The Long-Acting Serine Protease Inhibitor mPEG-SPA-MDSPI16 Alleviates LPS-Induced Acute Lung Injury
Abstract
:1. Introduction
2. Results
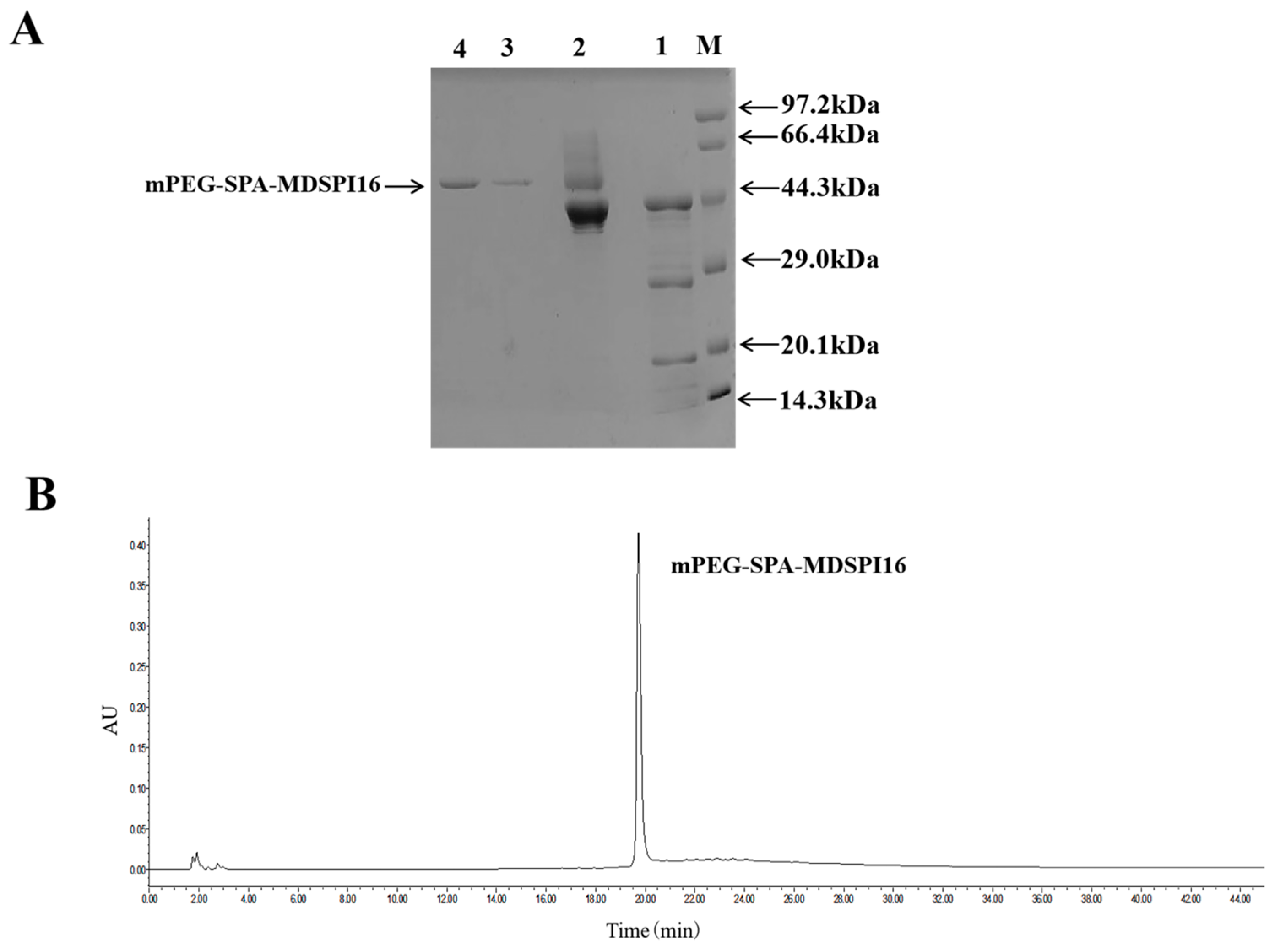
2.1. Preparation and Purification of mPEG-SPA-MDSPI16
2.2. Temperature and pH Stabilities of mPEG-SPA-MDSPI16
2.3. IC50 Analysis
2.4. Safety Analysis
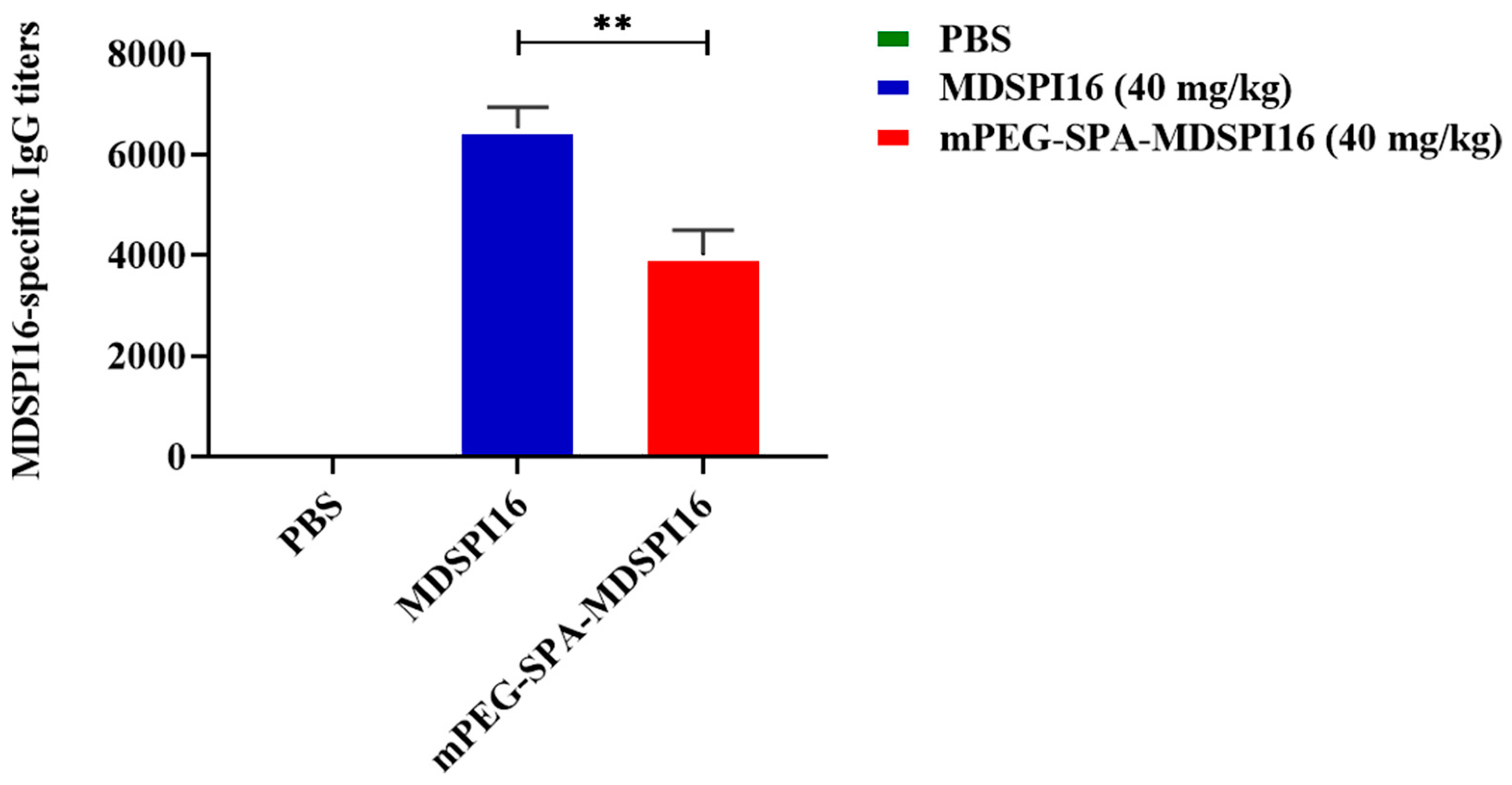
2.5. Immunogenic Analysis
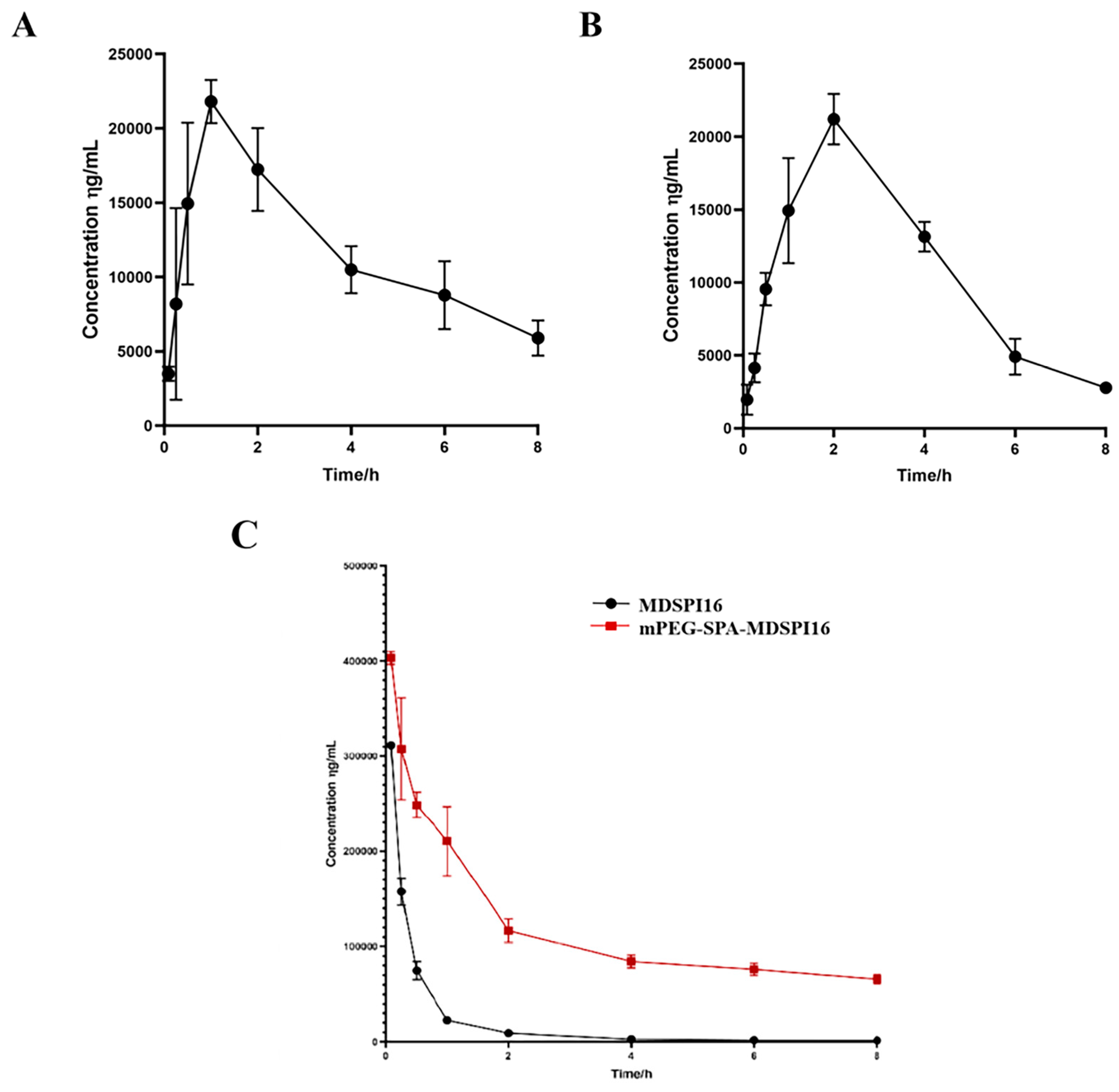
2.6. Pharmacokinetic Analysis
2.7. mPEG-SPA-MDSPI16 Ameliorated LPS-Induced Lung Inflammation and Physiological Changes in Mice
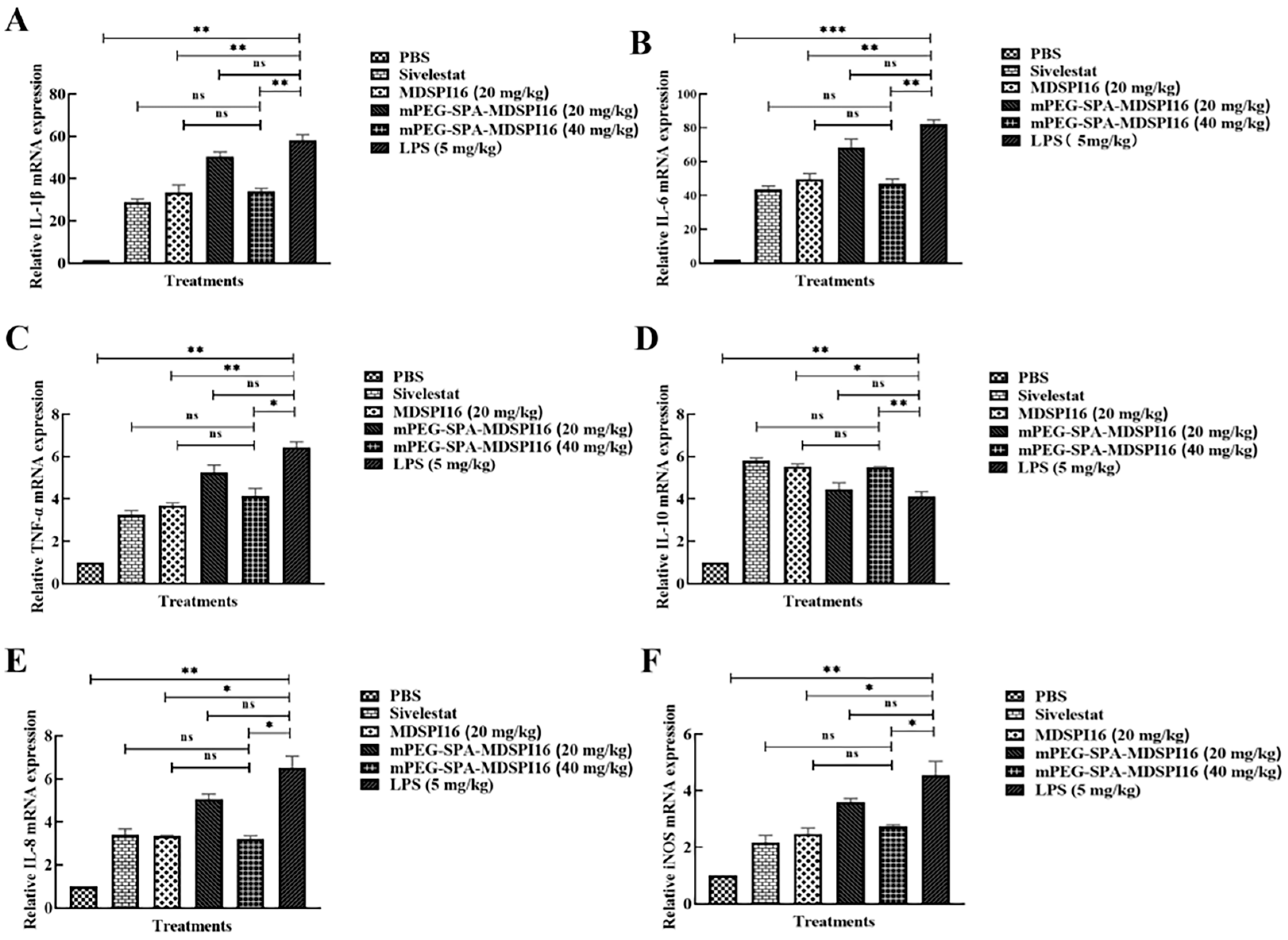
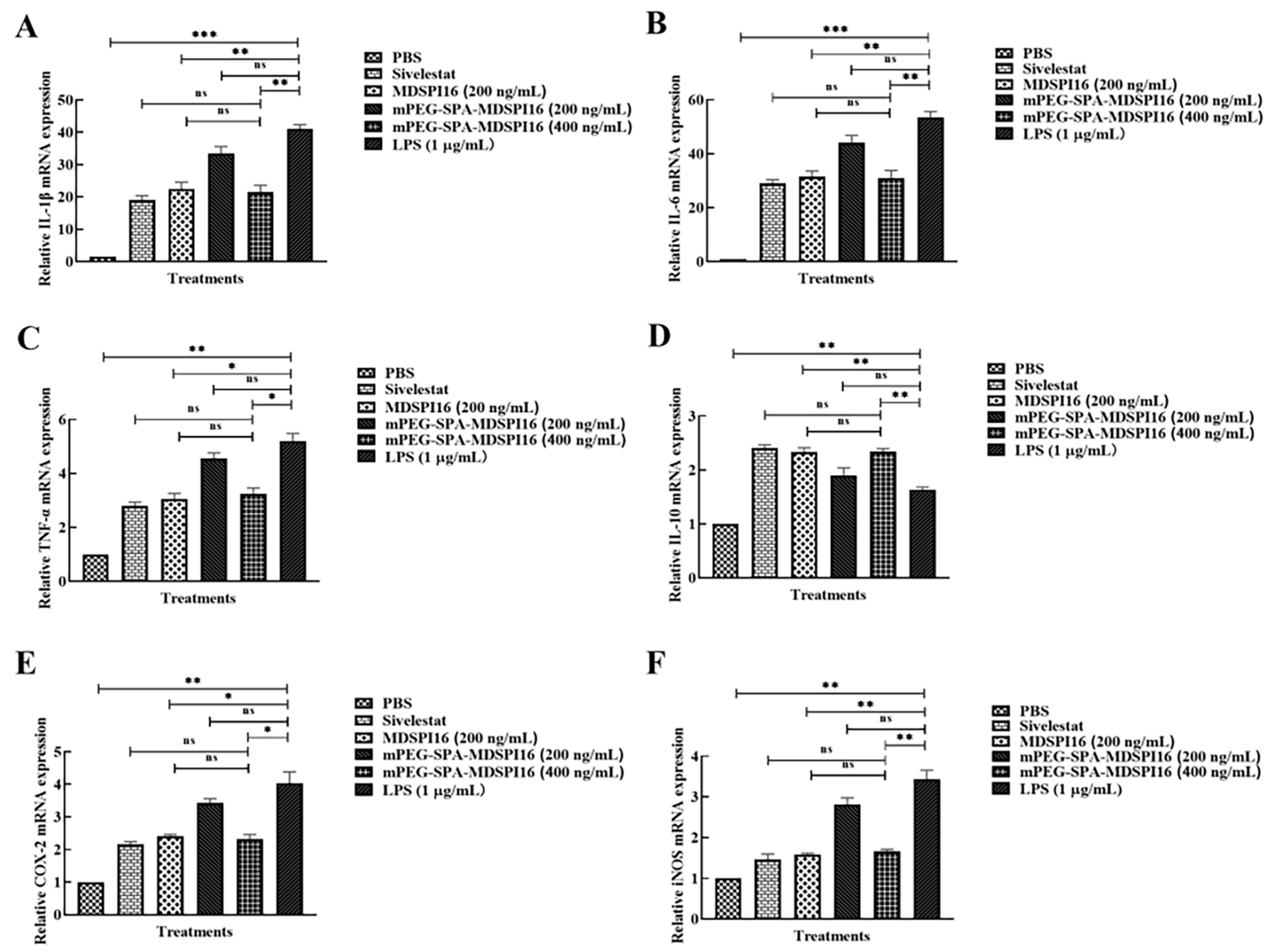
2.8. mPEG-SPA-MDSPI16 Attenuated LPS-Induced Inflammatory Cytokine Levels in Lung Tissue and BALF Samples
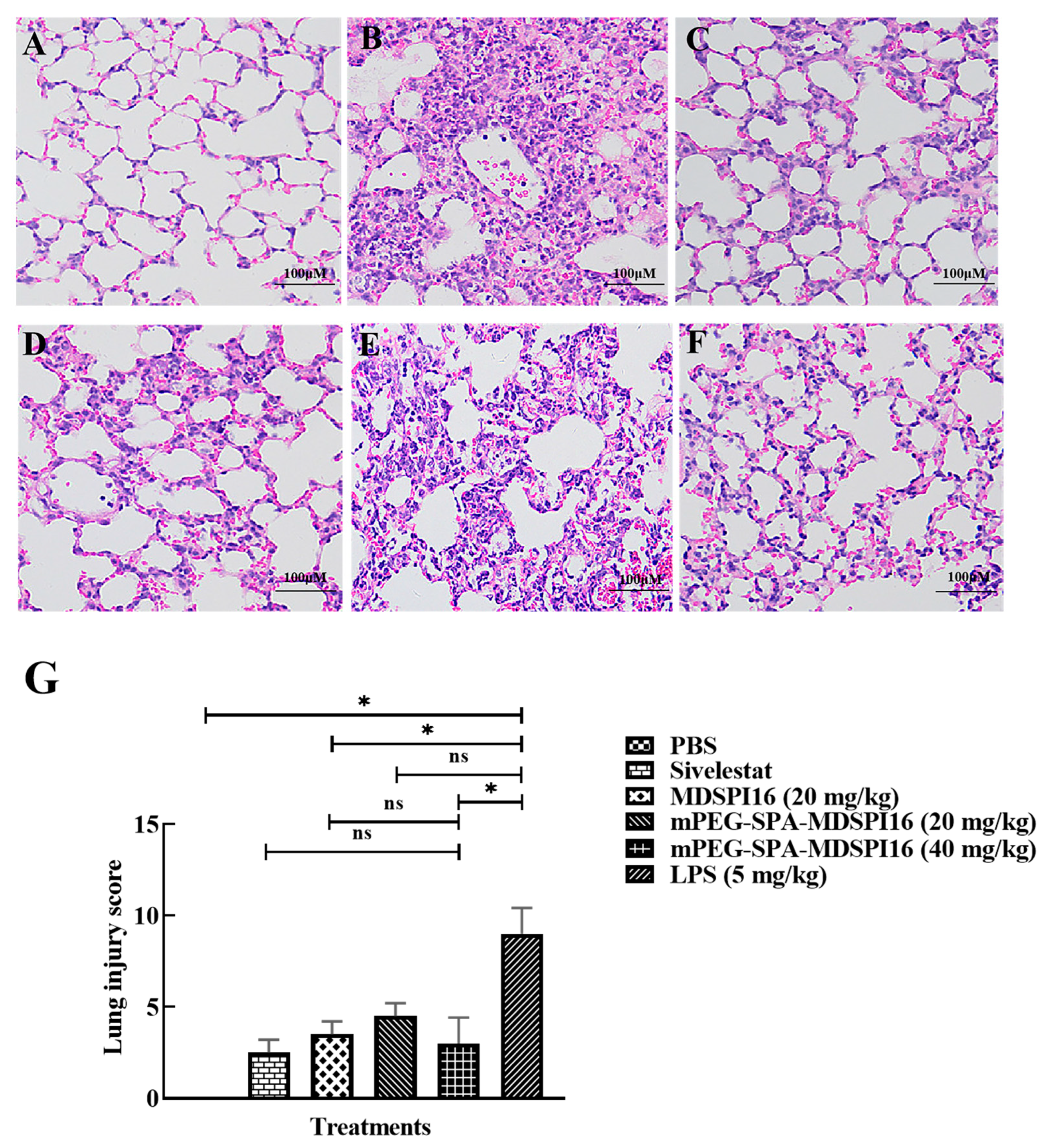
2.9. mPEG-SPA-MDSPI16 Ameliorated LPS-Induced Pathological Changes in Lung Tissues
2.10. mPEG-SPA-MDSPI16 Reversed the LPS-Induced Inflammatory Response in Mouse Bone Marrow Neutrophils
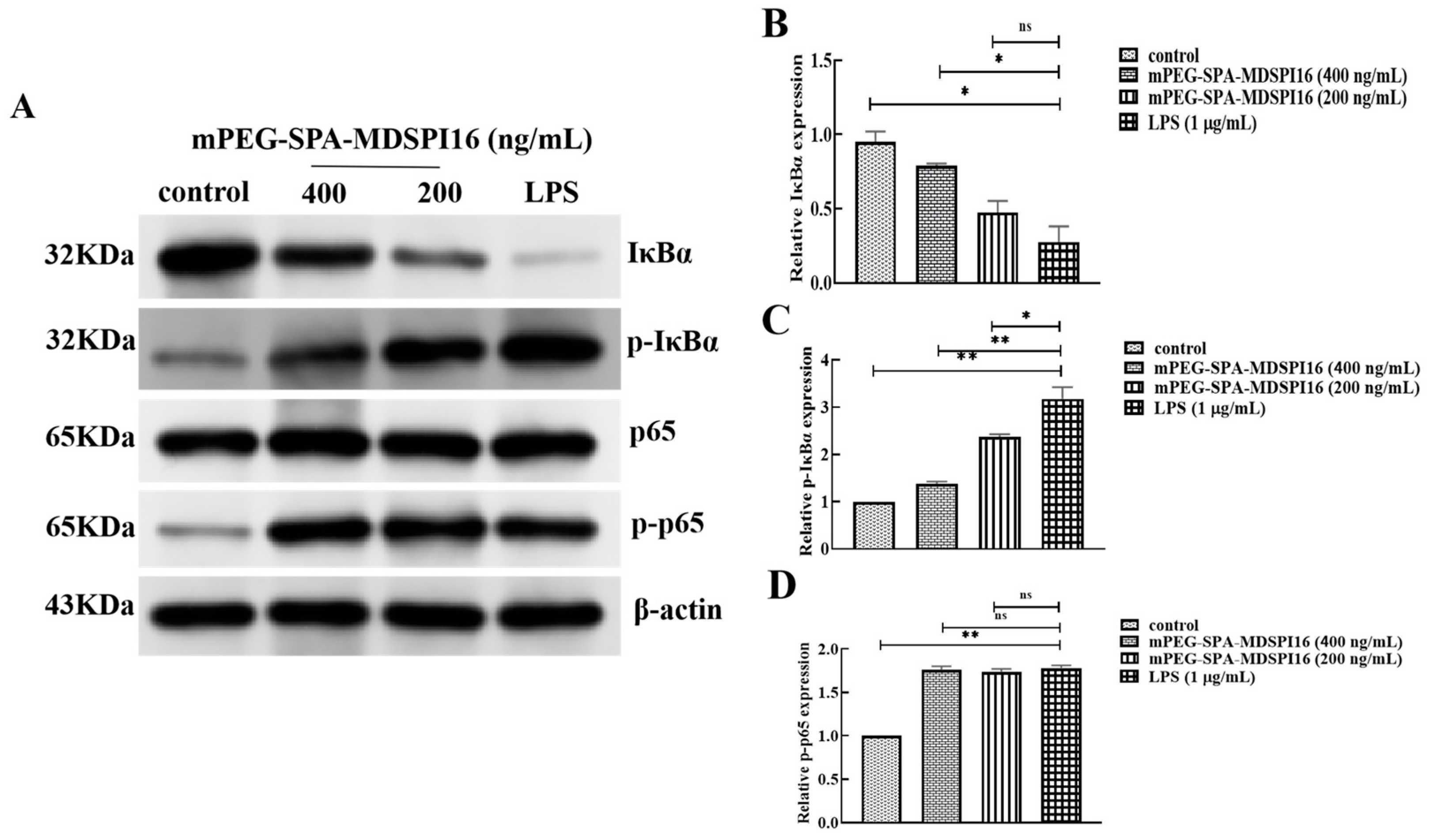
2.11. Effect of mPEG-SPA-MDSPI16 on the NF-κB Signaling Pathway
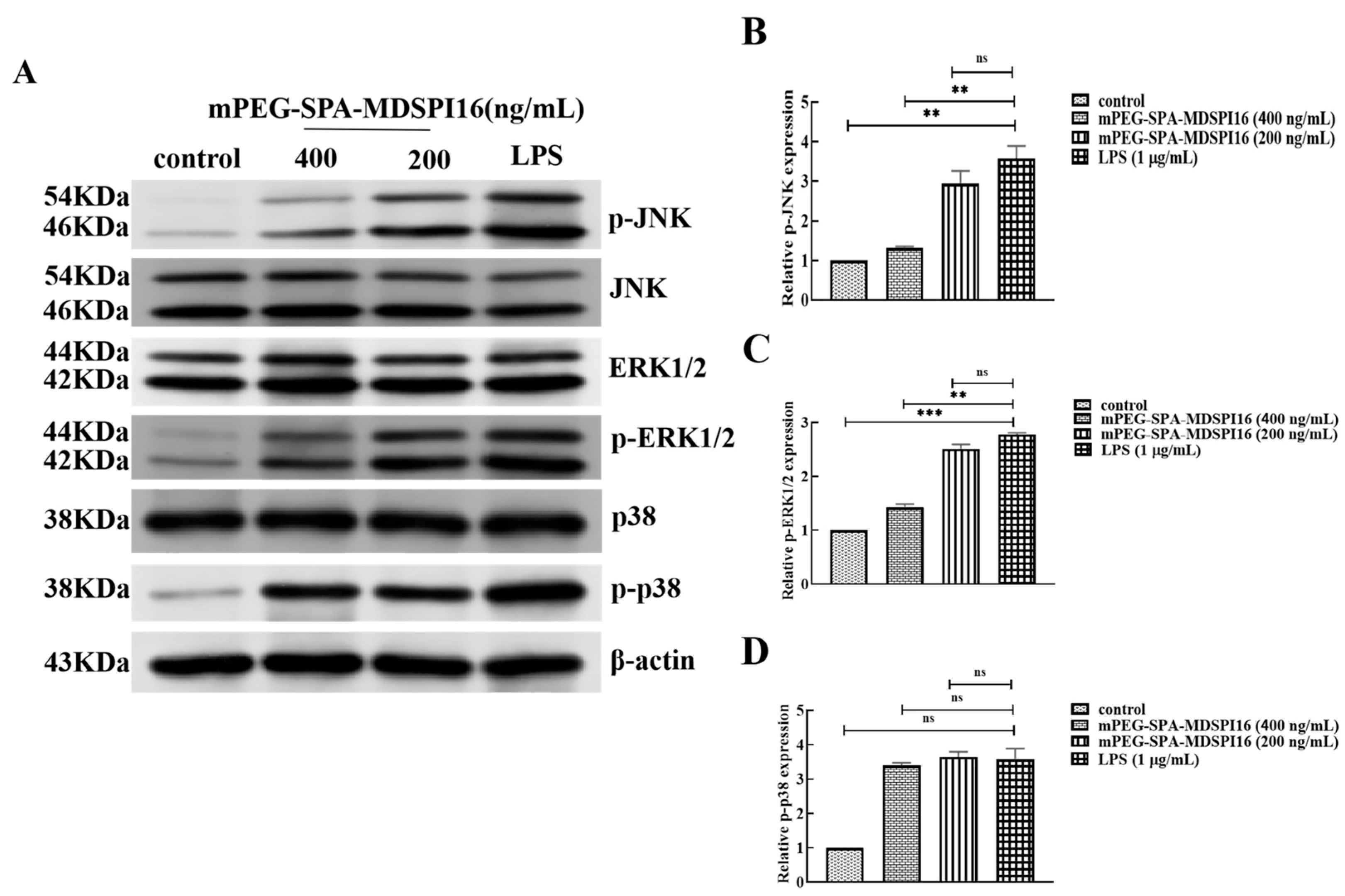
2.12. Effect of mPEG-SPA-MDSPI16 on the MAPK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Modification and Purification of mPEG-SPA-MDSPI16
4.4. Temperature and pH Stability
4.5. Determination of the IC50 of mPEG-SPA-MDSPI16
4.6. Safety Assay
4.6.1. In Vitro Safety Assay
4.6.2. In Vivo Safety Assay
4.7. Immunogenicity Assay
4.8. A Pharmacokinetic Study in Wistar Rats
4.9. Induction of ALI in Mice by Intratracheal Instillation of LPS
4.9.1. Mouse Model of ALI and Treatment
4.9.2. Lung Wet–Dry Weight (W/D) Ratio
4.9.3. RNA Isolation and Inflammatory Factor Analysis in Lung Tissue
4.9.4. Histopathology
4.9.5. Inflammatory Factor Analysis and Neutrophil Elastase Activity Analysis in Mouse BALF
4.10. LPS-Induced Cell Injury
4.10.1. Cell Culture and Treatments
4.10.2. Effect on the NF-κB and MAPK Signaling Pathways in Neutrophils
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci. 2022, 136, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Barkas, G.I.; Kotsiou, O.S. The Role of Osteopontin in Respiratory Health and Disease. J. Pers. Med. 2023, 13, 1259. [Google Scholar] [CrossRef]
- Domon, H.; Terao, Y. The Role of Neutrophils and Neutrophil Elastase in Pneumococcal Pneumonia. Front. Cell. Infect. Microbiol. 2021, 11, 615959. [Google Scholar] [CrossRef] [PubMed]
- El, A.C. Serine Protease Inhibitors to Treat Lung Inflammatory Diseases. Adv. Exp. Med. Biol. 2021, 1304, 215–226. [Google Scholar]
- Vivien, D.; Buisson, A. Serine protease inhibitors: Novel therapeutic targets for stroke? J. Cereb. Blood Flow Metab. 2000, 20, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Rogliani, P.; Ora, J.; Calzetta, L.; Cazzola, M. A comprehensive overview of investigational elastase inhibitors for the treatment of acute respiratory distress syndrome. Expert Opin. Investig. Drugs 2023, 32, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Tang, Y.; Wang, Y.L.; Cui, Q.; Inam, M.; Kong, L.C.; Ma, H.X. Serine protease inhibitor MDSPI16 ameliorates LPS-induced acute lung injury through its anti-inflammatory activity. Int. Immunopharmacol. 2020, 88, 107015. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bi, J.; Liang, B.; Wang, X.; Mo, R.; Feng, N.; Yan, F.; Wang, T.; Yang, S.; Zhao, Y.; et al. PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)(2). Int. J. Mol. Sci. 2023, 24, 3387. [Google Scholar] [CrossRef] [PubMed]
- Kurfurst, M.M. Detection and molecular weight determination of polyethylene glycol-modifiedhirudin by staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 1992, 200, 244–248. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, J.; Lee, K.C.; Na, D.H. Emerging PEGylated non-biologic drugs. Expert Opin. Emerg. Drugs. 2019, 24, 107–119. [Google Scholar] [CrossRef]
- Hu, T.; Manjula, B.N.; Li, D.; Brenowitz, M.; Acharya, S.A. Influence of intramolecular cross-links on the molecular, structural and functional properties of PEGylated haemoglobin. Biochem. J. 2007, 402, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, D.; Kang, A.; An, W.; Fan, B.; Ma, X.; Ma, G.; Su, Z.; Hu, T. PEG as a spacer arm markedly increases the immunogenicity of meningococcal group Y polysaccharide conjugate vaccine. J. Control. Release 2013, 172, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Javia, A.; Vanza, J.; Bardoliwala, D.; Ghosh, S.; Misra, L.A.; Patel, M.; Thakkar, H. Polymer-drug conjugates: Design principles, emerging synthetic strategies and clinical overview. Int. J. Pharm. 2022, 623, 121863. [Google Scholar] [CrossRef] [PubMed]
- Sahebnasagh, A.; Saghafi, F.; Safdari, M.; Khataminia, M.; Sadremomtaz, A.; Talaei, Z.; Ghaleno, H.R.; Bagheri, M.; Habtemariam, S.; Avan, R. Neutrophil elastase inhibitor (Sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J. Clin. Pharm. Ther. 2020, 45, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, S.; Li, B.; Yan, Q.; Zhao, Y.; Wang, Z.; Xiong, C.; Wei, J. Improvement of stability and in vivo antioxidant effect of human glutathione peroxidase mutant by PEGylation. Int. J. Pharm. 2021, 609, 121152. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, X.; Li, G.; Zeng, J.; Li, J.; Liu, J. SS-mPEG chemical modification of recombinant phospholipase C for enhanced thermal stability and catalytic efficiency. Int. J. Biol. Macromol. 2018, 111, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Gefen, T.; Vaya, J.; Khatib, S.; Harkevich, N.; Artoul, F.; Heller, E.D.; Pitcovski, J.; Aizenshtein, E. The impact of PEGylation on protein immunogenicity. Int. Immunopharmacol. 2013, 15, 254–259. [Google Scholar] [CrossRef]
- Seo, H.; Bae, H.-D.; Pyun, H.; Kim, B.-G.; Lee, S.-I.; Song, J.-S.; Lee, K. PEGylation improves the therapeutic potential of dimerized translationally controlled tumor protein blocking peptide in ovalbumin-induced mouse model of airway inflammation. Drug Deliv. 2022, 29, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Guo, W.; Li, Z.; Xie, X.; Qin, T.; Wu, Y.; Li, Z.; Chai, J.; Yi, F.; Tan, T.; Zhu, H.; et al. Urinary Trypsin Inhibitor Attenuates Acute Lung Injury by Improving Endothelial Progenitor Cells Functions. Cell. Physiol. Biochem. 2015, 36, 1059–1068. [Google Scholar] [CrossRef]
- Ju, M.; He, H.; Chen, S.; Liu, Y.; Liu, Y.; Pan, S.; Zheng, Y.; Xuan, L.; Zhu, D.; Luo, Z. Ulinastatin ameliorates LPS induced pulmonary inflammation and injury by blocking the MAPK/NF kappaB signaling pathways in rats. Mol. Med. Rep. 2019, 20, 3347–3354. [Google Scholar] [PubMed]
- Yuan, Q.; Jiang, Y.; Fang, Q. Improving effect of Sivelestat on lipopolysaccharide-induced lung injury in rats. APMIS 2014, 122, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeo, C.D.; Lee, H.Y.; Rhee, C.K.; Kim, I.K.; Lee, D.G.; Lee, S.H.; Kim, J.W. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J. Anesth. 2017, 31, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Y.; Shen, Y.; Huang, J.; Li, Y.; Wu, R.; Zhao, J. Minocycline and antipsychotics inhibit inflammatory responses in BV-2 microglia activated by LPS via regulating the MAPKs/JAK-STAT signaling pathway. BMC Psychiatry 2023, 23, 514. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Park, J.S.; Heo, J.D.; Kim, G.S. Scutellarein Inhibits LPS-Induced Inflammation through NF-kappaB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules 2022, 27, 3782. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.L.; Zheng, K.Y.; Wang, X.Y.; Li, M.W. Advances in the extracellular signal-regulated kinase signaling pathway in silkworms, Bombyx mori (Lepidoptera). Arch. Insect. Biochem. Physiol. 2023, 114, e22054. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Pei, Z.; Li, W.; Zhang, D.; Liu, L.; Kong, L.; Liu, S.; Jiang, X.; Ma, H. A serine protease inhibitor from Musca domestica larva exhibits inhibitory activity against elastase and chymotrypsin. Biotechnol. Lett. 2016, 38, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, K.S.; Kim, B.Y.; Choi, Y.S.; Yoon, H.J.; Jia, J.; Jin, B.R. Anti-fibrinolytic and anti-microbial activities of a serine protease inhibitor from honeybee (Apiscerana) venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 201, 11–18. [Google Scholar] [CrossRef]
- Dong, N.; Wang, C.; Zhang, T.; Zhang, L.; Xue, C.; Feng, X.; Bi, C.; Shan, A. Bioactivity and Bactericidal Mechanism of Histidine-Rich beta-Hairpin Peptide Against Gram-Negative Bacteria. Int. J. Mol. Sci. 2019, 20, 3954. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Junior, E.L.; Boldrini-França, J.; Takeda, A.A.S.; Costa, T.R.; Peigneur, S.; Cardoso, I.A.; de Oliveira, I.S.; Sampaio, S.V.; Fontes, M.R.d.M.; Tytgat, J.; et al. Towards toxin PEGylation: The example of rCollinein-1, a snake venom thrombin-like enzyme, as a PEGylated biopharmaceutical prototype. Int. J. Biol. Macromol. 2021, 190, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xu, Q.J.; Liu, L.; Guan, L.L.; Jiang, X.Y.; Inam, M.; Kong, L.C.; Ma, H.X. Preparation, Characterization and Pharmacokinetic Study of N-Terminal PEGylated D-Form Antimicrobial Peptide OM19r-8. J. Pharm. Sci. 2021, 110, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Tirunavalli, S.K.; Gourishetti, K.; Kotipalli, R.S.S.; Kuncha, M.; Kathirvel, M.; Kaur, R.; Jerald, M.K.; Sistla, R.; Andugulapati, S.B. Dehydrozingerone ameliorates Lipopolysaccharide induced acute respiratory distress syndrome by inhibiting cytokine storm, oxidative stress via modulating the MAPK/NF-κB pathway. Phytomedicine 2021, 92, 153729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Zhang, T.; Yang, Y.; Zhao, G.; Shaukat, A.; Wu, H.; Deng, G. Downregulation of TLR4 by miR-181a Provides Negative Feedback Regulation to Lipopolysaccharide-Induced Inflammation. Front. Pharmacol. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Huang, R.; Yan, L.; Li, D.-T.; Liu, Y.-Y.; Wei, X.-H.; Cui, Y.-C.; Pan, C.-S.; Fan, J.-Y.; Wang, X.; et al. Schisandrin Attenuates Lipopolysaccharide-Induced Lung Injury by Regulating TLR-4 and Akt/FoxO1 Signaling Pathways. Front. Physiol. 2018, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Barreto, T.R.; Costola-De-Souza, C.; Margatho, R.O.; Queiroz-Hazarbassanov, N.; Rodrigues, S.C.; Felício, L.F.; Palermo-Neto, J.; Zager, A. Repeated Domperidone treatment modulates pulmonary cytokines in LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2018, 56, 43–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z.; Jiang, A.M.; Wu, D.; Li, S.Q.; Liu, Z.Y.; Wei, Z.K.; Yang, Z.T.; Guo, C.M. Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways. Front. Pharmacol. 2021, 11, 591836. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, X.; Dai, N.; Liu, X.; Liu, S.; Zhang, H.; Kong, L.; Ma, H. The Long-Acting Serine Protease Inhibitor mPEG-SPA-MDSPI16 Alleviates LPS-Induced Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 4567. https://doi.org/10.3390/ijms25084567
Chen J, Zhou X, Dai N, Liu X, Liu S, Zhang H, Kong L, Ma H. The Long-Acting Serine Protease Inhibitor mPEG-SPA-MDSPI16 Alleviates LPS-Induced Acute Lung Injury. International Journal of Molecular Sciences. 2024; 25(8):4567. https://doi.org/10.3390/ijms25084567
Chicago/Turabian StyleChen, Jingrui, Xinjun Zhou, Nan Dai, Xiaoyu Liu, Shihan Liu, Haipeng Zhang, Lingcong Kong, and Hongxia Ma. 2024. "The Long-Acting Serine Protease Inhibitor mPEG-SPA-MDSPI16 Alleviates LPS-Induced Acute Lung Injury" International Journal of Molecular Sciences 25, no. 8: 4567. https://doi.org/10.3390/ijms25084567



