Transcription Factor McHB7 Improves Ice Plant Drought Tolerance through ABA Signaling Pathway
Abstract
:1. Introduction
2. Results
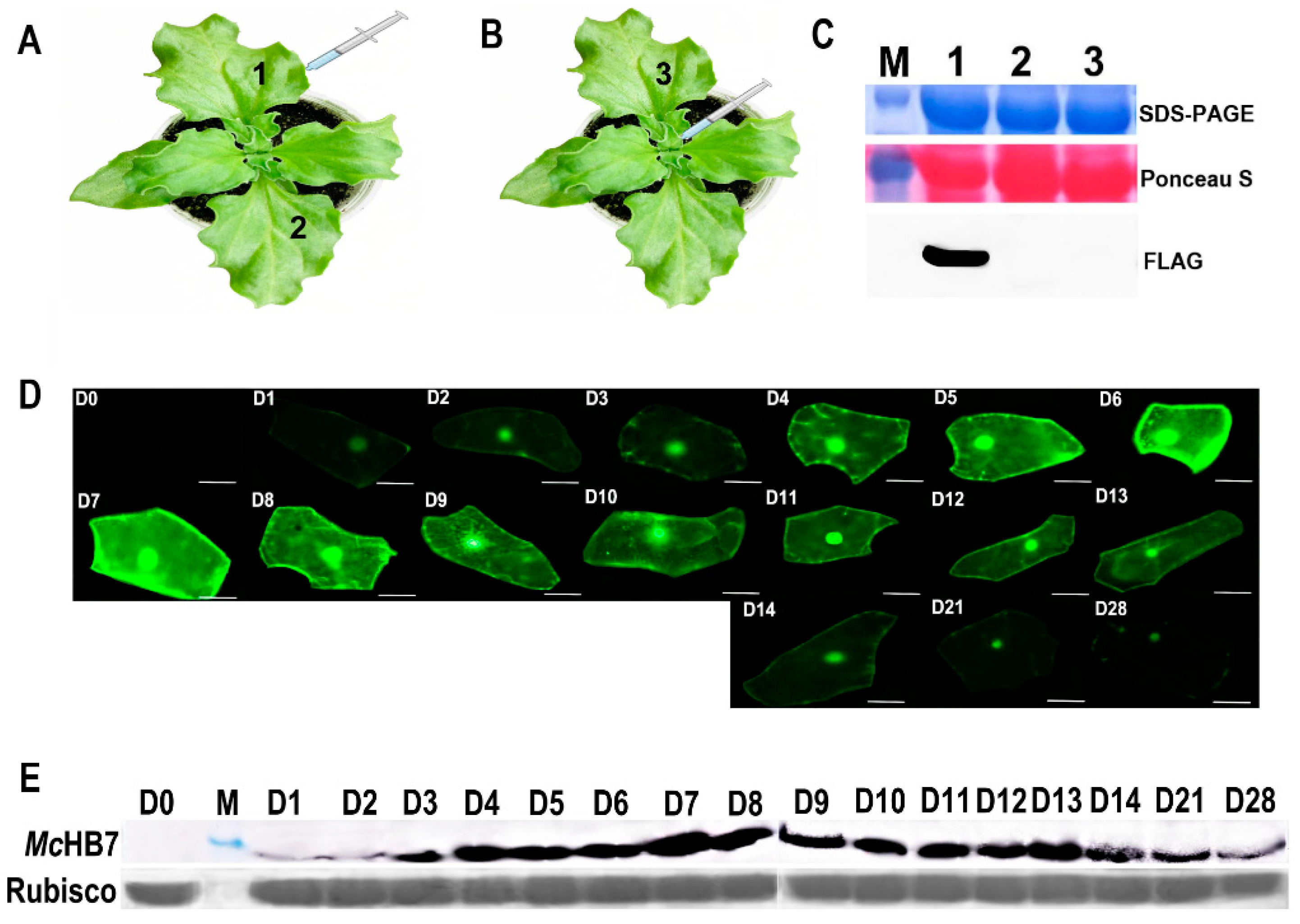
2.1. Transformation of Ice Plant Leaves with GFP and McHB7
2.2. McHB7 OE Ice Plants under Drought Stress
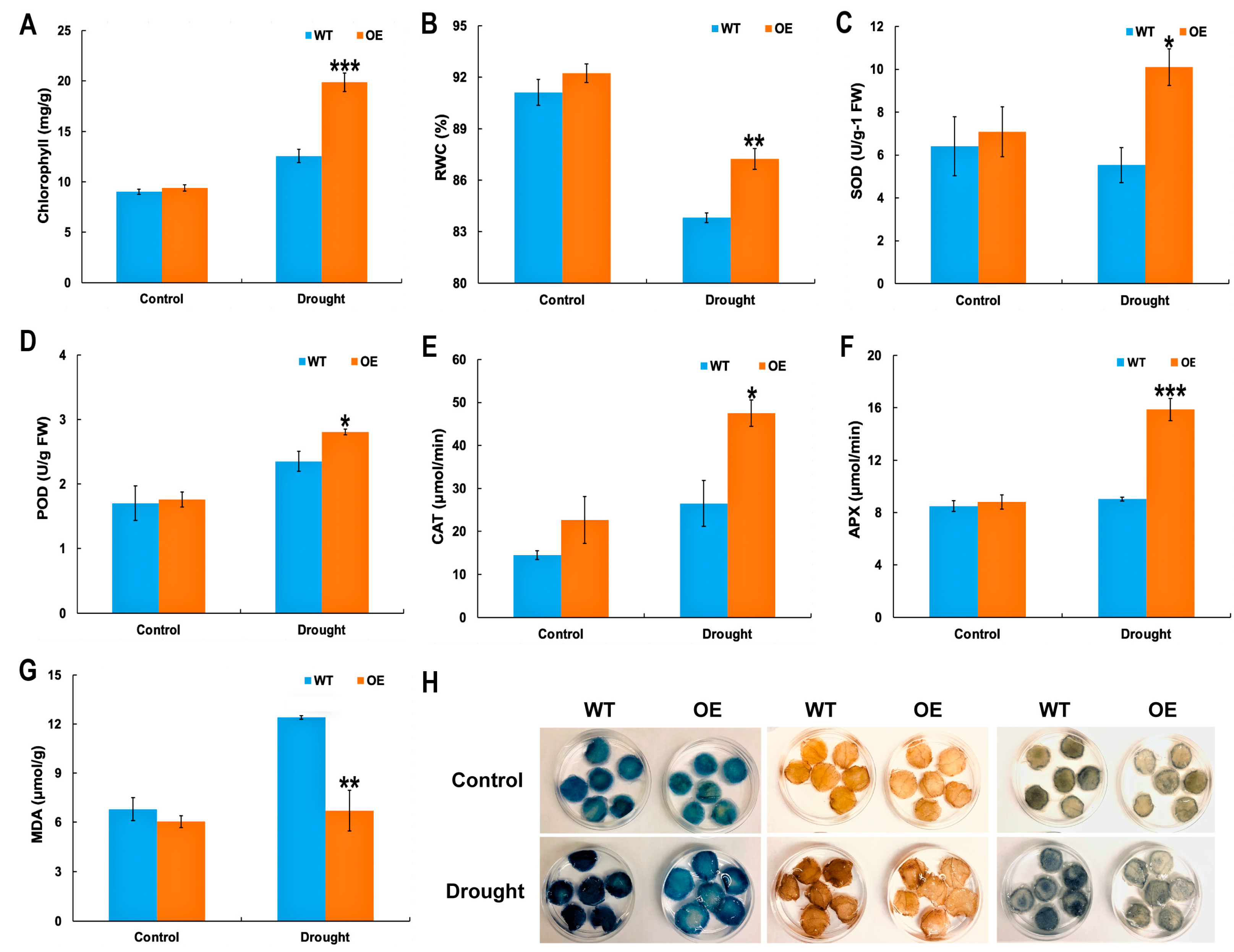
2.3. Physiological Indices of McHB7 OE Plants under Drought Stress
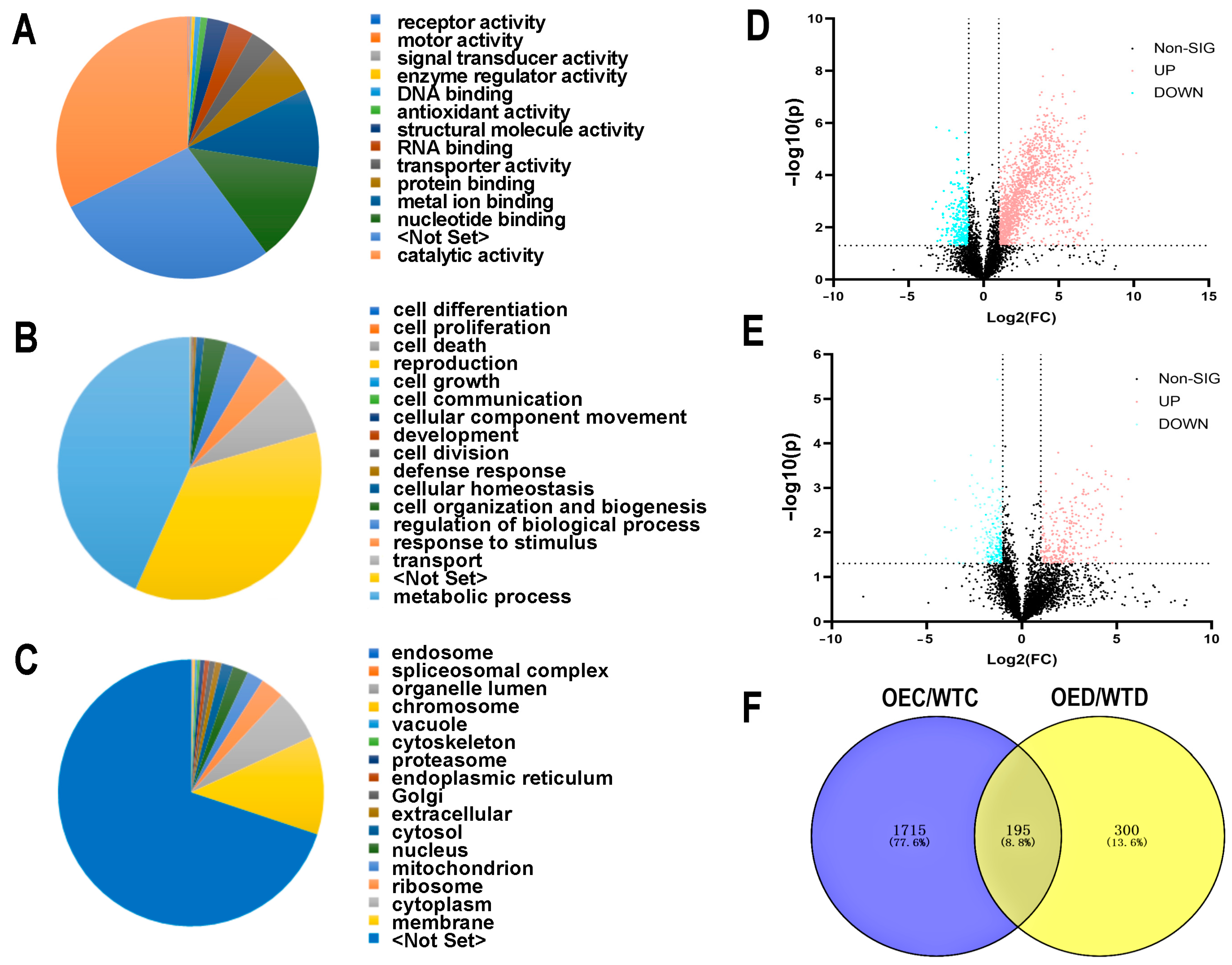
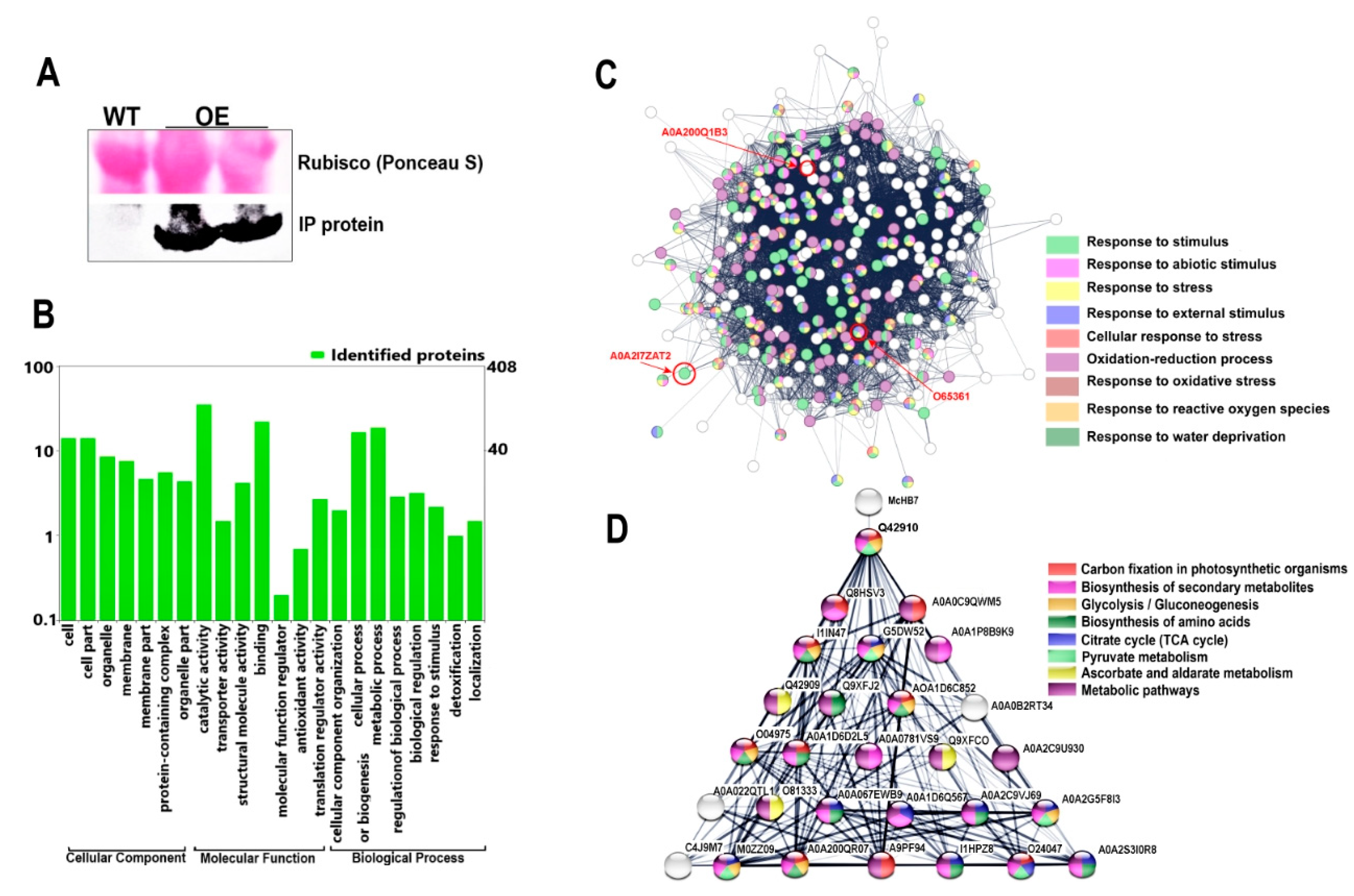
2.4. Protein Changes in WT and OE Plants after Drought Stress
2.5. McHB7 Protein Binds to Dehydration and ABA-Related Cis-Acting Elements and Has Self-Transactivation
2.6. Immunoprecipitation and Protein-Protein Interaction
2.7. Metabolomic Changes after Treatment with Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transformation of Ice Plant Leaves with pCAMBIA1300-GFP
4.3. Western Blot and RT-qPCR to Identify Transgenic Ice Plants
4.4. Drought Stress Treatment
4.5. Morphological and Physiological Measurements
4.6. Liquid Chromatography-Mass Spectrometry (LC-MS/MS) and Data Analysis
4.7. Yeast One-Hybrid Assay and In Vivo Transactivation Assay
4.8. Immunoprecipitation Coupled with LC-MS/MS
4.9. Metabolite Extraction and Metabolomic Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.L.; Jung, Y.; Kim, H.I.; Sung, N.Y.; Kim, M.J.; Han, I.J.; Kim, G.; Nho, E.Y.; Park, S.Y.; Han, Y.; et al. Antidiabetic effect of fermented Mesembryanthemum crystallinum L. in db/db mice involves regulation of PI3K-Akt pathway. Curr. Issues Mol. Biol. 2023, 45, 6415–6431. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Salt stress in Mesembryanthemum crystallinum L. cell suspensions activates adaptive mechanisms similar to those observed in the whole plant. Planta 1999, 207, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Dobri, R.-V.; Sfîcă, L.; Amihăesei, V.-A.; Apostol, L.; Țîmpu, S. Drought extent and severity on arable lands in Romania derived from normalized difference drought index. Remote Sens. 2021, 13, 1478. [Google Scholar] [CrossRef]
- Lata, C.; Yadav, A.; Prasad, M. Role of plant transcription factors in abiotic stress tolerance. In Abiotic Stress Response in Plants: Physiological, Biochemical and Genetic Perspectives; INTECH Open Access Publishers: Singapore, 2011; ISBN 978-953-307-672-0. [Google Scholar]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Kong, W.; Yoo, M.-J.; Zhu, D.; Noble, J.D.; Kelley, T.M.; Li, J.; Kirst, M.; Assmann, S.M.; Chen, S. Molecular changes in Mesembryanthemum crystallinum guard cells underlying the C3 to CAM transition. Plant Mol. Biol. 2020, 103, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Y.; Zhu, N.; Zhao, Y. The rice WUSCHEL-related homeobox genes are involved in reproductive organ development, hormone signaling and abiotic stress response. Gene 2014, 549, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Shchennikova, A.; Shulga, O.; Kochieva, E.; Skryabin, K.G. Homeobox genes encoding WOX transcription factors in the flowering parasitic plant Monotropa hypopitys. Vavilov J. Genet. Breed. 2017, 21, 234–240. [Google Scholar] [CrossRef]
- Li, R.; Ge, H.; Dai, Y.; Yuan, L.; Liu, X.; Sun, Q.; Wang, X. Genomewide analysis of homeobox gene family in apple (Malus domestica Borkh.) and their response to abiotic stress. J. Genet. 2019, 98, 13. [Google Scholar] [CrossRef]
- Zhao, P.; Cui, R.; Xu, P.; Wu, J.; Mao, J.L.; Chen, Y.; Zhou, C.Z.; Yu, L.H.; Xiang, C.B. AtHB17 enhances stress tolerance by coordinating photosynthesis associated nuclear gene and ATSIG5 expression in response to abiotic stress. Sci. Rep. 2017, 7, 45492. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Cheng, Z.; Zhu, D.; Jiang, T.; Chen, S. Overexpression of McHB7 transcription factor from Mesembryanthemum crystallinum improves plant salt tolerance. Int. J. Mol. Sci. 2022, 23, 7879. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Zhu, D.; Dufresne, D.; Jiang, T.; Chen, S. Proteomics of Homeobox7 enhanced salt tolerance in Mesembryanthemum Crystallinum. Int. J. Mol. Sci. 2021, 22, 6390. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Plant DNA repair and Agrobacterium T-DNA integration. Int. J. Mol. Sci. 2021, 22, 8458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, S.; Xi, X.; Cao, D.; Wang, Z.; Wang, R.; Liu, B. Comprehensive influences of overexpression of a MYB transcriptor regulating anthocyanin biosynthesis on transcriptome and metabolome of tobacco leaves. Int. J. Mol. Sci. 2019, 20, 5123. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.S.; Ge, X.L.; Wang, R.; Yang, H.F.; Bai, Y.E.; Guo, Y.H.; Zhang, J.; Lu, M.Z.; Zhao, S.T.; Wang, L.Q. An efficient Agrobacterium-mediated transformation method for hybrid poplar 84K (Populus alba × P. glandulosa) using calli as explants. Int. J. Mol. Sci. 2022, 23, 2216. [Google Scholar] [CrossRef]
- Dai, C.; Li, Y.; Li, L.; Du, Z.; Lin, S.; Tian, X.; Li, S.; Yang, B.; Yao, W.; Wang, J. An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol. Breed. 2020, 40, 96. [Google Scholar] [CrossRef]
- Agarie, S.; Umemoto, M.; Sunagawa, H.; Anai, T.; Cushman, J.C. An Agrobacterium-mediated transformation via organogenesis regeneration of a facultative CAM plant, the common ice plant Mesembryanthemum crystallinum L. Plant Prod. Sci. 2020, 23, 343–349. [Google Scholar] [CrossRef]
- Hwang, H.H.; Wang, C.H.; Chen, H.H.; Ho, J.F.; Chi, S.F.; Huang, F.C.; Yen, H.E. Effective Agrobacterium-mediated transformation protocols for callus and roots of halophyte ice plant (Mesembryanthemum crystallinum). Bot. Stud. 2019, 60, 1. [Google Scholar] [CrossRef]
- Sahoo, J.P.; Behera, L.; Sharma, S.S.; Praveena, J.; Nayak, S.K.; Samal, K.C. Omics studies and systems biology perspective towards abiotic stress response in plants. Am. J. Plant Sci. 2020, 11, 2172–2194. [Google Scholar] [CrossRef]
- Punia, A.; Kumari, M.; Chouhan, M.; Saini, V.; Joshi, R.; Kumar, A.; Kumar, R. Proteomic and metabolomic insights into seed germination of Ferula assa-foetida. J. Proteom. 2024, 9, 105176. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.E.; Overnäs, E.; Johansson, H.; Rada-Iglesias, A.; Engström, P. The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant Mol. Biol. 2012, 80, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B.; Estavillo, G.M.; Field, K.J.; Pornsiriwong, W.; Carroll, A.J.; Howell, K.A.; Woo, N.S.; Lake, J.A.; Smith, S.M.; Harvey Millar, A.; et al. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009, 58, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Ruiz de Galarreta, J.I. Genome-wide association study of agronomic and physiological traits related to drought tolerance in potato. Plants 2023, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.H.; Fang, Y.L.; Zhang, J.X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.C.; Grams, T.; Ratajczak, R. Effects of salinity, high irradiance, ozone, and ethylene on mode of photosynthesis, oxidative stress and oxidative damage in the C3/CAM intermediate plant Mesembryanthemum crystallinum L. Plant Cell Environ. 2004, 27, 187–197. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Suzuki, T.; Nishikawa, K.; Agarie, S.; Ishiguro, S.; Higashiyama, T. RNA-seq analysis of the response of the halophyte, Mesembryanthemum crystallinum (ice plant) to high salinity. PLoS ONE 2015, 10, e0118339. [Google Scholar] [CrossRef]
- Reyna-Llorens, I.; Aubry, S. As right as rain: Deciphering drought-related metabolic flexibility in the C4–CAM Portulaca. J. Exp. Bot. 2022, 73, 4615–4619. [Google Scholar] [CrossRef]
- Choi, J.H.; Jo, S.G.; Jung, S.K.; Park, W.T.; Kim, K.Y.; Park, Y.W.; Park, J.H. Immunomodulatory effects of ethanol extract of germinated ice plant (Mesembryanthemum crystallinum). Lab. Anim. Res. 2017, 33, 32–39. [Google Scholar] [CrossRef]
- Ma, L.; Lukasik, E.; Gawehns, F.; Takken, F.L. The use of agroinfiltration for transient expression of plant resistance and fungal effector proteins in Nicotiana benthamiana leaves. Methods Mol. Biol. 2012, 835, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Debler, J.W.; Henares, B.M.; Lee, R.C. Agroinfiltration for transient gene expression and characterisation of fungal pathogen effectors in cool-season grain legume hosts. Plant Cell Rep. 2021, 40, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Garber, R.L.; Kuroiwa, A.; Gehring, W.J. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. Embo J. 1983, 2, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Nantapong, N.; Murata, R.; Trakulnaleamsai, S.; Kataoka, N.; Yakushi, T.; Matsushita, K. The effect of reactive oxygen species (ROS) and ROS-scavenging enzymes, superoxide dismutase and catalase, on the thermotolerant ability of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2019, 103, 5355–5366. [Google Scholar] [CrossRef] [PubMed]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CA) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2017, 147, 279–294. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Zhu, L.; Zhang, N.; Si, H. StMAPKK5 positively regulates response to drought and salt stress in potato. Int. J. Mol. Sci. 2024, 25, 3662. [Google Scholar] [CrossRef]
- Song, M.; Fan, S.; Pang, C.; Wei, H.; Yu, S. Genetic analysis of the antioxidant enzymes, methane dicarboxylic aldehyde (MDA) and chlorophyll content in leaves of the short season cotton (Gossypium hirsutum L.). Euphytica 2014, 198, 153–162. [Google Scholar] [CrossRef]
- Libik-Konieczny, M.; Surowka, E.; Nosek, M.; Goraj, S.; Miszalski, Z. Pathogen-induced changes in malate content and NADP-dependent malic enzyme activity in C3 or CAM performing Mesembryanthemum crystallinum L. plants. Acta Physiol. Plant. 2012, 34, 1471–1477. [Google Scholar] [CrossRef]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Gupta, R.; Dogra, V.; Singh, R.; Abat, J.K.; Sarkar, A.; Mishra, Y.; Rai, V.; Sreenivasulu, Y.; Amalraj, R.S.; et al. Plant proteomics in India and Nepal: Current status and challenges ahead. Physiol. Mol. Biol. Plants. 2013, 19, 461–477. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Recent advances of metabolomics in plant biotechnology. Plant Biotechnol. Rep. 2012, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Yoon, D.W.; Lee, M.; So, D.; Hong, I.H.; Rhee, C.S.; Park, J.W.; Cho, J.Y.; Shin, H.W. Urinary metabolomic signatures in obstructive sleep apnea through targeted metabolomic analysis: A oilot study. Metabolomics 2017, 13, 761. [Google Scholar] [CrossRef]
- van Delden, S.H.; Nazarideljou, M.J.; Marcelis, L.F.M. Nutrient solutions for Arabidopsis thaliana: A study on nutrient solution composition in hydroponics systems. Plant Methods 2020, 16, 72. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Rha, E.Y.; Kim, J.M.; Yoo, G. Volume measurement of various tissues using the Image J software. J. Craniofac. Surg. 2015, 26, e505–e506. [Google Scholar] [CrossRef]
- Shim, I.S.; Momose, Y.; Yamamoto, A.; Usui, K.K. Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul. 2003, 39, 285–292. [Google Scholar] [CrossRef]
- Lin, J.S.; Lai, E.M. Protein-protein iteractions: Co-immunoprecipitation. Methods Mol. Biol. 2017, 1615, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Y.; Lai, S.; Li, Y.; Lin, Z.; Hao, L.; Dong, J.; Li, X.; Huang, K. Global trends and prospects in health economics of robotic surgery: A bibliometric analysis. Int. J. Surg. 2023, 109, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Madadizadeh, F.; Bahariniya, S. Frequency of the statistical methods and relation with acceptance period in archives of Iranian medicine articles: A Review from 2015-2019. Arch Iran Med. 2022, 25, 267–273. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cheng, Z.; Fan, G.; Zhu, D.; Tan, B.; Jiang, T.; Chen, S. Transcription Factor McHB7 Improves Ice Plant Drought Tolerance through ABA Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4569. https://doi.org/10.3390/ijms25084569
Zhang X, Cheng Z, Fan G, Zhu D, Tan B, Jiang T, Chen S. Transcription Factor McHB7 Improves Ice Plant Drought Tolerance through ABA Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(8):4569. https://doi.org/10.3390/ijms25084569
Chicago/Turabian StyleZhang, Xuemei, Zihan Cheng, Gaofeng Fan, Dan Zhu, Bowen Tan, Tingbo Jiang, and Sixue Chen. 2024. "Transcription Factor McHB7 Improves Ice Plant Drought Tolerance through ABA Signaling Pathway" International Journal of Molecular Sciences 25, no. 8: 4569. https://doi.org/10.3390/ijms25084569





