 |
| AD | Metabolic pathways | 6.67 × 10−5 |
| AD | ECM–receptor interaction | 1.19 × 10−4 |
| AD | Carbon metabolism | 2.16 × 10−4 |
| AD | Thermogenesis | 2.57 × 10−4 |
| Consensus | Ribosome | 1.56 × 10−30 |
| Consensus | Proteasome | 5.58 × 10−13 |
| Consensus | Bacterial invasion of epithelial cells | 1.66 × 10−9 |
| Consensus | Carbon metabolism | 9.65 × 10−9 |
| Consensus | Protein processing in endoplasmic reticulum | 2.41 × 10−8 |
| Consensus | Endocytosis | 5.19 × 10−8 |
| Consensus | Pathogenic Escherichia coli infection | 5.63 × 10−8 |
| Consensus | Phagosome | 1.58 × 10−7 |
| Consensus | Biosynthesis of amino acids | 1.63 × 10−7 |
| Consensus | Regulation of actin cytoskeleton | 2.09 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-R.; Huang, H.-C.; Chen, I.-L.; Li, S.-C. Exosomes Secreted by Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote the Ability of Cell Proliferation and Migration for Keratinocyte. Int. J. Mol. Sci. 2024, 25, 4758. https://doi.org/10.3390/ijms25094758
Yu H-R, Huang H-C, Chen I-L, Li S-C. Exosomes Secreted by Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote the Ability of Cell Proliferation and Migration for Keratinocyte. International Journal of Molecular Sciences. 2024; 25(9):4758. https://doi.org/10.3390/ijms25094758
Chicago/Turabian StyleYu, Hong-Ren, Hsin-Chun Huang, I-Lun Chen, and Sung-Chou Li. 2024. "Exosomes Secreted by Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote the Ability of Cell Proliferation and Migration for Keratinocyte" International Journal of Molecular Sciences 25, no. 9: 4758. https://doi.org/10.3390/ijms25094758






