Effect of Trichomonacide 6-Nitro-1H-benzimidazole Derivative Compounds on Expression Level of Metabolic Genes in Trichomonas vaginalis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity Trichomonacidal of Benzimidazole Derivative Compounds
2.2. Kinetic Growth of Trichomonas vaginalis
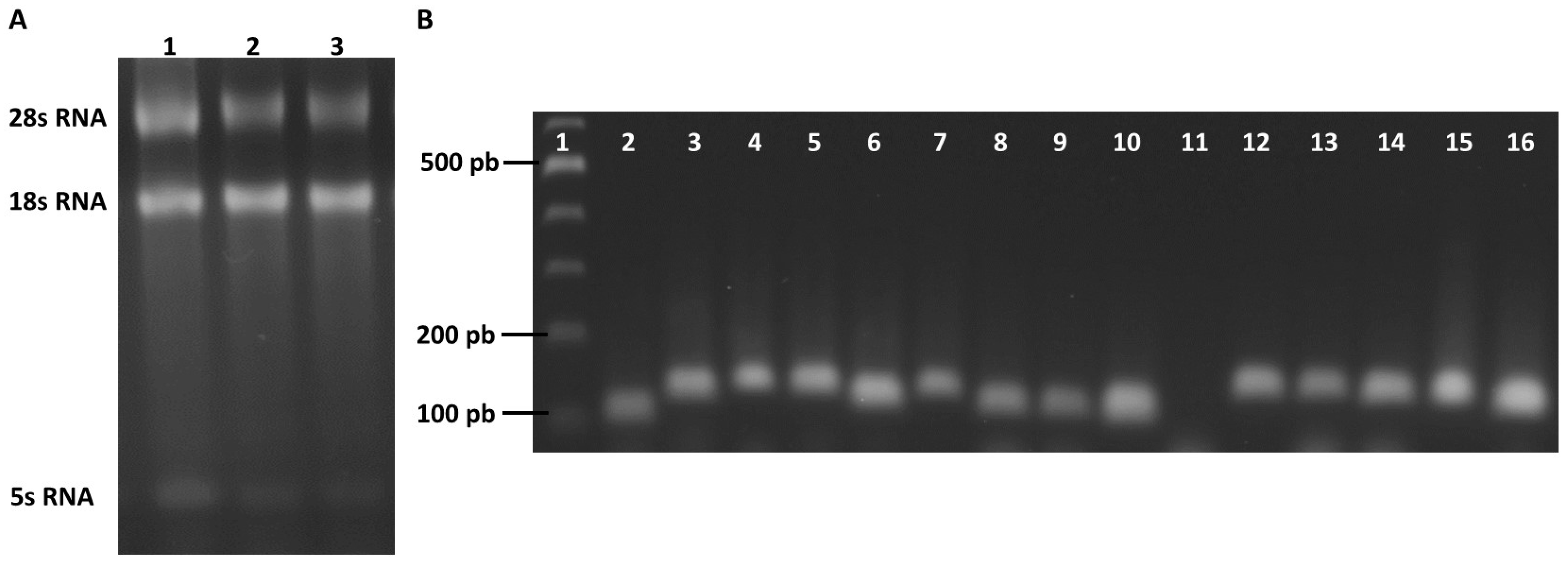
2.3. Selection of Reference Genes for RT-qPCR Analysis in Trichomonas vaginalis
2.4. Evaluation of the Specificity and Efficiency of the Primer Pairs
2.5. Expression Stabilities of Candidate Reference Genes
2.6. Validation Candidate Reference Genes
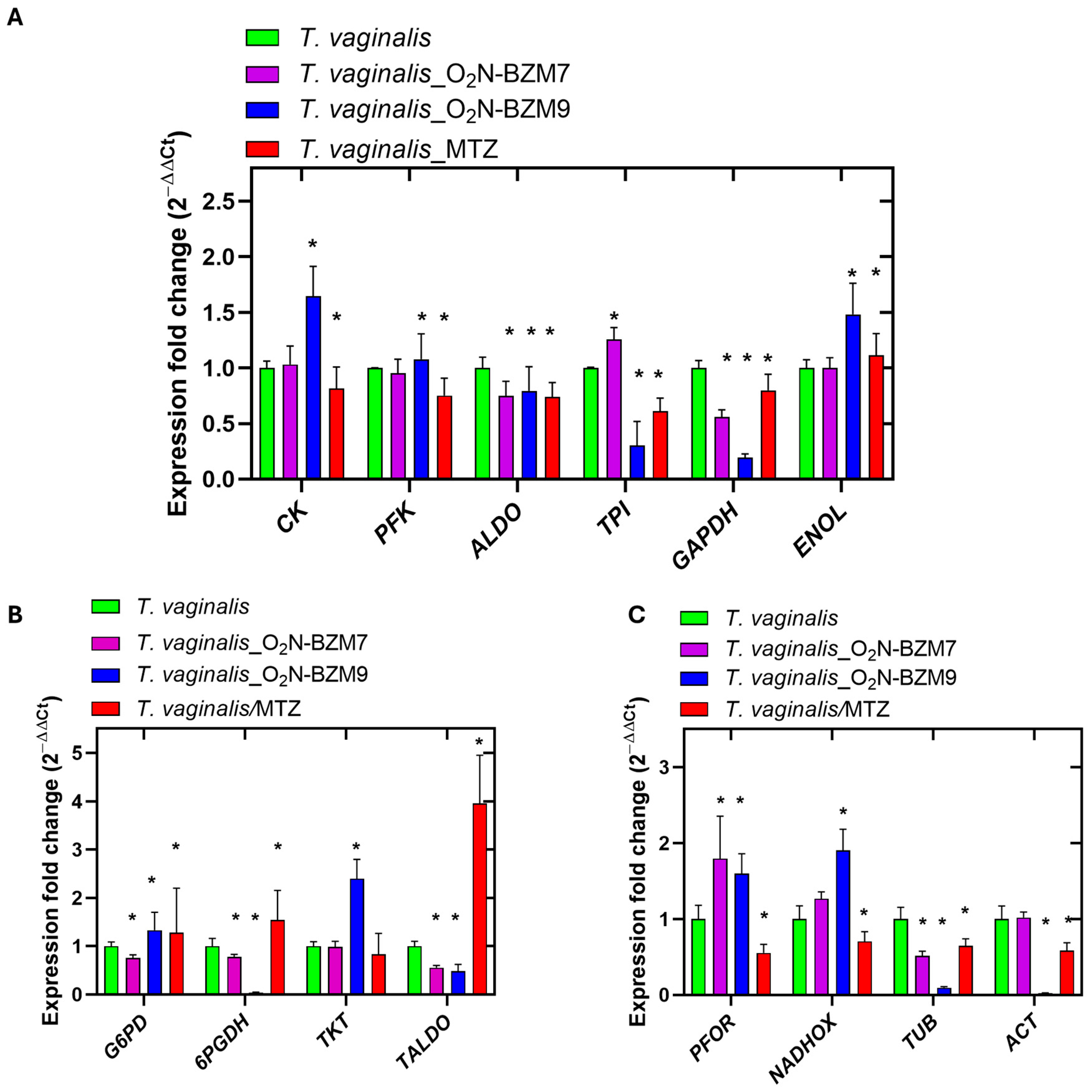
2.7. Level Expression Genes in Trichomonas vaginalis
3. Materials and Methods
3.1. Parasite and Culture Conditions
3.2. Trichomonacidal Activity of Compounds
3.3. Effects of Compounds on Trichomonas vaginalis Growth Kinetic
3.4. Validation and Selection of Reference Genes for RT-qPCR Analysis in Trichomonas vaginalis
3.4.1. Primer Design
3.4.2. PCR Efficiency and Specificity
3.4.3. Validating Candidate Reference Genes
3.4.4. RNA Extraction, cDNA Synthesis, and Quantitative PCR (qPCR)
3.5. Probit Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections: 2008. Reprod. Health Matters 2012, 20, 207–208. [Google Scholar] [CrossRef]
- Graham, S.; Smith, L.W.; Fairley, C.K.; Hocking, J. Prevalence of chlamydia, gonorrhoea, syphilis and trichomonas in Aboriginal and Torres Strait Islander Australians: A systematic review and meta-analysis. Sex Health 2016, 13, 99. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562P. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Videau, D.; Niel, G.; Siboulet, A.; Catalan, F. Secnidazole. A 5-nitroimidazole derivative with a long half-life. Br. J. Vener. Dis. 1978, 54, 77–80. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R.; Nyirjesy, P.; Kaufman, G.; Mena, L.A.; Lazenby, G.B.; Van Gerwen, O.T.; Graves, K.J.; Arbuckle, J.; Carter, B.A.; et al. Efficacy and safety of single oral dosing of secnidazole for trichomoniasis in women: Results of a phase 3, randomized, double-blind, placebo-controlled, delayed-treatment study. Clin. Infect. Dis. 2021, 73, e1282–e1289. [Google Scholar] [CrossRef]
- Watt, L.; Jennison, R.F. Clinical evaluation of metronidazole. A new systemic trichomonacide. Br. Med. J. 1960, 2, 902–905. [Google Scholar] [CrossRef]
- Robinson, S.C. Trichomonal vaginitis resistant to metranidazole. Can. Med. Assoc. J. 1962, 86, 665. [Google Scholar] [PubMed]
- Fung, H.B.; Doan, T. Tinidazole: A Nitroimidazole Antiprotozoal Agent. Clin. Ther. 2005, 27, 1859–1884. [Google Scholar] [CrossRef]
- Crowell, A.L.; Sanders-Lewis, K.A.; Secor, W.E. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob. Agents Chemother. 2003, 47, 1407–1409. [Google Scholar] [CrossRef]
- Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P.K.; Bhutani, H.; Paul, A.T.; Kumar, R.U.S. FDA Approved Drugs from 2015–June 2020: A Perspective. J. Med. Chem. 2021, 64, 2339–2381. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, S.L.; Delgaty, K.L.; Hayward-McClelland, S.F.; Petrin, D.P.; Garber, G.E. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin. Microbiol. Rev. 2004, 17, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Cenkowski, M.; Wudel, B.; Baragar, I.; Poliquin, V. Metronidazole-resistant trichomoniasis: Two Canadian cases. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2021, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gretchen, L. Johnson. Tinidazole (Tindamax) for Trichomoniasis and Bacterial Vaginosis. Am. Fam. Physician 2009, 79, 102–105. [Google Scholar]
- Seña, A.C.; Bachmann, L.H.; Hobbs, M.M. Persistent and recurrent Trichomonas vaginalis infections: Epidemiology, treatment and management considerations. Expert. Rev. Anti Infect. Ther. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Helms, D.J.; Mosure, D.J.; Secor, W.E.; Workowski, K.A. Management of Trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am. J. Obstet. Gynecol. 2008, 198, 370.e1–370.e7. [Google Scholar] [CrossRef] [PubMed]
- Asensio Sánchez, T.; Dávila, I.; Moreno, E.; Laffond, E.; Macías, E.; Ruiz, A.; Lorente, F. Anaphylaxis due to metronidazole with positive skin prick test. J. Investig. Allergol. Clin. Immunol. 2008, 18, 136–142. [Google Scholar]
- Piskin, G.; Mekkes, J.R. Stevens–Johnson syndrome from metronidazole. Contact Dermat. 2006, 55, 192–193. [Google Scholar] [CrossRef]
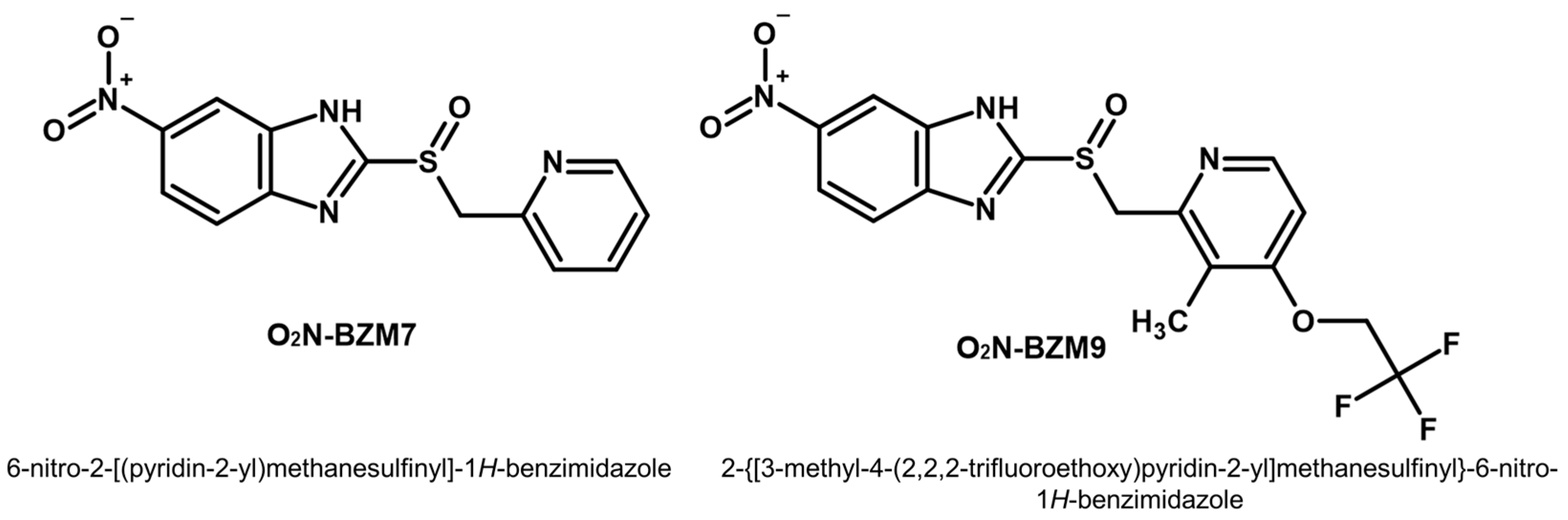
- Chung, N.T.; Dung, V.C.; Duc, D.X. Recent achievements in the synthesis of benzimidazole derivatives. RSC Adv. 2023, 13, 32734–32771. [Google Scholar] [CrossRef]
- Hernández-Ochoa, B.; Martínez-Rosas, V.; Morales-Luna, L.; Calderón-Jaimes, E.; Rocha-Ramírez, L.M.; Ortega-Cuellar, D.; Rufino-González, Y.; González-Valdez, A.; Arreguin-Espinosa, R.; Enríquez-Flores, S.; et al. Pyridyl Methylsulfinyl Benzimidazole Derivatives as Promising Agents against Giardia lamblia and Trichomonas vaginalis. Molecules 2022, 27, 8902. [Google Scholar] [CrossRef]
- Syvret, R.G.; Butt, K.M.; Nguyen, T.P.; Bulleck, V.L.; Rieth, R.D. Novel process for generating useful electrophiles from common anions using Selectfluor® fluorination agent. J. Org. Chem. 2002, 67, 4487–4493. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.; Karaghiosoff, K. Reagents for selective fluoromethylation: A challenge in organofluorine chemistry. Angew. Chem. Int. Ed. Engl. 2020, 59, 12268–12281. [Google Scholar] [CrossRef]
- O’Hagan, D. Fluorine in health care: Organofluorine containing blockbuster drugs. J. Fluor. Chem. 2010, 131, 1071–1081. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Soria-Arteche, O.; Hernández-Campos, A.; Yépez-Mulia, L.; Trejo-Soto, P.J.; Hernández-Luis, F.; Gres-Molina, J.; Maldonado, L.A.; Castillo, R. Synthesis and antiprotozoal activity of nitazoxanide-N-methylbenzimidazole hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 6838–6841. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, H.J.; Fehrmann, R.S.; de Bont, E.S.; Hofstra, R.M.W.; Gerbens, F.; Kamps, W.A.; de Vries, E.G.E.; van der Zee, A.G.J.; Meerman, G.J.T.; Elst, A.T. Evidence based selection of housekeeping genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nepost, C.; Laroche, D.G.; Courtin, C.; Laplanche, J.L.; Etain, B.; Marie-Claire, C. Selecting reference genes in RT-qPCR based on equivalence tests: A network based approach. Sci. Rep. 2019, 9, 16231. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Wilkening, S.; Bader, A. Quantitative real-time polymerase chain reaction: Methodical analysis and mathematical model. J. Biomol. Tech. 2004, 15, 107–111. [Google Scholar] [PubMed]
- Fernández-Aparicio, M.; Huang, K.; Wafula, E.K.; Honaas, L.A.; Wickett, N.J.; Timko, M.P.; de Pamphilis, C.W.; Yoder, J.I.; Westwood, J.H. Application of qRT-PCR and RNA-Seq analysis for the identification of housekeeping genes useful for normalization of gene expression values during Striga hermonthica development. Mol. Biol. Rep. 2013, 40, 3395–3407. [Google Scholar] [CrossRef] [PubMed]
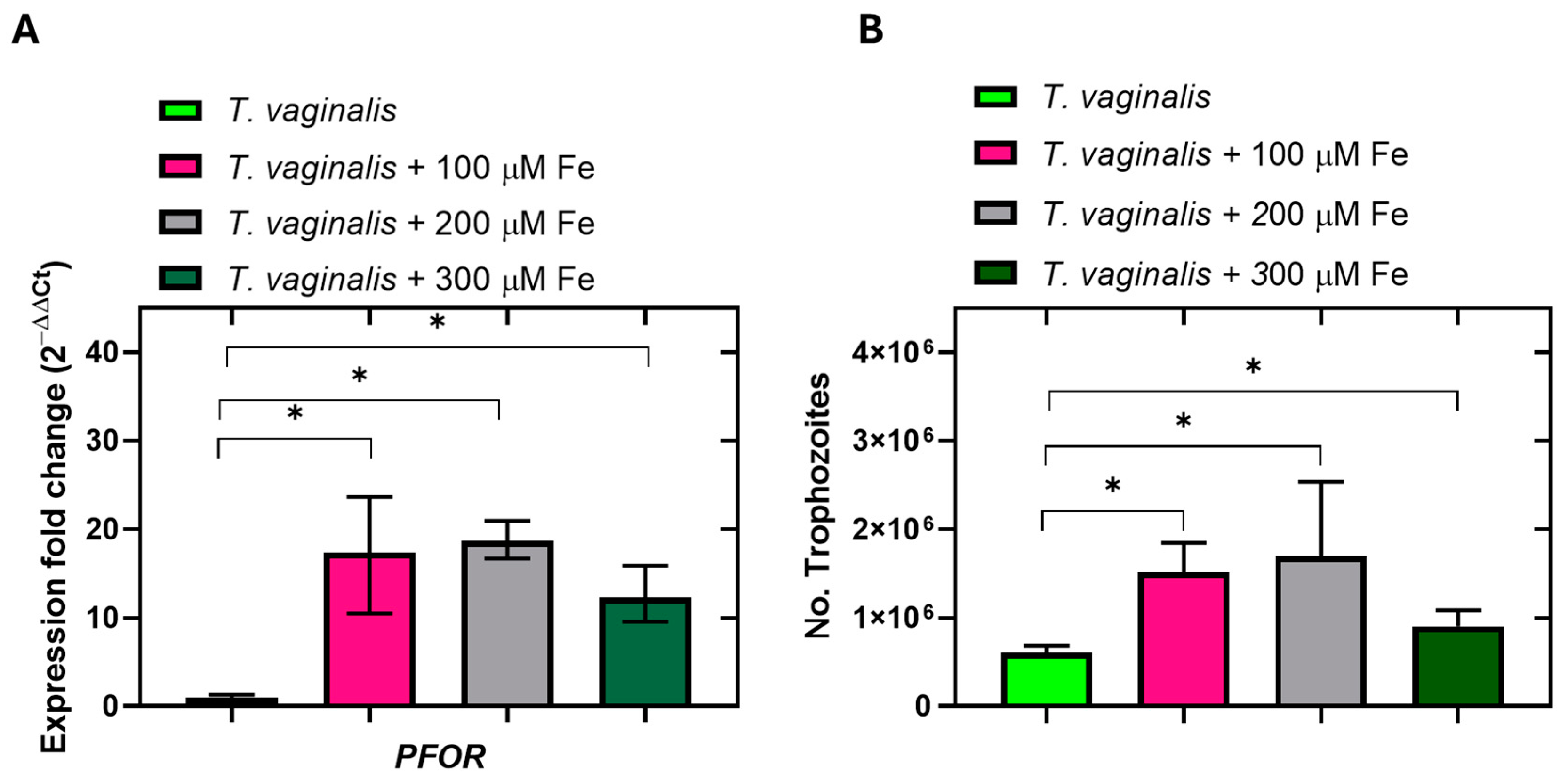
- Gorrell, T.E. Effect of culture medium iron content on the biochemical composition and metabolism of Trichomonas vaginalis. J. Bacteriol. 1985, 161, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rivas, L.A.; Arroyo, R. Iron restriction increases the expression of a cytotoxic cysteine proteinase TvCP2 by a novel mechanism of tvcp2 mRNA alternative polyadenylation in Trichomonas vaginalis. Biochim. Biophys. Acta. Gene Regul. Mech. 2023, 1866, 194935. [Google Scholar] [CrossRef] [PubMed]
- Lehker, M.W.; Alderete, J.F. Iron regulates growth of Trichomonas vaginalis and the expression of immunogenic trichomonad proteins. Mol. Microbiol. 1992, 6, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Dias-Lopes, G.; Saboia-Vahia, L.; Margotti, E.T.; Fernandes, N.S.; Castro, C.L.F.; Oliveira, F.O.; Peixoto, J.F.; Britto, C.; Silva-Filho, F.C.E.; Cuervo, P.; et al. Morphologic study of the effect of iron on pseudocysts formation un Trichomonas vaginalis and its interaction with human epithelial cells. Memórias Inst. Oswaldo Cruz 2017, 112, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Energy metabolism of protozoa without mitochondria. Annu. Rev. Microbiol. 1988, 42, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Miiller, M. Energy metabolism of anaerobic protists. In Biochemical Protozoology; Coombs, G.H., North, M.J., Eds.; Taylor and Francis: London, UK, 1991; pp. 80–91. [Google Scholar]
- Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Nava-Zuazo, C.; Castillo-Villanueva, A.; Méndez, S.T.; Torres-Arroyo, A.; Gómez Manzo, S.; Marcial-Quino, J.; Ponce-Macotela, M.; Rufino-González, Y.; et al. Novel giardicidal compounds bearing proton pump inhibitor scaffold proceeding through triosephosphate isomerase inactivation. Sci. Rep. 2017, 7, 7810. [Google Scholar] [CrossRef]
- Vique-Sánchez, J.L.; Caro-Gómez, L.A.; Brieba, L.G.; Benítez-Cardoza, C.G. Developing a new drug against trichomoniasis, new inhibitory compounds of the protein triosephosphate isomerase. Parasitol. Int. 2020, 76, 102086. [Google Scholar] [CrossRef]
- López-Velázquez, G.; Fernández-Lainez, C.; de la Mora-de la Mora, J.I.; Caudillo De La Portilla, D.; Reynoso-Robles, R.; González Maciel, A.; Ridaura, C.; García-Torres, I.; Gutiérrez-Castrellón, P.; Olivos-García, A.; et al. On the molecular and cellular effects of omeprazole to further support its effectiveness as an antigiardial drug. Sci. Rep. 2019, 9, 8922. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Müller, M. Glycerol, a metabolic end product of Trichomonas vaginalis and Tritrichomonas foetus. Mol. Biochem. Parasitol. 1986, 20, 45–55. [Google Scholar] [CrossRef]
- Lindmark, D.G.; Eckenrode, B.L.; Halberg, L.A.; Dinbergs, I.D. Carbohydrate, energy and hydrogenosomal metabolism of Tritrichomonas foetus and Trichomonas vaginalis. J. Protozool. 1989, 36, 214–216. [Google Scholar] [CrossRef]
- Tielens, A.G.; van Grinsven, K.W.; Henze, K.; van Hellemond, J.J.; Martin, W. Acetate formation in the energy metabolism of parasitic helminths and protists. Int. J. Parasitol. 2010, 40, 387–397. [Google Scholar] [CrossRef]
- Huang, K.Y.; Chen YY, M.; Fang, Y.K.; Cheng, W.H.; Cheng, C.C.; Chen, Y.C.; Wu, T.E.; Ku, F.M.; Chen, S.C.; Lin, R.; et al. Adaptive responses to glucose restriction enhance cell survival, antioxidant capability, and autophagy of the protozoan parasite Trichomonas vaginalis. Biochim. Biophys. Acta 2014, 1840, 53–64. [Google Scholar] [CrossRef]
- Diaz-Ramos, A.; Roig-Borrellas, A.; Garcia-Melero, A.; Lopez-Alemany, R. α-Enolase, a multifunctional protein: Its role on pathophysiological situations. J. Biomed. Biotechnol. 2012, 2012, 156795. [Google Scholar] [CrossRef]
- Leitsch, D.; Janssen, B.D.; Kolarich, D.; Johnson, P.J.; Duchêne, M. Trichomonas vaginalis flavin reductase 1 and its role in metronidazole resistance. Mol. Microbiol. 2014, 91, 198–208. [Google Scholar] [CrossRef]
- Linstead, D.J.; Bradley, S. The purification and properties of two soluble reduced nicotinamide: Acceptor oxidoreductases from Trichomonas vaginalis. Mol. Biochem. Parasitol. 1988, 27, 125–133. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Leitsch, D. Identification of the NADH-oxidase gene in Trichomonas vaginalis. Parasitol. Res. 2020, 119, 683–686. [Google Scholar] [CrossRef]
- Tanabe, M. Trichomonas vaginalis: NADH oxidase activity. Exp. Parasitol. 1979, 48, 143–145. [Google Scholar] [CrossRef]
- Arroyo, R.; González-Robles, A.; Martínez-Palomo, A.; Alderete, J.F. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol. Microbiol. 1993, 7, 299–309. [Google Scholar] [CrossRef]
- Furtado, M.B.; Benchimol, M. Observation of membrane fusion on the interaction of Trichomonas vaginalis with human vaginal epithelial cells. Parasitol. Res. 1998, 84, 213–220. [Google Scholar] [CrossRef]
- Kusdian, G.; Woehle, C.; Martin, W.F.; Gould, S.B. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell. Microbiol. 2013, 15, 1707–1721. [Google Scholar] [CrossRef]
- Lorenzo-Benito, S.; Rivera-Rivas, L.A.; Sánchez-Ayala, L.; Ortega-López, J.; Montes-Flores, O.; Talamás-Lara, D.; Arroyo, R. Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis. Genes 2022, 13, 1067. [Google Scholar] [CrossRef]
- Benchimol, M. Trichomonads under Microscopy. Microsc. Microanal. 2004, 10, 528–550. [Google Scholar] [CrossRef]
- Wang, K.H.; Chang, J.Y.; Li, F.A.; Wu, K.Y.; Hsu, S.H.; Chen, Y.J.; Chu, T.L.; Lin, J.; Hsu, H.M. An Atypical F-Actin Capping Protein Modulates Cytoskeleton Behaviors Crucial for Trichomonas vaginalis Colonization. Microbiol. Spectr. 2023, 11, e0059623. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant. Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]





| Gen Symbol | 5′-3′Sequence | Amplicon Size | Tm | RT-qPCR E (%) | Slope | R2 |
|---|---|---|---|---|---|---|
| CK | FW: ACAACAGGAGCCGGAGATG RV: AGCAGCACAACCTCTCTTTG | 97 | 59.18 58.40 | ND | ND | ND |
| PFK | FW: TGCAGTTCTCTCTAGTGGCC RV: CACGGAAGCCACCAGTAATG | 116 | 59.10 58.91 | 101 | −3.296 | 0.964 |
| ALDO | FW: AAGTCACTCGGTCTCTGCAA RV: TTGACGGAGGCTGTGATGAT | 125 | 58.96 59.1 | 98.5 | −3.356 | 0.991 |
| TPI | FW: GGCAAGTGGGACGATGTTG RV: TTAGCAGCAAGGATGTCACG | 122 | 59.12 58.27 | 94.4 | −3.464 | 0.995 |
| GAPDH | FW: CCAAGTTGTCGCTATCCACG RV: TGCTTAGCCTCATCGACTGT | 114 | 59 58.81 | 90.3 | −3.577 | 0.996 |
| ENOL | FW: ACAGGTGTTGGTGAAGCTCT RV: AGCACATTCCCTTGAGAGCT | 124 | 59.16 59 | 110 | −3.055 | 0.975 |
| PK | FW: CCACAAGCAAACACTCGACA RV: CTCCAACTTGCCAACACGAA | 109 | 59 59 | 114.8 | −3.042 | 0.989 |
| G6PD | FW: ATTCTCACGTCTCCACCAGG RV: GTCATCGTAGCCACCAGAGA | 109 | 59.1 58.9 | 117 | −3.068 | 0.959 |
| 6PGDH | FW: CGATGGTGGCAACTCTCACT RV: CTCTTCACCGCCGGAGATAC | 122 | 60 60.1 | 104 | −3.226 | 0.991 |
| TALDO | FW: TCCTCAAGATTGTCCCAGGC RV: TCTTGATTCCGGCTTCGTGA | 123 | 59.3 59.3 | 111 | −3.07 | 0.994 |
| ACT | FW: GTCAAGCTTCTCACAGAGCG RV: GGCCTTCTCCATTTCAGCAT | 123 | 58.9 58.2 | 90.4 | −3.574 | 0.998 |
| TUB | FW: CTTCCGTGGCCGTATGTCAT RV: GCAGATAGCGGACTTGACGT | 115 | 60 60 | 97.1 | −3.392 | 0.998 |
| PFOR | FW: CCAGATCACACCACTCGACT RV: TTCCCAGTTCTTGCCCTCTT | 121 | 59.1 58.9 | 105 | −3.188 | 0.942 |
| NADHOX | FW: ATTGGCTTGGCGTCCTTGAT RV: TCGACGAGAACTGCACCTTC | 118 | 60.3 60.0 | 118 | −3.045 | 0.92 |
| GeNorm | M | Comparative ΔCt Method | NormFinder | Ranking | |||
|---|---|---|---|---|---|---|---|
| ACT | 0.234 | PK | 0.73 | PK | 0.195 | PK | 2.43 |
| GAPDH | 0.234 | TUB | 0.75 | TALDO | 0.387 | TUB | 3.57 |
| TUB | 0.289 | TALDO | 0.75 | TUB | 0.490 | TALDO | 3.83 |
| TPI | 0.320 | ALDO | 0.76 | ALDO | 0.495 | GAPDH | 4.56 |
| ALDO | 0.337 | TPI | 0.79 | TPI | 0.578 | ACT | 4.98 |
| TALDO | 0.400 | GAPDH | 0.82 | GAPDH | 0.649 | ALDO | 5.03 |
| PK | 0.480 | ACT | 0.85 | ACT | 0.690 | TPI | 5.62 |
| PFK | 0.540 | PFK | 0.93 | PFK | 0.693 | NADHOX | 6.45 |
| PFOR | 0.616 | PFOR | 0.95 | PFOR | 0.721 | G6PD | 6.84 |
| G6PD | 0.726 | G6PD | 1.02 | G6PD | 0.828 | ENOL | 7.18 |
| 6PGDH | 0.807 | 6PGDH | 1.06 | 6PGDH | 0.893 | PFOR | 7.48 |
| ENOL | 0.861 | ENOL | 1.07 | ENOL | 0.928 | 6PGDH | 7.95 |
| NADHOX | 0.897 | NADHOX | 1.08 | NADHOX | 0.944 | PFK | 8.66 |
| GenBank Accession Number | Gene Annotation | Gen Symbol | Function |
|---|---|---|---|
| XM_001579622.1 | Carbohydrate kinase | CK | Kinase in glycolysis |
| XM_001581728.2 | Phosphofructokinase | PFK | Kinase in glycolysis |
| XM_001315350.2 | Aldolase IIB | ALDO | Oxidoreductase in glycolysis |
| XM_001320301.2 | Triosephosphate isomerase | TPI | Isomerase in glycolysis |
| XM_001581066.2 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Oxidoreductase in glycolysis |
| XM_001325471.2 | Enolase | ENOL | Lyase in glycolysis |
| XM_001329865.2 | Pyruvate kinase | PK | Oxidoreductase in glycolysis |
| XM_001321943.2 | Glucose-6-phosphate 1-dehydrogenase | G6PD | Oxidoreductase in pentose phosphate |
| XM_001323727.2 | Phosphogluconate dehydrogenase | 6PGDH | Oxidoreductase in pentose phosphate |
| XM_001326902.1 | Transketolase | TKT | Transferase in glycolysis |
| XM_001330311.2 | Transaldolase | TALDO | Transferase in glycolysis |
| XM_001321286.2 | Pyruvate–ferredoxin oxidoreductase proprotein | PFOR | Oxidoreductase in hydrogenosome |
| XM_001315387.2 | Diflavin flavoprotein A (NADH oxidase) | NADHOX | O2-Detoxifying enzyme |
| XM_001321203.2 | Tubulin beta 4 chain | TUB | Cytoskeletal structural protein (Flagella, median body, ventral disc) |
| XM_001301716.2 | Actin | ACT | Cytoskeletal structural protein (all cell) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Cardona, J.Y.; Calderón-Jaimes, E.; Ortega-Cuellar, D.; Sánchez-Carrillo, A.; Castillo-Rodríguez, R.A.; Canseco-Ávila, L.M.; Rocha-Ramírez, L.M.; Martínez-Rosas, V.; Gómez-Manzo, S.; Hernández-Ochoa, B. Effect of Trichomonacide 6-Nitro-1H-benzimidazole Derivative Compounds on Expression Level of Metabolic Genes in Trichomonas vaginalis. Int. J. Mol. Sci. 2024, 25, 4568. https://doi.org/10.3390/ijms25084568
Gutiérrez-Cardona JY, Calderón-Jaimes E, Ortega-Cuellar D, Sánchez-Carrillo A, Castillo-Rodríguez RA, Canseco-Ávila LM, Rocha-Ramírez LM, Martínez-Rosas V, Gómez-Manzo S, Hernández-Ochoa B. Effect of Trichomonacide 6-Nitro-1H-benzimidazole Derivative Compounds on Expression Level of Metabolic Genes in Trichomonas vaginalis. International Journal of Molecular Sciences. 2024; 25(8):4568. https://doi.org/10.3390/ijms25084568
Chicago/Turabian StyleGutiérrez-Cardona, Jocelyn Yamin, Ernesto Calderón-Jaimes, Daniel Ortega-Cuellar, Adrián Sánchez-Carrillo, Rosa Angélica Castillo-Rodríguez, Luis Miguel Canseco-Ávila, Luz María Rocha-Ramírez, Víctor Martínez-Rosas, Saúl Gómez-Manzo, and Beatriz Hernández-Ochoa. 2024. "Effect of Trichomonacide 6-Nitro-1H-benzimidazole Derivative Compounds on Expression Level of Metabolic Genes in Trichomonas vaginalis" International Journal of Molecular Sciences 25, no. 8: 4568. https://doi.org/10.3390/ijms25084568





