Oral Microbially-Induced Small Extracellular Vesicles Cross the Blood–Brain Barrier
Abstract
:1. Introduction
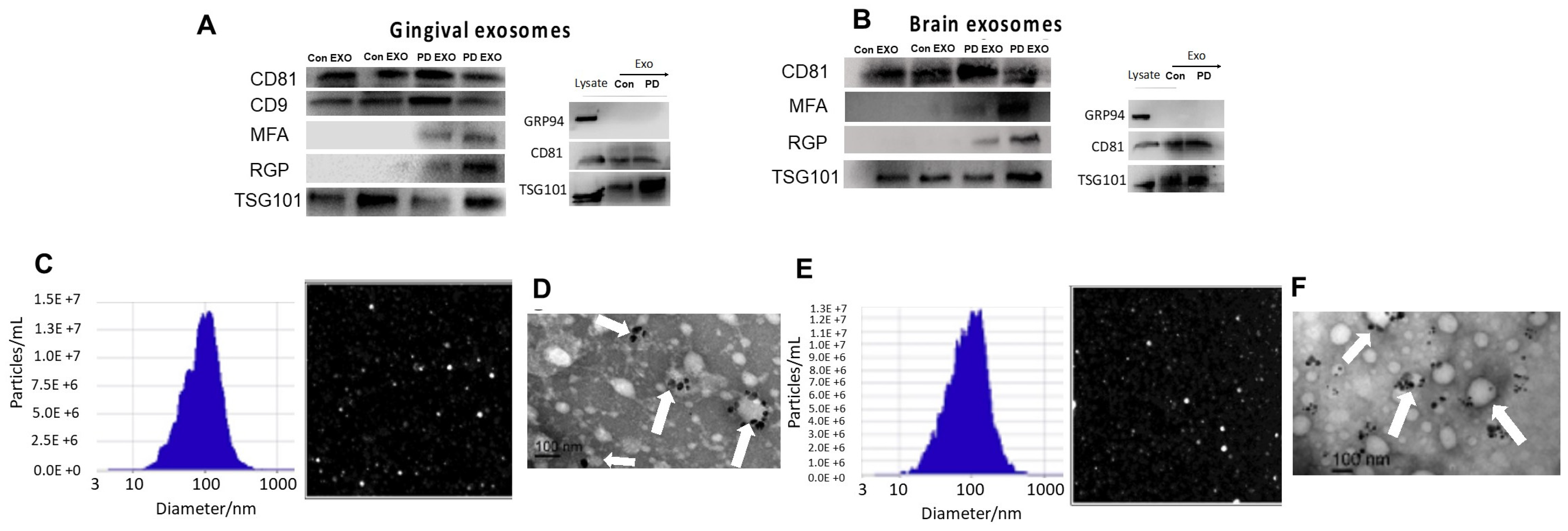
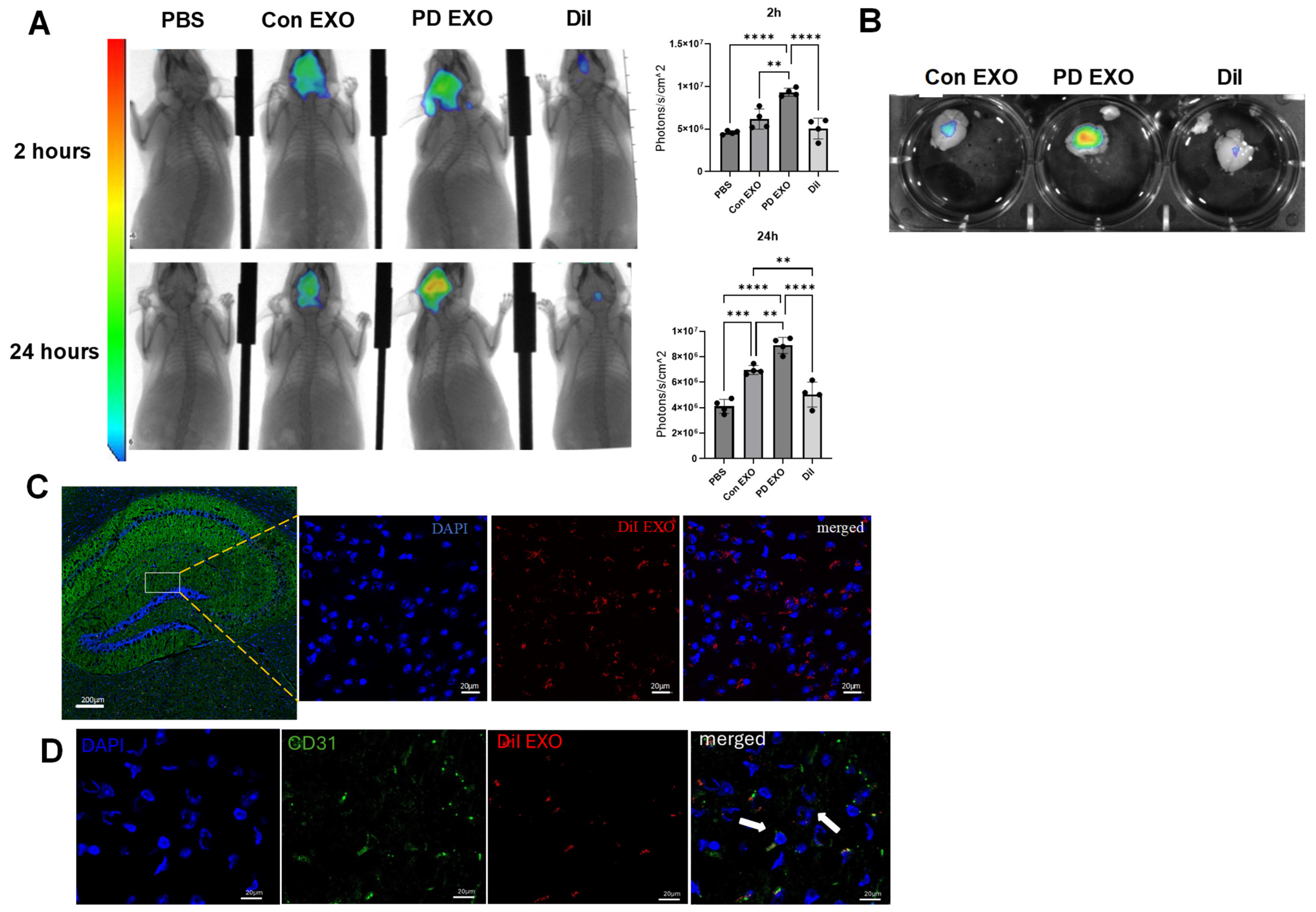
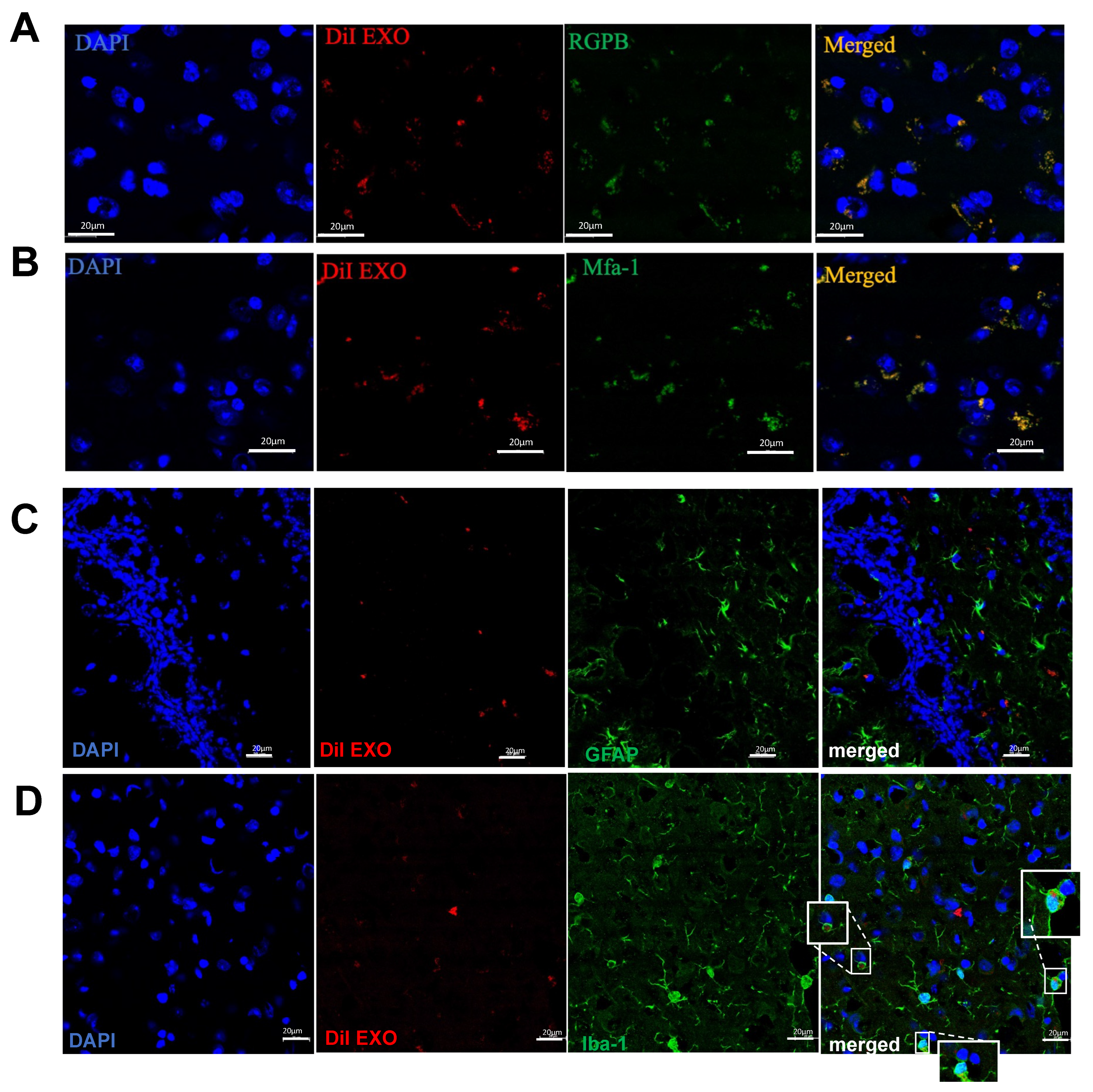
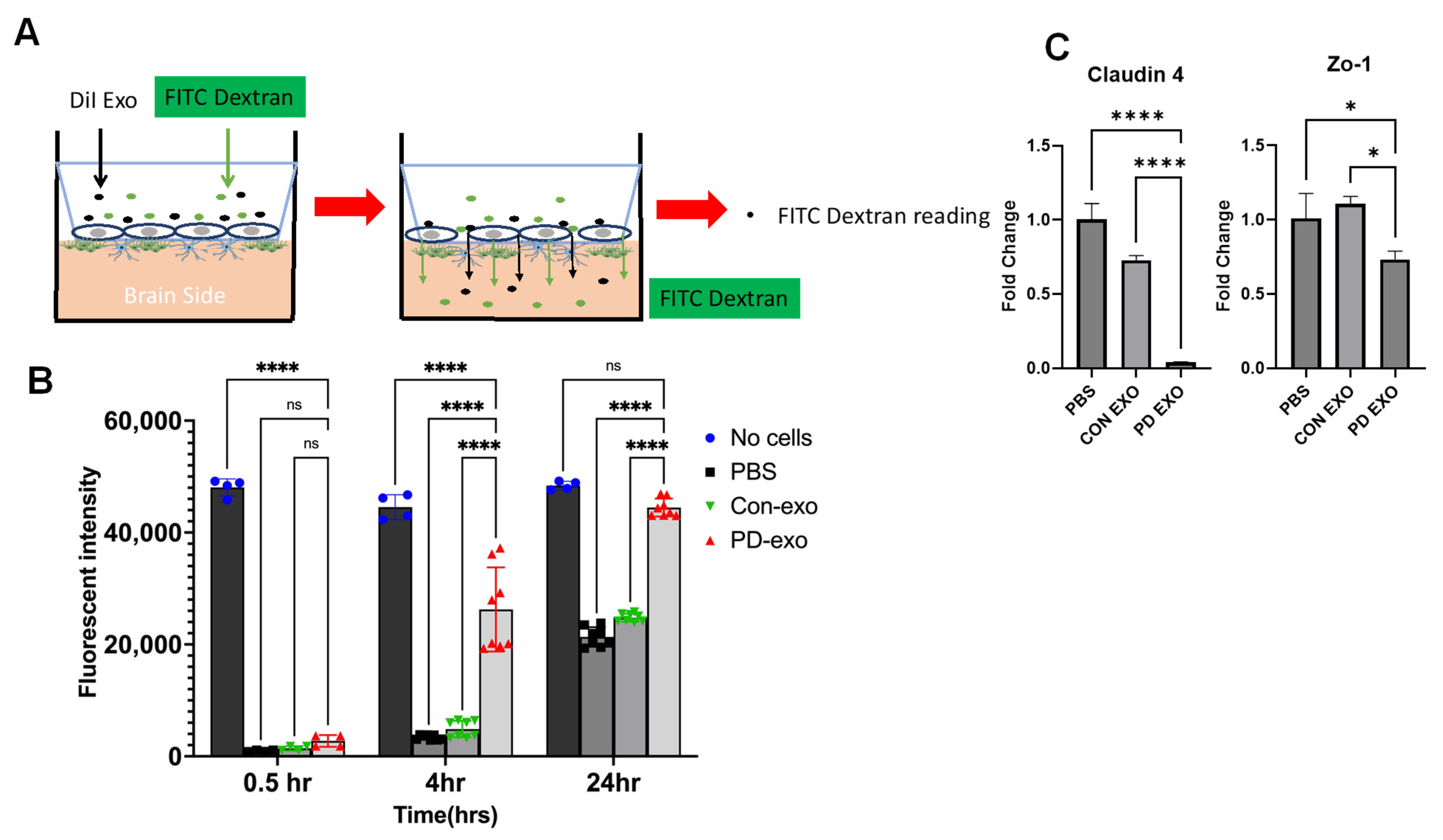
2. Results
3. Discussion
4. Methods and Materials
4.1. Experimental PD in Mice Model
4.2. Periodontitis Patients and Healthy Volunteers
4.3. Brain Exosome Isolation
4.4. Gingival Exosome Isolation
4.5. Nanoparticle Tracking Analysis of Exosomes
4.6. Electron Microscopy
4.7. Western Blotting and Antibodies
4.8. Biodistribution of Gingival EXO after Intragingival Injection
4.9. Gingival EXO Colocalization with Pg Mfa-1, Pg Gingipains, Microglial Cells, Astrocytes, and Endothelial Cells in Brain In Vivo
4.10. Human In Vitro Blood–Brain Barrier Model Culture and PD EXO Treatment
4.11. FITC-Dextran Permeability Assay in Blood Brain Barrier Model
Real-Time PCR
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, C.A.; Hardy, J.; Schott, J.M. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.; Dye, B.; Genco, R. Advances in Surveillance of Periodontitis: The Centers for Disease Control and Prevention Periodontal Disease Surveillance Project. J. Periodontol. 2012, 83, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Kadowaki, T.; Nakanishi, H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem. Int. 2022, 154, 105282. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Fang, J.; Ma, W.; Guo, J.; Shan, Z.; Ma, D.; Hu, Q.; Wen, L.; Wang, Z. Porphyromonas gingivalis Gingipains Destroy the Vascular Barrier and Reduce CD99 and CD99L2 Expression To Regulate Transendothelial Migration. Microbiol. Spectr. 2023, 11, e0476922. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Stähli, A.; Zhu, X.; Alberi, L.A.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease—A causative relationship? Periodontology 2000 2022, 89, 59–82. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Nara, P.L.; Sindelar, D.; Penn, M.S.; Potempa, J.; Griffin, W.S.T. Porphyromonas gingivalis Outer Membrane Vesicles as the Major Driver of and Explanation for Neuropathogenesis, the Cholinergic Hypothesis, Iron Dyshomeostasis, and Salivary Lactoferrin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, 1417–1450. [Google Scholar] [CrossRef]
- Ryder, M.I.; Xenoudi, P. Alzheimer disease and the periodontal patient: New insights, connections, and therapies. Periodontology 2000 2021, 87, 32–42. [Google Scholar] [CrossRef]
- Gong, T.; Chen, Q.; Mao, H.; Zhang, Y.; Ren, H.; Xu, M.; Chen, H.; Yang, D. Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front. Cell. Infect. Microbiol. 2022, 12, 925435. [Google Scholar] [CrossRef]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef]
- Noble, J.M.; Borrell, L.N.; Papapanou, P.N.; Elkind, M.S.V.; Scarmeas, N.; Wright, C.B. Periodontitis is associated with cognitive impairment among older adults: Analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhu, Z.; Plassman, B.L.; Wu, B. Dose-Response Meta-Analysis on Tooth Loss With the Risk of Cognitive Impairment and Dementia. J. Am. Med. Dir. Assoc. 2021, 22, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Nadim, R.; Tang, J.; Dilmohamed, A.; Yuan, S.; Wu, C.; Bakre, A.T.; Partridge, M.; Ni, J.; Copeland, J.R.; Anstey, K.J.; et al. Influence of periodontal disease on risk of dementia: A systematic literature review and a meta-analysis. Eur. J. Epidemiol. 2020, 35, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Palacio, L.; Goyer, M.; Maggiorani, D.; Espinosa, A.; Villeneuve, N.; Bourbonnais, S.; Moquin-Beaudry, G.; Le, O.; Demaria, M.; Davalos, A.R.; et al. Restored immune cell functions upon clearance of senescence in the irradiated splenic environment. Aging Cell 2019, 18, e12971. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.; Elashiry, M.; Liu, Y.; El-Awady, A.; Hamrick, M.; Cutler, C.W. Porphyromonas gingivalis Provokes Exosome Secretion and Paracrine Immune Senescence in Bystander Dendritic Cells. Front. Cell. Infect. Microbiol. 2021, 11, 669989. [Google Scholar] [CrossRef] [PubMed]
- Ridder, K.; Keller, S.; Dams, M.; Rupp, A.-K.; Schlaudraff, J.; Del Turco, D.; Starmann, J.; Macas, J.; Karpova, D.; Devraj, K.; et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLOS Biol. 2014, 12, e1001874. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.; Elashiry, M.; Liu, Y.; Morandini, A.C.; El-Awady, A.; Elashiry, M.M.; Hamrick, M.; Cutler, C.W. Microbially-Induced Exosomes from Dendritic Cells Promote Paracrine Immune Senescence: Novel Mechanism of Bone Degenerative Disease in Mice. Aging Dis. 2023, 14, 136–151. [Google Scholar] [CrossRef]
- Elashiry, M.; Elashiry, M.M.; Elsayed, R.; Rajendran, M.; Auersvald, C.; Zeitoun, R.; Rashid, M.H.; Ara, R.; Meghil, M.M.; Liu, Y.; et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J. Extracell. Vesicles 2020, 9, 1795362. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Moir, R.D.; Lathe, R.; Tanzi, R.E. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Petric, M.; Kozak, M.; Rewcastle, N.; McLachlan, D.C. Herpes-simplex viral genome and senile and presenile dementias of alzheimer and pick. Lancet 1980, 315, 1038. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; De Strooper, B.; Arancibia-Cárcamo, I.L. Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci. 2021, 44, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Evans, S.A.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.M.; Patel, A.D.; Pirtskhalava, T.; Inman, C.L.; et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 2021, 20, e13296. [Google Scholar] [CrossRef] [PubMed]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- An, J.Y.; Quarles, E.K.; Mekvanich, S.; Kang, A.; Liu, A.; Santos, D.; Miller, R.A.; Rabinovitch, P.S.; Cox, T.C.; Kaeberlein, M. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience 2017, 39, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.-C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta 2015, 1852, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef] [PubMed]
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 2012, 189, 3178–3187. [Google Scholar] [CrossRef]
- Zeituni, A.E.; McCaig, W.; Scisci, E.; Thanassi, D.G.; Cutler, C.W. The Native 67-Kilodalton minor fimbria of Porphyromonas gingivalis is a novel glycoprotein with DC-SIGN-targeting motifs. J. Bacteriol. 2010, 192, 4103–4110. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elashiry, M.; Carroll, A.; Yuan, J.; Liu, Y.; Hamrick, M.; Cutler, C.W.; Wang, Q.; Elsayed, R. Oral Microbially-Induced Small Extracellular Vesicles Cross the Blood–Brain Barrier. Int. J. Mol. Sci. 2024, 25, 4509. https://doi.org/10.3390/ijms25084509
Elashiry M, Carroll A, Yuan J, Liu Y, Hamrick M, Cutler CW, Wang Q, Elsayed R. Oral Microbially-Induced Small Extracellular Vesicles Cross the Blood–Brain Barrier. International Journal of Molecular Sciences. 2024; 25(8):4509. https://doi.org/10.3390/ijms25084509
Chicago/Turabian StyleElashiry, Mahmoud, Angelica Carroll, Jessie Yuan, Yutao Liu, Mark Hamrick, Christopher W. Cutler, Qin Wang, and Ranya Elsayed. 2024. "Oral Microbially-Induced Small Extracellular Vesicles Cross the Blood–Brain Barrier" International Journal of Molecular Sciences 25, no. 8: 4509. https://doi.org/10.3390/ijms25084509




