Assessment of Changes in the Structure of Zooplankton Communities to Infer Water Quality of the Caspian Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Field Sampling
2.3. Laboratory Processing
3. Results
3.1. Hydrophysical and Hydrochemical Characteristics of Study Sites
3.2. Species Richness
3.3. Quantitative Variables and Composition of the Dominant Species
3.4. Structural Variables
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Meier, M.H.E.; Müller-Karulis, B.; Andersson, H.C.; Dieterich, C.; Eilola, K.; Gustafsson, B.G.; Höglund, A.; Hordoir, R.; Kuznetsov, I.; Neumann, T.; et al. Impact of Climate Change on Ecological Quality Indicators and Biogeochemical Fluxes in the Baltic Sea: A Multi-Model Ensemble Study. Ambio 2012, 41, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Brander, K.M.; Ottersen, G.; Bakker, J.P.; Beaugrand, G.; Herr, H.; Garthe, S.; Gilles, A.; Kenny, A.; Siebert, U.; Skjolda, H.R.; et al. Environmental Impacts—Marine Ecosystems. In North Sea Region Climate Change Assessment, Regional Climate Studies; Quante, M., Colijn, F., Eds.; Springer: Kongens Lyngby, Denmark, 2016; pp. 241–274. [Google Scholar]
- Barber, D.G.; Asplin, M.G.; Papakyriakou, T.N.; Miller, L.; Else, B.G.T.; Iacozza, J.; Mundy, C.J.; Gosslin, M.; Asselin, N.C.; Ferguson, S.; et al. Consequences of change and variability in sea ice on marine ecosystem and biogeochemical processes during the 2007–2008. Canadian International Polar Year program. Clim. Chang. 2012, 115, 135–159. [Google Scholar] [CrossRef]
- Holstein, A.; Kappas, M.; Propastin, P.; Renchin, T. Oil spill detection in the Kazakhstan sector of the Caspian Sea with the help of ENVISAT ASAR data. Environ. Earth Sci. 2018, 77, 198. [Google Scholar] [CrossRef] [Green Version]
- Dolotov, Y.; Kaplin, P. Black and Caspian Seas. Coastal Ecology and Geomorphology. In Encyclopedia of Coastal Science; Schwartz, M.L., Ed.; Encyclopedia of Earth Science Series Springer: Dordrecht, The Netherlands, 2018; ISBN 978-1-4020-3880-8. [Google Scholar]
- Karbassi, A.R.; Amirnezhad, R. Geochemistry of heavy metals and sedimentation rate in a bay adjacent to the Caspian Sea. Int. J. Environ. Sci. Technol. 2004, 1, 191. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, W.; Yu, L.; Feng, H. Metal Pollution in Coastal Sediments. Curr. Pollut. Rep. 2015, 1, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Korshenko, A.; Gul, A.G. Pollution of the Caspian Sea. In The Caspian Sea Environment. The Handbook of Environmental Chemistry; Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 109–142. [Google Scholar]
- Ivanov, V.P.; Sokolsky, A.F. Scientific Basis for the Strategy of Protecting the Biological Resources of the Caspian SEA from Oil Pollution; CaspNIRKH Publishing House: Astrakhan, Russia, 2000; 180p. (In Russian) [Google Scholar]
- Huang, J.; Xie, Y.; Guan, X.; Li, D.; Ji, F. The dynamics of the warming hiatus over the Northern Hemisphere. Clim. Dyn. 2017, 48, 429–446. [Google Scholar] [CrossRef]
- Sharmad, T.; Bidhendi, G.R.N.; Karbassi, A.R.; Moatar, F.; Adabi, M.H. Historical changes in distribution and partitioning of natural and anthropogenic shares of heavy metals in sediment core from the southern Caspian Sea. Environ. Earth Sci. 2012, 67, 799–811. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Hosseini, S.V.; Miandare, H.K.; Faizbakhsh, R.; Harsij, M.; Regenstein, J.M. Determination of Cd, Cu, Mn and Zn concentrations in Iranian Caspian Sea caviar of Acipenser persicus using anodic stripping voltammetry. Iran J. Sci. Technol. Trans. Sci. 2017, 41, 139. [Google Scholar] [CrossRef]
- Guseinova, S.A.; Abdusamadov, A.S. Forecast of the Caspian Sea level and its implications for coastal areas. South Russ. Ecol. Dev. 2015, 10, 119–126. [Google Scholar] [CrossRef]
- Ecological Monitoring Studies of the Environment of the North-Eastern Caspian During the Development of Oil Fields of NCOC N.V; in the period from 2006 to 2016; Skolsky, V.A. (Ed.) North Caspian Operating Company N.V., Kazakh Agency of Applied Ecology: Almaty, Kazakhstan, 2018; 400p, ISBN 978-601-332-146-2. (In Russian) [Google Scholar]
- Pollutants in the Waters of the Volga-Caspian Basin; Brekhovskikh, V.F.; Ostrovskaya, E.V. (Eds.) Publisher IP Sorokin Roman Vasilyevich: Astrakhan, Russia, 2017; 408p, ISBN 978-5-91910-527-5. [Google Scholar]
- Adams, S.M.; Greeley, M.S. Multi-response indicators to assess the health of aquatic ecosystems. Water Air Soil Pollut. 2000, 123, 103–115. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Gharib, S.M. Phytoplankton abundance and structure as indicator of water quality in the drainage system of the Burullus Lagoon, southern Mediterranean coast. Egypt Environ. Monit. Assess. 2016, 188, 530. [Google Scholar] [CrossRef] [PubMed]
- Barinova, S.S.; Medvedeva, L.A.; Kondratieva, L.M.; Shesterkin, V.P. Bio-indication in the Amur River, Russian Far East. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1171–1187. [Google Scholar]
- Gerasimova, T.N.; Pogozhev, P.I. The role of zooplankton in phytoplankton biomass decline and water transparency regulation in a water body subject to high organic and mineral load. Water Res. 2010, 37, 796–806. [Google Scholar] [CrossRef]
- Filho, S.L.N.; França, E.J.; Júnior, M.M.; Moura, A.N. Interactions between benthic microalgae, nutrients and benthic macroinvertebrates in reservoirs from the semi-arid Neotropical region. Fundam. Appl. Limnol. 2019, 192, 237–254. [Google Scholar] [CrossRef]
- Barinova, S. Algal Diversity Dynamics, Ecological Assessment, and Monitoring in the River Ecosystems of the Eastern Mediterranean; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Andronikova, I.N. Zooplankton Structural and Functional Organization of Lacustrine Ecosystems of Different Trophic Types; Nauka: St. Petersburg, Russia, 1996; 189p, ISBN 5-02-026713-9. (In Russian) [Google Scholar]
- Krupa, E.G.; Barinova, S.S. Environmental Variables Regulating the Phytoplankton Structure in High Mountain Lakes. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1251–1261. [Google Scholar]
- Barinova, S.; Krupa, E. Bioindication of ecological state and water quality by phytoplankton in the Shardara Reservoir, Kazakhstan. Environ. Ecol. Res. 2017, 5, 73–92. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.M.; Romanova, S.M.; Malybekov, A.B. Hydrobiological assessment of the high mountain Kolsay Lakes (Kungey Alatau, Southeastern Kazakhstan) ecosystems in climatic gradient. Br. J. Environ. Clim. Chang. 2016, 6, 259–278. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.S.; Assylbekova, S.Z.; Isbekov, K.B. Structural indicators of zooplankton of the Shardara Reservoir (Kazakhstan) and the main influencing factors. Turk. J. Fish. Aquat. Sci. 2018, 18, 659–669. [Google Scholar] [CrossRef]
- Alimov, A.F.; Golubkov, M.S. Lake Eutrophication and Community Structure. Inl. Water Biol. 2014, 3, 185–191. [Google Scholar] [CrossRef]
- Ochocka, A.; Pasztaleniec, A. Sensitivity of plankton indices to lake trophic conditions. Environ. Monit. Assess. 2016, 188, 622. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M. Zooplankton size structure in the Kolsay mountain lakes (Kungei Alatau, southeastern Kazakhstan) and its relationships with environmental factors. Water Res. 2019, 46, 403–414. [Google Scholar] [CrossRef]
- Klymiuk, V.; Barinova, S. Phytoplankton cell size in saline lakes. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1077–1085. [Google Scholar]
- Barinova, S.; Bilous, O.; Ivanova, N. New statistical approach to spatial analysis of ecosystem of the Sasyk Reservoir, Ukraine. Int. J. Ecotoxicol. Ecobiol. 2016, 1, 118–126. [Google Scholar] [CrossRef]
- Warwick, R.M. A new method for detecting pollution effects on marine macrobenthos communities. Marine Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Clarke, K.R. Comparison of dominance curves. J. Exp. Mar. Biol. Ecol. 1990, 138, 143–157. [Google Scholar] [CrossRef]
- Krupa, E.G. The size structure of zooplankton as an indicator of the ecological status of the Caspian Sea. In Marine Biological Studies: Achievements and Prospects, Proceedings of the Materials of the All-Russian Conference, Sevastopol, Russian, 2016; ECOSY-Hydrophysics: Sevastopol, Russia, 2016; Volume 3, pp. 119–123. (In Russian) [Google Scholar]
- Krupa, E.G.; Barinova, S.M. The use of structural indicators of hydrocenoses in assessing the ecological status of water bodies in Kazakhstan. In Bioindication in Monitoring Freshwater Ecosystems: The Third International Conference; Institute of Lake Science of RAS: St. Petersburg, Russia, 2017; pp. 165–170. [Google Scholar]
- Krupa, E.G.; Grishaeva, O.V. The structure of species dominance in the macrozoobenthos of the Small Aral Sea as an indicator of changes in water salinity. In Bioindication in Monitoring Freshwater Ecosystems: Abstracts of the Second International Conference; Lyubavich: St. Petersburg, Russia, 2011; p. 96. [Google Scholar]
- Diamant, A.; Westernhagen, H. MARS: Biological indicators of natural and man-made changes in marine and coastal waters: Scientific coordinators introduction. Helgol. Marine Res. 2003, 57, 146–156. [Google Scholar] [CrossRef]
- Barinova, S.; Bondarenko, A.; Ryabushko, L.; Kapranov, S. Microphytobenthos as an indicator of water quality and organic pollution in the western coastal zone of the Sea of Azov. Oceanol. Hydrobiol. Stud. 2019, 48, 21–35. [Google Scholar] [CrossRef]
- Parizanganeh, A.; Lakhan, V.C.; Jalalian, H.A. Geochemical and statistical approach for assessing heavy metal pollution in sediments from the southern Caspian coast. Int. J. Environ. Sci. Technol. 2007, 4, 351–358. [Google Scholar] [CrossRef]
- Monitoring of the Environment of the Northeastern Caspian Sea When Developing Oil Deposits (Results of Agip KCO Studies, 1993–2006); Ogar, N.P. (Ed.) Agip KCO: Almaty, Kazakhstan, 2014; 263p, ISBN 5-02-033731-5. (In Russian) [Google Scholar]
- Caspian Sea. Hydrology and Hydrochemistry; Baydin, S.S., Kosareva, A.N., Eds.; Science: Moscow, Russia, 1986; 261p. (In Russian) [Google Scholar]
- Zooplankton and Its Products. Guidelines for the Collection and Processing of Materials in Hydrobiological Research in Freshwater Water Bodies; Winberg, G.G., Lavrenteva, G.M., Eds.; GosNIIORH: Leningrad, Russia, 1984; 34p. (In Russian) [Google Scholar]
- Borutsky, E.V.; Stepanova, L.A.; Koss, M.S. Determinant for Calanoida in Fresh Waters of the USSR; Science: St. Petersburg, Russia, 1991; 504p. (In Russian) [Google Scholar]
- Kutikova, L.A. Rotifer in USSR Fauna; Science: Leningrad, Russia, 1970; 744p. (In Russian) [Google Scholar]
- Key to Freshwater Invertebrates of Russia and Adjacent Lands; Tsalolihin, S.Y. (Ed.) Zoological Institute: St. Petersburg, Russia, 1995; 628p. (In Russian) [Google Scholar]
- Magurran, E. Ecological Diversity and its Measurement; Mir: Moscow, Russia, 1998; 184p, ISBN 5-03-002404-2. (In Russian) [Google Scholar]
- Glantz, S.A. Primer of BIOSTATISTICS; Praktik: Moscow, Russia, 1998; 459p, ISBN 5-89816-009-4. (In Russian) [Google Scholar]
- Krupa, E.G.; Dobrokhotova, O.V.; Stuge, T.S. Fauna Calanoida (Crustacea, Copepoda) of Kazakhstan and Adjacent Territories; EtalonPrint: Almaty, Kazakhstan, 2016; 208p, ISBN 978-601-80265-8-4. (In Russian) [Google Scholar]
- Krupa, E.; Kokhno, L.; Kiyko, O. Zooplankton. In Environmental Monitoring Studies of the Environment of the Northeast Caspian Sea During the Development of Oil Fields by NCOC N.V. in the Period from 2006 to 2016; Skolsky, V.A., Ed.; North Caspian Operating Company N.V., Kazakh Agency of Applied Ecology: Almaty, Kazakhstan, 2018; pp. 139–159. ISBN 978-601-332-146-2. (In Russian) [Google Scholar]
- Shadrin, N. Acartia tonsa (Copepoda) in the Black and Caspian Seas: Review and some lessons. J. Biodiv. 2013, 22, 229–236. [Google Scholar] [CrossRef]
- Kurasheva, E.K.; Tinenkova, D.K.H. Population abundance, biomass and distribution of Acartia clausi Gisbrecht (Calanoida, Acartiidae) in the North and Middle Caspian. Hydrobiol. J. 1988, 24, 23–27. (In Russian) [Google Scholar]
- Krupa, E.G.; Sagandykova, R.R.; Klimov, F.V. Characteristics of zooplankton of the northeastern part of the Caspian Sea in a seasonal aspect. In Some Aspects of Hydroecological Problems of Kazakhstan; Burlibaev, M., Ed.; Kaganat: Almaty, Kazakhstan, 2011; pp. 127–134. ISBN 978-601-78-596-8. (In Russian) [Google Scholar]
- Leonov, A.V.; Nazarov, N.A. Nutrient input into the Caspian Sea with river runoff. Water Res. 2001, 28, 656–665. [Google Scholar] [CrossRef]
- Krupa, E.G.; Orlova, I.V. Structure of zooplankton in the Kazakhstan sector of the northern and middle Caspian. In Some Aspects of the Hydroecological Problems of Kazakhstan; Burlibaev, M., Ed.; Kaganat: Almaty, Kazakhstan, 2011; pp. 120–126. ISBN 978-601-78-596-8. (In Russian) [Google Scholar]
- Krupa, E.G. Zooplankton of Limnic and Lotic Ecosystems of Kazakhstan. Structure, patterns of formation—Saarbrucken; Palmarium Academic Publishing: Saarbrucken, Germany, 2012; 346p. [Google Scholar]
- Kuczyńska-Kippen, N.; Joniak, T. Zooplankton diversity and macrophyte biometry in shallow water bodies of various trophic state. Hydrobiologia 2016, 774, 39–51. [Google Scholar] [CrossRef]
- Thakar, M.K.; Luthra, D.; Khattar, J.S. Forensic studies of phytoplankton ecology of two water bodies of Kurukshetra area of Haryana, State in India. Egypt. J. Forensic Sci. 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Meire, P.M.; Dereu, J. Use of the Abundance/Biomass Comparison Method for detecting environmental stress: Some considerations based on intertidal macrozoobenthos and bird communities. J. Appl. Ecol. 1990, 27, 210–223. [Google Scholar] [CrossRef]
- Shitikov, V.K.; Golovatyuk, L.V. ABC-method and specificity of species dominance in bottom river communities. Volga Ecol. J. 2013, 1, 88–97. (In Russian) [Google Scholar]
- Barinova, S. On the classification of water quality from an ecological point of view. Int. J. Environ. Sci. Nat. Res. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Krupa, E.G.; Mademarova, N.A. Structure of the phytoplankton of the Northern and Middle Caspian. News Nat. Acad. Sci. Republ. Kaz. 2016, 1, 70–77. (In Russian) [Google Scholar]
- Gasiūnaitė, Z.R.; Olenina, I. Zooplankton-phytoplankton interactions: A possible explanation of the seasonal succession in the Kuršiu˛ Marios lagoon. Hydrobiologia 1998, 363, 333–339. [Google Scholar] [CrossRef]
- Moschonas, G.; Gowen, R.J.; Paterson, R.F.; Mitchell, E.; Stewart, B.M.; McNeill, S.; Glibert, P.M.; Davidson, K. Nitrogen dynamics and phytoplankton community structure: The role of organic nutrients. Biogeochemistry 2017, 134, 125–145. [Google Scholar] [CrossRef]
- Dmitrieva, O.A.; Semenova, A.S. Seasonal dynamics of phyto- and zooplankton and their relationships in hypertrophic reservoir. Inl. Water Biol. 2011, 4, 308–315. [Google Scholar] [CrossRef]
- Dmitrieva, O.A.; Semenova, A.S. Seasonal dynamics and trophic relationships of phyto- and zooplankton in the Vistula Lagoon of the Baltic Sea. Oceanology 2012, 52, 851–856. (In Russian) [Google Scholar] [CrossRef]



| Water Area | Season, Year | Depth, m | Transparency, m | pH | Temperature, °C | Salinity, ‰ |
|---|---|---|---|---|---|---|
| Northeastern Caspian | spring 2010 | 5.2 ± 0.5 | 1.5 ± 0.1 | 8.07 ± 0.02 | 21.7 ± 0.2 | 7.4 ± 0.1 |
| summer 2010 | 5.3 ± 0.4 | 1.6 ± 0.1 | 7.92 ± 0.08 | 26.9 ± 0.4 | 8.4 ± 0.2 | |
| Northern Caspian | spring 2008 | 7.1 ± 1.0 | 2.5 ± 0.4 | 8.94 ± 0.07 | 20.5 ± 0.3 | 7.2 ± 0.9 |
| summer 2008 | 7.0 ± 1.1 | 2.8 ± 0.7 | 8.67 ± 0.06 | 23.4 ± 0.2 | 8.2 ± 0.5 | |
| Middle Caspian | spring 2008 | 38.4 ± 6.1 | 10.6 ± 0.9 | 8.83 ± 0.02 | 18.3 ± 1.2 | 9.7 ± 1.0 |
| summer 2008 | 34.9 ± 7.0 | 9.4 ± 1.0 | 8.71 ± 0.09 | 25.2 ± 0.3 | 11.4 ± 0.2 |
| Water Area | Season, Year | Rotifera | Cladocera | Copepoda | Others | Total |
|---|---|---|---|---|---|---|
| Northeastern Caspian | spring 2010 | 6 | 6 | 4 | 3 | 19 |
| Northeastern Caspian | summer 2010 | 5 | 4 | 4 | 6 | 19 |
| Northern Caspian | spring 2008 | 9 | 7 | 10 | 6 | 32 |
| Northern Caspian | summer 2008 | 8 | 3 | 4 | 6 | 21 |
| Middle Caspian | spring 2008 | 4 | 6 | 4 | 2 | 16 |
| Middle Caspian | summer 2008 | 3 | 3 | 1 | 4 | 11 |
| Total | 12 | 7 | 10 | 8 | 37 |
| Water Area | Season, Year | Abundance, 103 Specimen/m3 | Biomass of Holoplankton, mg/m3 | Biomass of Zooplankton (with Jellyfish), mg/m3 |
|---|---|---|---|---|
| Northeastern Caspian | spring 2010 | 150.8 ± 40.8 | 690.2 ± 119.0 | no jellyfish |
| summer 2010 | 57.6 ± 18.3 | 245.1 ± 60.8 | 1278.5 ± 351.4 | |
| Northern Caspian | spring 2008 | 60.2 ± 20.1 | 208.4 ± 63.8 | 488.6 ± 294.5 |
| summer 2008 | 14.2 ± 3.1 | 53.2 ± 10.4 | 1766.5 ± 685.2 | |
| Middle Caspian | spring 2008 | 3.8 ± 0.7 | 20.2 ± 10.7 | no jellyfish |
| summer 2008 | 3.6 ± 0.7 | 33.4 ± 6.1 | no jellyfish |
| Water Area | Season, Year | The Dominant Species | ||
|---|---|---|---|---|
| by Abundance (%) | by Biomass without Jellyfish (%) | by Biomass with Jellyfish (%) | ||
| Northeastern Caspian | spring 2010 | Brachionus quadridentatus (52.0) Calanipeda aquedulcis (22.0) Acartia tonsa (10.0) | Podonevadne camptonyx (40.9) Calanipeda aquedulcis (17.6) Podonevadne trigona (12.7) Acartia tonsa (9.5) | no jellyfish |
| summer 2010 | Acartia tonsa (34.9) Brachionus quadridentatus (29.8) Brachionus plicatilis (25.6) | Acartia tonsa (67.1) Calanipeda aquedulcis (6.0) Brachionus plicatilis (5.8) | Blackfordia virginica (78.7) Acartia tonsa (12.9) | |
| Northern Caspian | spring 2008 | Brachionusquadridentatus (53.1) Bivalvia gen. sp. (13.7) Acartia tonsa (7.8) | Asplanchna priodonta (23.4) Brachionus quadridentatus (14.0) Calanipeda aquae-dulcis (13.7) Acartia tonsa (12.4) | Blackfordia virginica (57.4) Asplanchna priodonta (10.0) Brachionus quadridentatus (6.0) Calanipeda aquae-dulcis (5.8) |
| summer 2008 | Acartia tonsa (49.7) Brachionus plicatilis (13.6) Brachionus quadridentatus (11.2) | Acartia tonsa (53.4) Podonevadne trigona (7.7) | Blackfordia virginica (96.7) | |
| Middle Caspian | spring 2008 | Synchaeta cecilia (39.3) Acartia tonsa (30.9) Cirripedia gen. sp. (14.3) Bivalvia gen. sp. (6.6) | Acartia tonsa (54.4) Evadne anonyx (14.3) | no jellyfish |
| summer 2008 | Acartia tonsa (93.8) | Acartia tonsa (92.2) | no jellyfish | |
| Water Area | Season, Year | Species Number | * Shannon Ab (bit/Specimen) | * Shannon Bi (bit/mg) | * An average Individual Mass of a Specimen (mg) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | |||
| Northeastern Caspian | spring 2010 | 10.5 ± 0.5 | 1.95 ± 0.12 | no jellyfish | 2.20 ± 0.09 | no jellyfish | 0.0070 ± 0.0013 | no jellyfish |
| summer 2010 | 10.8 ± 0.4 | 1.55 ± 0.13 | 1.56 ± 0.13 | 1.54 ± 0.19 | 1.32 ± 0.20 | 0.0043 ± 0.0011 | 0.0399 ± 0.0101 | |
| Northern Caspian | spring 2008 | 14.2 ± 0.98 | 1.85 ± 0.17 | 1.96 ± 0.16 | 2.35 ± 0.15 | 2.19 ± 0.18 | 0.0048 ± 0.0008 | 0.0071 ± 0.0021 |
| summer 2008 | 10.4 ± 1.3 | 1.74 ± 0.18 | 1.81 ± 0.18 | 1.67 ± 0.20 | 0.63 ± 0.23 | 0.0046 ± 0.0007 | 0.1980 ± 0.0700 | |
| Middle Caspian | spring 2008 | 8.3 ± 0.6 | 1.60 ± 0.19 | no jellyfish | 1.81 ± 0.16 | no jellyfish | 0.0122 ± 0.0017 | no jellyfish |
| summer 2008 | 4.4 ± 0.3 | 0.44 ± 0.12 | no jellyfish | 0.51 ± 0.09 | no jellyfish | 0.0099 ± 0.0001 | no jellyfish | |
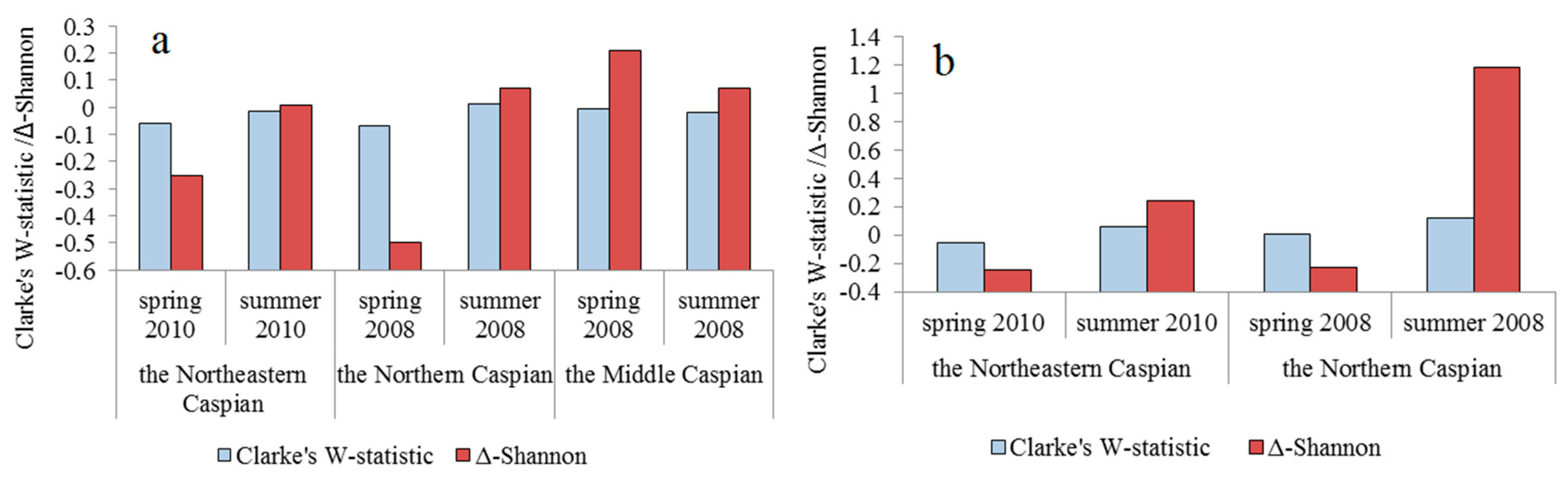
| Water Area | Season, Year | Clarke’s W-Statistics | Δ-Shannon | ||
|---|---|---|---|---|---|
| without Jellyfish | with Jellyfish | without Jellyfish | with Jellyfish | ||
| Northeastern Caspian | spring 2010 | −0.050 ± 0.033 | no jellyfish | −0.25 ± 0.15 | no jellyfish |
| summer 2010 | −0.017 ± 0.021 | 0.012 ± 0.008 | −0.01 ± 0.02 | −0.24 ± 0.22 | |
| Northern Caspian | spring 2008 | −0.068 ± 0.029 | −0.038 ± 0.030 | −0.42 ± 0.18 | −0.24 ± 0.20 |
| summer 2008 | 0.016 ± 0.027 | 0.178 ± 0.050 | 0.07 ± 0.11 | 1.18 ± 0.28 | |
| Middle Caspian | spring 2008 | −0.004 ± 0.005 | no jellyfish | −0.21 ± 0.14 | no jellyfish |
| summer 2008 | −0.019 ± 0.015 | no jellyfish | −0.08 ± 0.07 | no jellyfish | |
| Variables | * Northeastern Caspian (t-critical 2.14) | * Northern Caspian (t-critical 2.16) | * Middle Caspian (t-critical 2.16) | ||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | |
| An average individual mass of a specimen, mg | −2.71 | 0.22 | −3.36 | 1.23 | 1.30 |
| Shannon Ab, bit/specimen | 0.58 | 12.30 | 1.79 | 1.83 | 4.67 |
| Shannon Bi, bit/mg | 4.71 | 12.17 | 4.11 | 2.79 | 6.39 |
| Δ-Shannon | 3.80 | 1.40 | −0.03 | −1.25 | −0.53 |
| Clarke’s W-statistics | −3.00 | −1.00 | −1.30 | −0.44 | −0.40 |
| Abundance, 103 specimen/m3 | 2.13 | 2.13 | 2.44 | 2.44 | 0.92 |
| Biomass, mg/m3 | −1.66 | 2.51 | −1.76 | 3.20 | 2.19 |
| Water Area | Season, Year | Paired Variables | Spearman’s Rank Correlation Coefficients | |
|---|---|---|---|---|
| without Jellyfish | with Jellyfish | |||
| Northeastern Caspian | spring 2010 | Clarke’s W-statistics—Δ-Shannon | 0.882 | no jellyfish |
| Clarke’s W-statistics—m * | 0.72 | |||
| Clarke’s W-statistics—Shannon Bi | −0.779 | |||
| Shannon Bi—m | − | |||
| summer 2010 | Clarke’s W-statistics—Δ-Shannon | 0.964 | 0.979 | |
| Clarke’s W-statistics—m | − | 0.732 | ||
| Clarke’s W-statistics—Shannon Bi | −0.780 | −0.946 | ||
| Shannon Bi—m | −0.571 | −0.846 | ||
| Northern Caspian | spring 2008 | Clarke’s W-statistics—Δ-Shannon | 0.864 | 0.9 |
| Clarke’s W-statistics—m | 0.7 | 0.645 | ||
| Clarke’s W-statistics—Shannon Bi | −0.691 | −0.718 | ||
| Shannon Bi—m | −0.700 | −0.745 | ||
| summer 2008 | Clarke’s W-statistics—Δ-Shannon | 0.955 | 0.827 | |
| Clarke’s W-statistics—m | − | 0.772 | ||
| Clarke’s W-statistics—Shannon Bi | − | −0.782 | ||
| Shannon Bi—m | − | −0.964 | ||
| Middle Caspian | spring 2008 | Clarke’s W-statistics—Δ-Shannon | 0.951 | no jellyfish |
| Clarke’s W-statistics—m | 0.797 | |||
| Clarke’s W-statistics—Shannon Bi | − | |||
| Shannon Bi—m | − | |||
| summer 2008 | Clarke’s W-statistics—Δ-Shannon | 0.954 | no jellyfish | |
| Clarke’s W-statistics—m | − | |||
| Clarke’s W-statistics—Shannon Bi | − | |||
| Shannon Bi—m | − | |||
| Water Area | Season, Year | Content of Nutrients, mg/dm3 | ||||
|---|---|---|---|---|---|---|
| NH4+ | NO2− | NO3− | Total of Nitrogen | PO4- | ||
| Northern Caspian | spring 2008 | 0.113 | 0.0016 | 0.135 | 0.250 | 0.004 |
| summer 2008 | 0.091 | 0.0032 | 0.125 | 0.219 | 0.004 | |
| Middle Caspian | spring 2008 | 0.091 | 0.0015 | 0.127 | 0.220 | 0.002 |
| summer 2008 | 0.127 | 0.0019 | 0.128 | 0.257 | 0.004 | |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, E. Assessment of Changes in the Structure of Zooplankton Communities to Infer Water Quality of the Caspian Sea. Diversity 2019, 11, 122. https://doi.org/10.3390/d11080122
Krupa E. Assessment of Changes in the Structure of Zooplankton Communities to Infer Water Quality of the Caspian Sea. Diversity. 2019; 11(8):122. https://doi.org/10.3390/d11080122
Chicago/Turabian StyleKrupa, Elena. 2019. "Assessment of Changes in the Structure of Zooplankton Communities to Infer Water Quality of the Caspian Sea" Diversity 11, no. 8: 122. https://doi.org/10.3390/d11080122
APA StyleKrupa, E. (2019). Assessment of Changes in the Structure of Zooplankton Communities to Infer Water Quality of the Caspian Sea. Diversity, 11(8), 122. https://doi.org/10.3390/d11080122




