Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analyses
3. Results
3.1. Decapod Species Composition
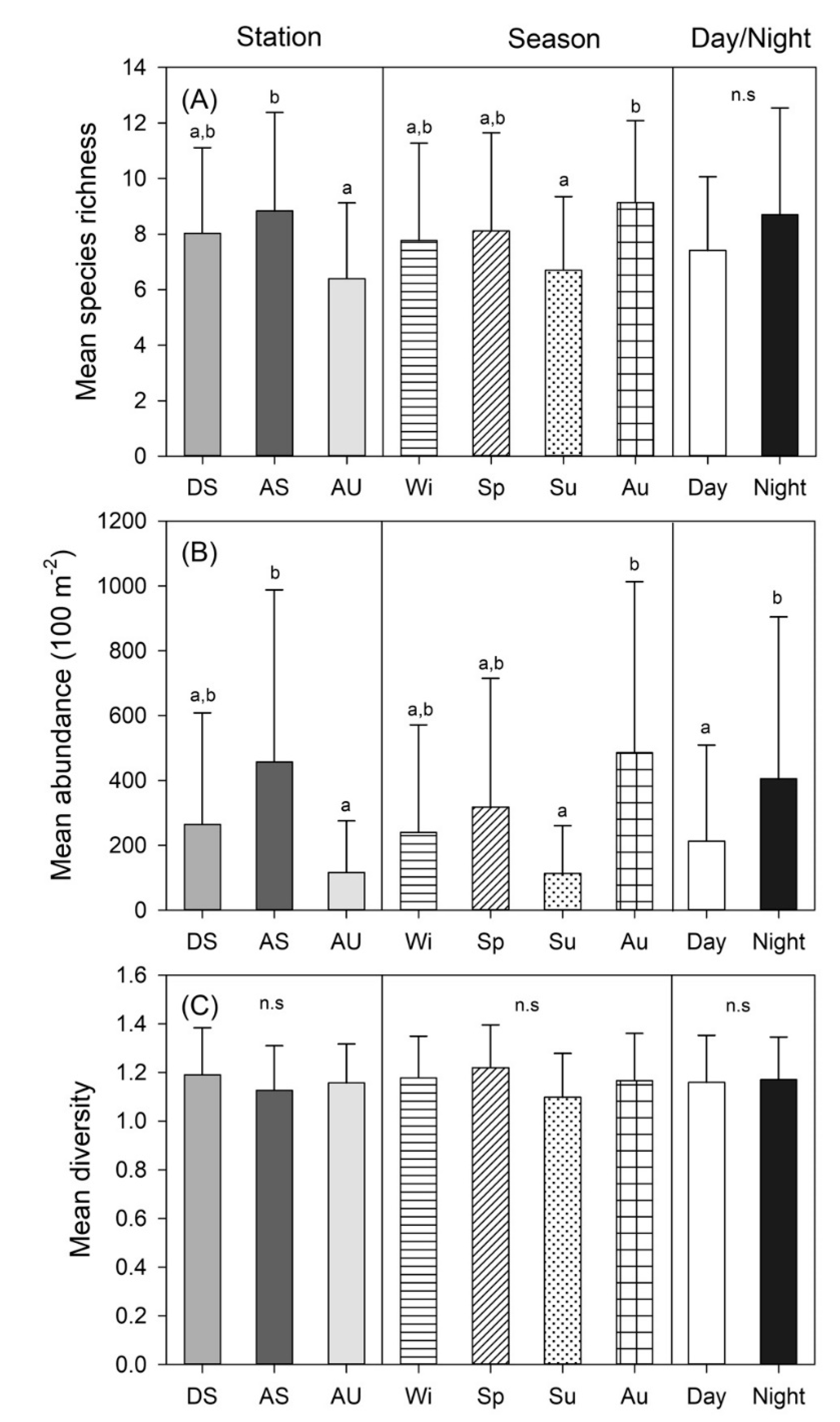
3.2. Spatio-temporal Changes in Species Richness, Abundance, and Diversity
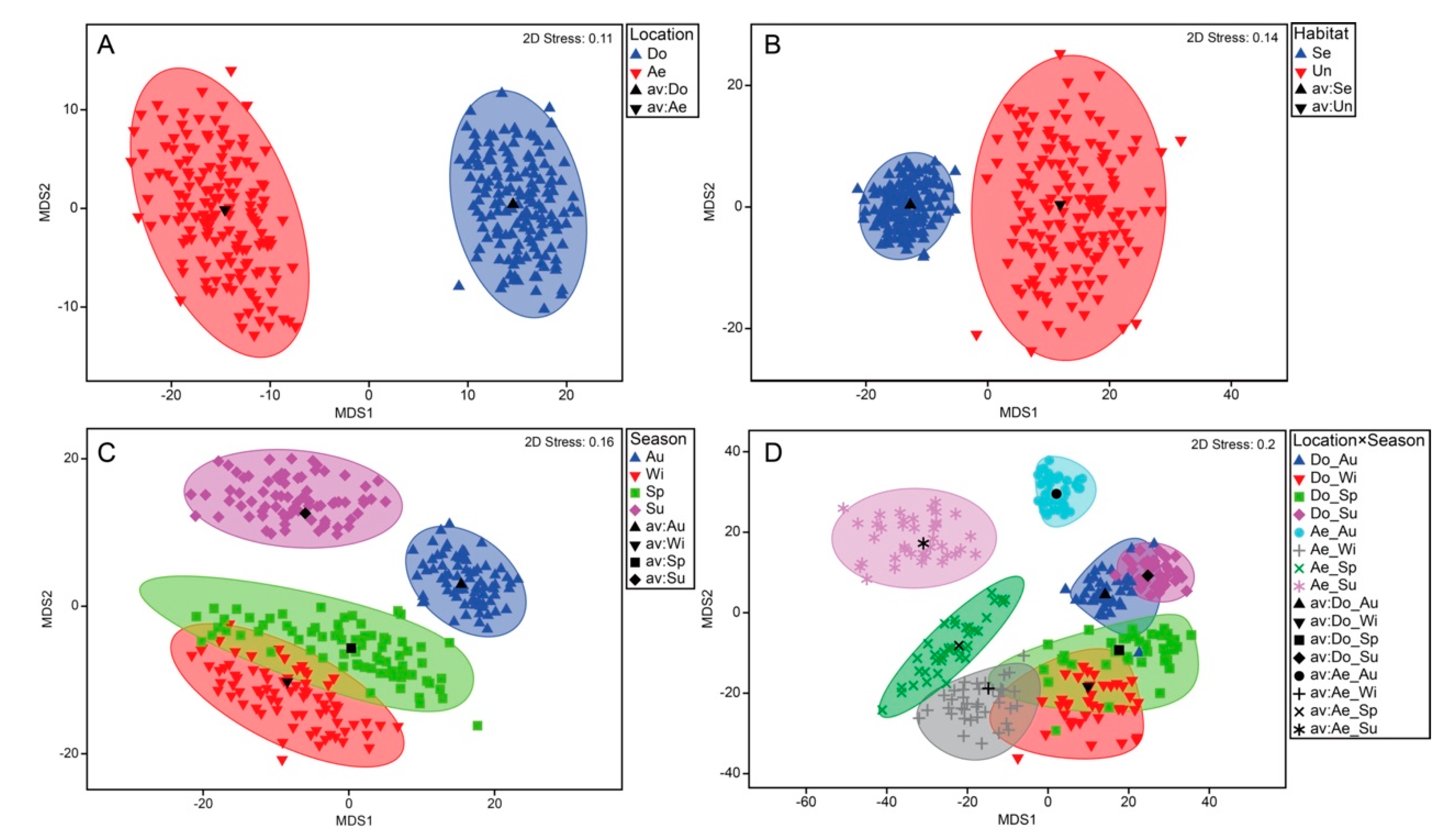
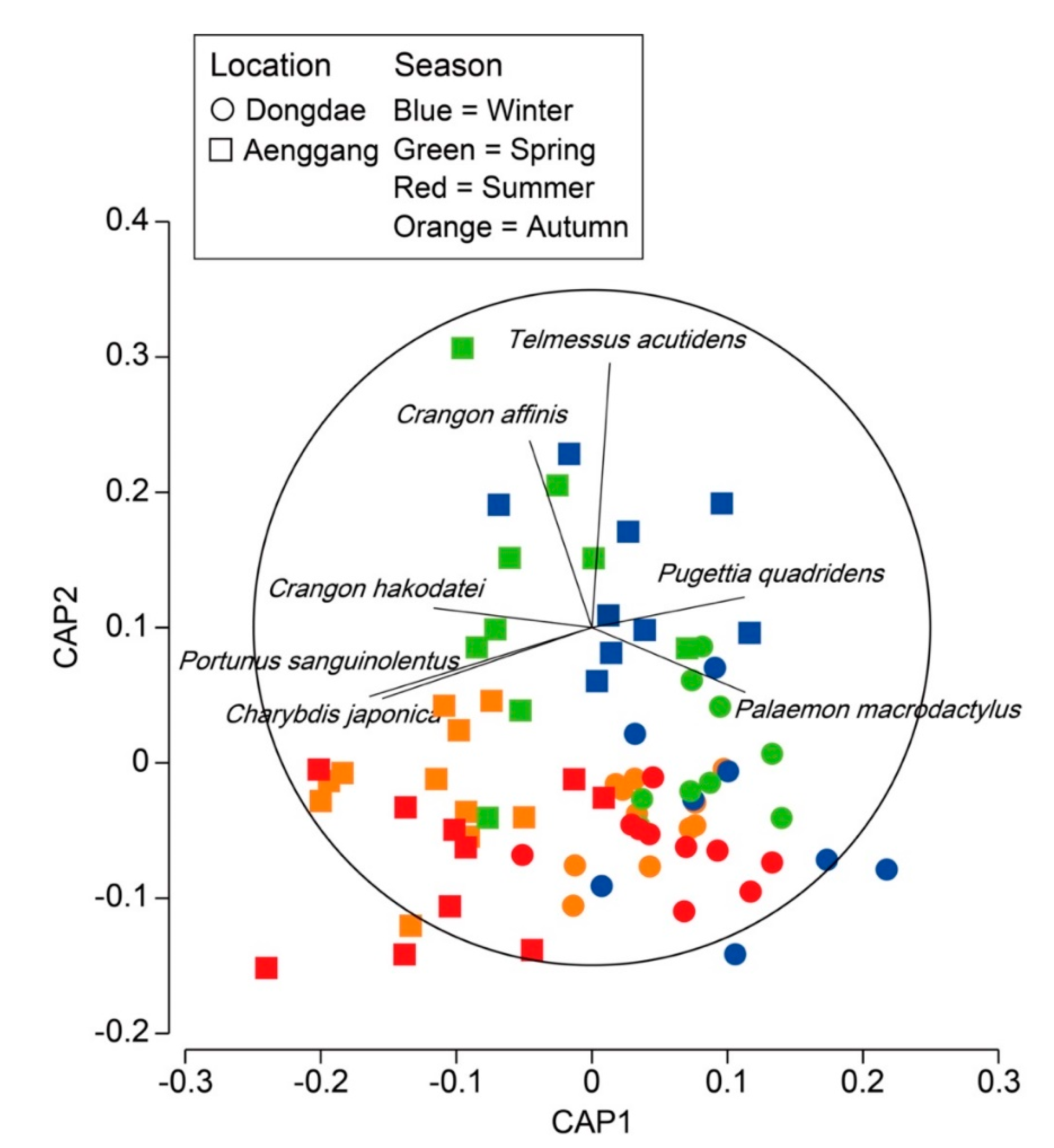
3.3. Decapod Assemblage Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thayer, G.; Wolfe, D.; Williams, R. The Impact of Man on Seagrass Systems: Seagrasses must be considered in terms of their interaction with the other sources of primary production that support the estuarine trophic structure before their significance can be fully appreciated. Am. Sci. 1975, 63, 288–296. [Google Scholar]
- Duarte, C.M.; Marbà, N.; Gacia, E.; Fourqurean, J.W.; Beggins, J.; Barrón, C.; Apostolaki, E.T. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochem. Cy. 2010, 24, GB4032. [Google Scholar] [CrossRef]
- Klumpp, D.; Howard, R.K.; Pollard, D. Trophodynamics and nutritional ecology of seagrass communities. In Biology of Seagrasses; Larkum, A., McComb, A., Shepherd, S., Eds.; Elsevier Science Pub.: Amsterdam, The Netherlands, 1989; pp. 394–437. [Google Scholar]
- Huh, S.-H.; AN, Y.-R. Seasonal variation of shrimp (Crustacea: Decapoda) community in the eelgrass (Zostera marina) bed in Kwangyang Bay, Korea. Korean J. Fish. Aquat. Sci. 1997, 30, 532–542. [Google Scholar]
- Connolly, R.; Jenkins, G.; Loneragan, N. Seagrass Dynamics and Fisheries Sustainability; CSIRO Publishing: Clayton, VIC, Australia, 1999. [Google Scholar]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Blaber, S.; Brewer, D.; Salini, J. Fish communities and the nursery role of the shallow inshore waters of a tropical bay in the Gulf of Carpentaria, Australia. Estuar. Coast. Shelf Sci. 1995, 40, 177–193. [Google Scholar] [CrossRef]
- Beyst, B.; Hostens, K.; Mees, J. Factors influencing the spatial variation in fish and macrocrustacean communities in the surf zone of sandy beaches in Belgium. J. Mar. Biolog. Assoc. U.K. 2002, 82, 181–187. [Google Scholar] [CrossRef][Green Version]
- Nagelkerken, I.; Roberts, C.M.; Van der Velde, G.; Dorenbosch, M.; Van Riel, M.C.; Cocheret de la Morinière, E.; Nienhuis, P.H. How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Mar. Ecol. Prog. Ser. 2002, 244, 299–305. [Google Scholar] [CrossRef]
- Orth, R.J.; Heck, K.L.; van Montfrans, J. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 1984, 7, 339–350. [Google Scholar] [CrossRef]
- Edgar, G.J.; Shaw, C. The production and trophic ecology of shallow-water fish assemblages in southern Australia, I. Species richness, size-structure and production of fishes in Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 1995, 194, 53–81. [Google Scholar] [CrossRef]
- Guidetti, P.; Bussotti, S. Fish fauna of a mixed meadow composed by the seagrasses Cymodocea nodosa and Zostera noltii in the Western Mediterranean. Oceanol. Acta 2000, 23, 759–770. [Google Scholar] [CrossRef]
- Nelson, W. The role of predation by decapod crustaceans in seagrass ecosystems. Kiel. Meeresforsch 1981, 5, 529–536. [Google Scholar]
- Polte, P.; Asmus, H. Influence of seagrass beds (Zostera noltii) on the species composition of juvenile fishes temporarily visiting the intertidal zone of the Wadden Sea. J. Sea Res. 2006, 55, 244–252. [Google Scholar] [CrossRef]
- Edgar, G.J. The influence of plant structure on the species richness, biomass and secondary production of macrofaunal assemblages associated with Western Australian seagrass beds. J. Exp. Mar. Biol. Ecol. 1990, 137, 215–240. [Google Scholar] [CrossRef]
- Orth, R.J. The Importance of Sediment Stability in Seagrass Communities. In Ecology of Marine Benthos; Coull, B., Ed.; University of South Carolina Press: Columbia, SC, USA, 1977; pp. 281–300. [Google Scholar]
- Wells, F.; Rose, R.; Lang, S. An analysis of benthic marine invertebrate communities in subtidal seagrass and sand habitats in Shark Bay, Western Australia. Rec. West. Aust. Mus. 1985, 12, 47–56. [Google Scholar]
- Boström, C.; Mattila, J. The relative importance of food and shelter for seagrass-associated invertebrates: A latitudinal comparison of habitat choice by isopod grazers. Oecologia 1999, 120, 162–170. [Google Scholar] [CrossRef]
- Heck, K., Jr.; Wetstone, G. Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J. Biogeogr. 1977, 4, 135–142. [Google Scholar] [CrossRef]
- Attrill, M.J.; Strong, J.A.; Rowden, A.A. Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 2000, 23, 114–121. [Google Scholar] [CrossRef]
- Mateo-Ramírez, Á.; Urra, J.; Marina, P.; Rueda, J.L.; García Raso, J.E. Crustacean decapod assemblages associated with fragmented Posidonia oceanica meadows in the Alboran Sea (Western Mediterranean Sea): Composition, temporal dynamics and influence of meadow structure. Mar. Ecol. 2016, 37, 344–358. [Google Scholar] [CrossRef]
- Mateo Ramírez, Á.; García Raso, J.E. Temporal changes in the structure of the crustacean decapod assemblages associated with Cymodocea nodosa meadows from the Alboran Sea (Western Mediterranean Sea). Mar. Ecol. 2012, 33, 302–316. [Google Scholar] [CrossRef]
- Mateo-Ramírez, Á.; Urra, J.; Rueda, J.; Marina, P.; Raso, J.G. Decapod assemblages associated with shallow macroalgal communities in the northwestern Alboran Sea: Microhabitat use and temporal variability. J. Sea Res. 2018, 135, 84–94. [Google Scholar] [CrossRef]
- Ávila, E.; Yáñez, B.; Vazquez-Maldonado, L.E. Influence of habitat structure and environmental regime on spatial distribution patterns of macroinvertebrate assemblages associated with seagrass beds in a southern Gulf of Mexico coastal lagoon. Mar. Biol. Res. 2015, 11, 755–764. [Google Scholar] [CrossRef]
- Kwak, S.N.; Park, J.M.; Im, S.O.; Jawad, L.A. Influences of diel and tidal cycles on fish assemblage in eelgrass (Zostera marina) bed of southern Korea during autumn. Acta Oceanol. Sin. 2018, 37, 40–47. [Google Scholar] [CrossRef]
- Unsworth, R.K.; Collier, C.J.; Henderson, G.M.; McKenzie, L.J. Tropical seagrass meadows modify seawater carbon chemistry: Implications for coral reefs impacted by ocean acidification. Environ. Res. Lett. 2012, 7, 024026. [Google Scholar] [CrossRef]
- Heck, K.L., Jr.; Orth, R.J. Seagrass habitats: The roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In Estuarine Perspectives; Kennedy, V.S., Ed.; Academic Press: New York, NY, USA, 1980; pp. 449–464. [Google Scholar]
- Box, A.; Martin, D.; Deudero, S. Changes in seagrass polychaete assemblages after invasion by Caulerpa racemosa var. cylindracea (Chlorophyta: Caulerpales): Community structure, trophic guilds and taxonomic distinctness. Sci. Mar. 2010, 74, 317–329. [Google Scholar] [CrossRef]
- Hindell, J.S.; Jenkins, G.P.; Keough, M.J. Evaluating the impact of predation by fish on the assemblage structure of fishes associated with seagrass (Heterozostera tasmanica) (Martens ex Ascherson) den Hartog, and unvegetated sand habitats. J. Exp. Mar. Biol. Ecol. 2000, 255, 153–174. [Google Scholar] [CrossRef]
- Huston, M.A. Biological Diversity: The Coexistence of Species; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Blanchet, H.; de Montaudouin, X.; Lucas, A.; Chardy, P. Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: Diversity, abundance, biomass and structuring factors. Estuar. Coast. Shelf Sci. 2004, 61, 111–123. [Google Scholar] [CrossRef]
- Gratwicke, B.; Speight, M. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish. Biol. 2005, 66, 650–667. [Google Scholar] [CrossRef]
- Hull, S.L. Seasonal changes in diversity and abundance of ostracods on four species of intertidal algae with differing structural complexity. Mar. Ecol. Prog. Ser. 1997, 161, 71–82. [Google Scholar] [CrossRef]
- May, R.M. Will a large complex system be stable? Nature 1972, 238, 413–414. [Google Scholar] [CrossRef]
- Bloomfield, A.; Gillanders, B. Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 2005, 28, 63–77. [Google Scholar] [CrossRef]
- Park, J.M.; Kwak, S.N. Seasonal and habitat structures of crustacean decapod assemblages associated with Zostera marina beds in northern Jinhae Bay, Korea. J. Mar. Biol. Assoc. U.K. 2019, 99, 461–471. [Google Scholar] [CrossRef]
- Jelbart, J.E.; Ross, P.M.; Connolly, R.M. Fish assemblages in seagrass beds are influenced by the proximity of mangrove forests. Mar. Biol. 2007, 150, 993–1002. [Google Scholar] [CrossRef]
- Mazumder, D.; Saintilan, N.; Williams, R.J. Fish assemblages in three tidal saltmarsh and mangrove flats in temperate NSW, Australia: A comparison based on species diversity and abundance. Wetl. Ecol. Manag. 2006, 14, 201–209. [Google Scholar] [CrossRef]
- Lazzari, M.A. Epibenthic fishes and decapod crustaceans in northern estuaries: A comparison of vegetated and unvegetated habitats in Maine. Estuaries 2002, 25, 1210–1218. [Google Scholar] [CrossRef]
- Krumme, U. Diel and tidal movements by fish and decapods linking tropical coastal ecosystems. In Ecological Connectivity among Tropical Coastal Ecosystems; Negelkerken, I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 271–324. [Google Scholar]
- Gray, C.; Chick, R.; McElligott, D. Diel changes in assemblages of fishes associated with shallow seagrass and bare sand. Estuarine Coast. Shelf Sci. 1998, 46, 849–859. [Google Scholar] [CrossRef]
- Bauer, R.T. Diel and seasonal variation in species composition and abundance of caridean shrimps (Crustacea, Decapoda) from seagrass meadows on the north coast of Puerto Rico. Bull. Mar. Sci. 1985, 36, 150–162. [Google Scholar]
- Huh, S.H.; Kwak, S.N. Species composition and seasonal variations of fishes in eelgrass (Zostera marina) bed in Kwangyang Bay. Korean J. Ichthyol. 1997, 9, 202–220. [Google Scholar]
- Yun, S.G.; Huh, S.H.; Kwak, S.N. Species composition and seasonal variations of benthic macrofauna in eelgrass, Zostera marina, bed. Korean J. Fish. Aquat. Sci. 1997, 30, 744–752. [Google Scholar]
- Park, J.M.; Kwak, S.N. Seagrass fish assemblages in the Namhae Island, Korea: The influences of seagrass vegetation and biomass. J. Sea Res. 2018, 139, 41–49. [Google Scholar] [CrossRef]
- Bell, J.D.; Westoby, M. Importance of local changes in leaf height and density to fish and decapods associated with seagrasses. J. Exp. Mar. Biol. Ecol. 1986, 104, 249–274. [Google Scholar] [CrossRef]
- Heck, K.; Able, K.; Fahay, M.; Roman, C. Fishes and decapod crustaceans of Cape Cod eelgrass meadows: Species composition, seasonal abundance patterns and comparison with unvegetated substrates. Estuaries 1989, 12, 59–65. [Google Scholar] [CrossRef]
- Worthington, D.G.; Ferrell, D.J.; McNeill, S.E.; Bell, J.D. Effects of the shoot density of seagrass on fish and decapods: Are correlation evident over larger spatial scales? Mar. Biol. 1992, 112, 139–146. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Barbera-Cebrian, C.; Ramos-Espla, A.A. Influence of the structure of Posidonia oceanica meadows modified by bottom trawling on crustacean assemblages: Comparison of amphipods and decapods. Sci. Mar. 2000, 64, 319–326. [Google Scholar] [CrossRef][Green Version]
- Kwak, S.N.; Klumpp, D.W. Temporal variation in species composition and abundance of fish and decapods of a tropical seagrass bed in Cockle Bay, North Queensland, Australia. Aquat. Bot. 2004, 78, 119–134. [Google Scholar] [CrossRef]
- Kwak, S.N.; Huh, S.H.; Kim, H.W. Change in fish assemblage inhabiting around Dae Island in Gwangyang Bay, Korea. J. Korean Soc. Mar. Environ. Saf. 2012, 18, 175–184. [Google Scholar] [CrossRef]
- De La Rosa, I.L.; Rodríguez, A.; Raso, J.E.G. Seasonal variation and structure of a decapod (Crustacea) assemblage living in a Caulerpa prolifera meadow in Cadiz Bay (SW Spain). Estuar. Coast. Shelf Sci. 2006, 66, 624–633. [Google Scholar] [CrossRef]
- Horton, T.; Kroh, A.; Ahyong, S.; Bailly, N.; Boyko, C.B.; Brandão, S.N.; Gofas, S.; Hooper, J.N.A.; Hernandez, F.; Holovachov, O.; et al. World Register of Marine Species (WoRMS); WoRMS Editorial Board: Ostend, Belgium, 2020. [Google Scholar]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; Illinois University Press: Urbana, IL, USA, 1949. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman: New York, NY, USA, 1981. [Google Scholar]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Plymouth Marine Laboratory: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Huh, S.-H.; An, Y.-R. Seasonal variation of crab (Crustacea: Decapoda) community in the eelgrass (Zostera marina) bed in Kwangyang Bay, Korea. Korean J. Fish. Aquat. Sci. 1998, 31, 535–544. [Google Scholar]
- Howard, R.K. The trophic ecology of caridean shrimps in an eelgrass community. Aquat. Bot. 1984, 18, 155–174. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, D.; Zhang, P.D.; Zhang, X.M.; Li, W.T.; Wu, Z.X. Seasonal variation in species composition and abundance of demersal fish and invertebrates in a Seagrass Natural Reserve on the eastern coast of the Shandong Peninsula, China. Chin. J. Oceanol. Limnol. 2016, 34, 330–341. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawamura, T.; Yamashita, Y. Introduction: The coastal ecosystem complex as a unit of structure and function of biological productivity in coastal areas. Fish. Sci. 2018, 84, 149–152. [Google Scholar] [CrossRef]
- Bell, J. Ecology of fish assemblages and fisheries associated with seagrasses. Biol. Seagrasses 1989, 536–564. [Google Scholar]
- Guidetti, P. Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky–algal reefs and unvegetated sand habitats in the Adriatic Sea. Estuar. Coast. Shelf Sci. 2000, 50, 515–529. [Google Scholar] [CrossRef]
- Klumpp, D.W.; Kwak, S.N. Composition and abundance of benthic macrofauna of a tropical sea-grass bed in north Queensland, Australia. Pac. Sci. 2005, 59, 541–561. [Google Scholar] [CrossRef]
- Stoner, A. The role of seagrass biomass in the organization of benthic macrofaunal assemblages. Bull. Mar. Sci. 1980, 30, 537–551. [Google Scholar]
- Hori, M.; Suzuki, T.; Monthum, Y.; Srisombat, T.; Tanaka, Y.; Nakaoka, M.; Mukai, H. High seagrass diversity and canopy-height increase associated fish diversity and abundance. Mar. Biol. 2009, 156, 1447–1458. [Google Scholar] [CrossRef]
- Moranta, J.; Palmer, M.; Morey, G.; Ruiz, A.; Morales-Nin, B. Multi-scale spatial variability in fish assemblages associated with Posidonia oceanica meadows in the Western Mediterranean Sea. Estuar. Coast. Shelf Sci. 2006, 68, 579–592. [Google Scholar] [CrossRef]
- De La Rosa, I.L.; Raso, J.G.; Rodríguez, A. Evolution of a decapod community (Crustacea) of shallow soft bottoms with seaweeds from southern Europe. J. Mar. Biol. Assoc. U.K. 2002, 82, 85–95. [Google Scholar] [CrossRef]
- Pinn, E.H.; Ansell, A.D. The effect of particle size on the burying ability of the brown shrimp Crangon crangon. J. Mar. Biol. Assoc. U.K. 1993, 73, 365–377. [Google Scholar] [CrossRef]
- Ouellette, C.; Boghen, A.D.; Courtenay, S.C.; St-Hilaire, A. Influence of peat substrate on the distribution and behaviour patterns of sand shrimp, Crangon septemspinosa, under experimental conditions. J. Appl. Ichthyol. 2003, 19, 359–365. [Google Scholar] [CrossRef]
- Berglund, A. Niche differentiation between two littoral prawns in Gullmar Fjord, Sweden: Palaemon adspersus and P. squilla. Ecography 1980, 3, 111–115. [Google Scholar] [CrossRef]
- Baden, S.P.; Pihl, L. Abundance, biomass and production of mobile epibenthic fauna in Zostera marina (L.) meadows, western Sweden. Ophelia 1984, 23, 65–90. [Google Scholar] [CrossRef]
- Berglund, A.; Bengtsson, J. Biotic and abiotic factors determining the distribution of two prawn species: Palaemon adspersus and P. squilla. Oecologia 1981, 49, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Morton, B. The temperature tolerances of Tetraclita squamosa (Crustacea: Cirripedia) and Septifer virgatus (Bivalvia: Mytilidae) on a sub-tropical rocky shore in Hong Kong. J. Zool. 1994, 234, 325–339. [Google Scholar] [CrossRef]
- Matheson, R.E.; Camp, D.K.; Sogard, S.M.; Bjorgo, K.A. Changes in seagrass-associated fish and crustacean communities on Florida Bay mud banks: The effects of recent ecosystem changes? Estuaries 1999, 22, 534. [Google Scholar] [CrossRef]
- Yu, C.; Song, H.; Yao, G. The quantity distribution and biological property of Charybdis japonica in the East China Sea. J.Shanghai Fish. Univ. 2005, 14, 40–45. [Google Scholar]
- Rasheed, S.; Mustaquim, J. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fish. Res. 2010, 103, 56–62. [Google Scholar] [CrossRef]
- McCarthy, L.C.; Loftus, W.F.; Rehage, J.S. Segregation of palaemonid shrimp along an Everglades estuarine gradient: Do multiple species have similar trophic function? Bull. Mar. Sci. 2012, 88, 843–861. [Google Scholar] [CrossRef]
- Hamrin, S.F. Vertical distribution and habitat partitioning between different size classes of vendace, Coregonus albula, in thermally stratified lakes. Can. J. Fish. Aquat. Sci. 1986, 43, 1617–1625. [Google Scholar] [CrossRef]
- Rountree, R.A.; Able, K.W. Diel variation in decapod crustacean and fish assemblages in New Jersey polyhaline marsh creeks. Estuar. Coast. Shelf Sci. 1993, 37, 181–201. [Google Scholar] [CrossRef]
- Guest, M.A.; Connolly, R.M.; Loneragan, N.R. Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat. Fish. Res. 2003, 64, 185–196. [Google Scholar] [CrossRef]
- Hampel, H.; Cattrijsse, A.; Vincx, M. Tidal, diel and semi-lunar changes in the faunal assemblage of an intertidal salt marsh creek. Estuar. Coast. Shelf Sci. 2003, 56, 795–805. [Google Scholar] [CrossRef]
- Dall, W.; Hill, B.; Rothlisberg, P.; Sharples, D. The Biology of the Penaeidae; Academic Press: San Diego, CA, USA, 1990; Volume 27, pp. 1–489. [Google Scholar]
- Breckenridge, J.K.; Bollens, S.M. Vertical distribution and migration of decapod larvae in relation to light and tides in Willapa Bay, Washington. Estuar. Coas. 2011, 34, 1255–1261. [Google Scholar] [CrossRef]
- Robertson, A.I.; Howard, R.K. Diel trophic interactions between vertically-migrating zooplankton and their fish predators in an eelgrass community. Mar. Biol. 1978, 48, 207–213. [Google Scholar] [CrossRef]
- Robertson, A.; Klumpp, D. Feeding habits of the southern Australian garfish Hyporhamphus melanochir: A diurnal herbivore and nocturnal carnivore. Mar. Ecol. Progr. Ser. 1983, 10, 197–201. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Wylie, E.; Smith, D.J.; Bell, J.J. Diel trophic structuring of seagrass bed fish assemblages in the Wakatobi Marine National Park, Indonesia. Estuar. Coast. Shelf Sci. 2007, 72, 81–88. [Google Scholar] [CrossRef]

| Dongdae Bay | Aenggang Bay | ||||
|---|---|---|---|---|---|
| Taxa | Species Name | DS(t) | DS(r) | AS | AU |
| Penaeoidea | Metapenaeopsis tenella | 3.3 | 3.3 | 3.9 | 1.1 |
| Mierspenaeopsis hardwickii | 0.6 | ||||
| Penaeus japonicus | 0.6 | ||||
| Trachysalambria curvirostris | 3.9 | 0.6 | |||
| Sergestoidea | Acetes chinensis | 5.6 | 1.7 | 3.9 | |
| Acetes japonicus | 0.6 | 0.6 | |||
| Caridea | Alpheus brevicristatus | 2.2 | 0.6 | 1.1 | |
| Alpheus digitalis | 0.6 | 1.1 | |||
| Crangon affinis | 1.7 | 2.2 | 76.7 | 92.8 | |
| Crangon hakodatei | 0.6 | 24.4 | 14.4 | ||
| Eualus leptognathus | 1380.6 | 544.4 | 347.2 | 9.4 | |
| Eualus middendorffi | 3.9 | 1.7 | |||
| Hayashidonus japonicus | 7.8 | 3.3 | |||
| Heptacarpus futilirostris | 61.7 | 0.6 | 16.7 | 1.1 | |
| Heptacarpus pandaloides | 2462.2 | 925.0 | 5067.2 | 882.2 | |
| Heptacarpus rectirostris | 17.8 | 7.2 | 108.9 | 0.6 | |
| Latreutes anoplonyx | 334.4 | 578.3 | 2220.6 | 106.1 | |
| Latreutes laminirostris | 415.6 | 185.6 | 123.9 | 52.8 | |
| Latreutes planirostris | 0.6 | 3.3 | |||
| Leptochela gracilis | 0.6 | ||||
| Lysmata vittata | 0.6 | 0.6 | |||
| Palaemon carinicauda | 1.1 | 1.7 | |||
| Palaemon macrodactylus | 224.4 | 46.7 | 4.4 | 5.6 | |
| Palaemon orientis | 18.9 | 3.9 | |||
| Palaemon ortmanni | 92.8 | 30.6 | 40.0 | 8.3 | |
| Brachyura | Arcania undecimspinosa | 0.6 | |||
| Charybdis (Charybdis) japonica | 5.0 | 8.3 | 94.4 | 20.0 | |
| Charybdis (Charybdis) sagamiensis | 3.3 | ||||
| Hemigrapsus penicillatus | 20.0 | 11.1 | 6.1 | 0.6 | |
| Hemigrapsus sanguineus | 0.6 | ||||
| Paradorippe granulata | 0.6 | ||||
| Pilumnus minutus | 0.6 | ||||
| Portunus sanguinolentus | 5.0 | 28.9 | 2.8 | ||
| Portunus trituberculatus | 1.7 | 1.1 | |||
| Pugettia quadridens | 33.3 | 26.1 | 23.3 | 1.7 | |
| Telmessus acutidens | 11.1 | 1.1 | 35.0 | 2.8 | |
| Thalamita sima | 8.3 | 1.1 | |||
| Xanthidae sp. | 0.6 | ||||
| Total | 5106.7 | 2380.0 | 8252.8 | 1213.9 | |
| Number of species | 25 | 21 | 30 | 23 | |
| Species Richness | Abundance | Diversity | |||||
|---|---|---|---|---|---|---|---|
| Source | df | F | p | F | p | F | p |
| Main test | |||||||
| Station (St) | 2 | 3.373 | 0.030 | 5.930 | 0.005 | 1.269 | 0.289 |
| Season (Se) | 3 | 3.035 | 0.037 | 7.318 | 0.001 | 0.385 | 0.764 |
| Day/Night (D/N) | 1 | 0.004 | 0.953 | 4.513 | 0.039 | 0.266 | 0.608 |
| Interactions | |||||||
| St × Se | 6 | 1.085 | 0.383 | 2.245 | 0.052 | 1.178 | 0.117 |
| St × D/N | 2 | 2.319 | 0.108 | 5.186 | 0.009 | 1.132 | 0.330 |
| Se × D/N | 3 | 0.885 | 0.455 | 1.228 | 0.309 | 0.221 | 0.882 |
| St × Se × D/N | 5 | 0.298 | 0.912 | 1.212 | 0.316 | 0.470 | 0.797 |
| Source | df | MS | Pseudo-F | p | COV |
|---|---|---|---|---|---|
| Main test | |||||
| Lo | 1 | 15294.0 | 9.538 | 0.001 | 21.269 |
| Ha (Lo) | 1 | 3747.8 | 2.337 | 0.031 | 12.847 |
| Se | 3 | 6363.6 | 3.969 | 0.001 | 16.936 |
| D/N | 1 | 2968.8 | 1.851 | 0.086 | 6.761 |
| Interactions | |||||
| Lo × Se | 3 | 4450.4 | 2.775 | 0.001 | 18.522 |
| Lo × D/N | 1 | 3102.2 | 1.935 | 0.074 | 11.045 |
| Ha (Lo) × Se | 3 | 2404.0 | 1.499 | 0.073 | 14.443 |
| Ha (Lo) × D/N | 1 | 2666.6 | 1.663 | 0.114 | 12.936 |
| Se × D/N | 3 | 2618.4 | 1.633 | 0.065 | 10.017 |
| Lo × Se × D/N | 3 | 1568.2 | 0.978 | 0.497 | -2.915 |
| Ha (Lo) × Se × D/N | 2 | 2297.5 | 1.433 | 0.151 | 18.001 |
| Residual | 54 | 1603.5 | 40.044 |
| Winter | Spring | Summer | Autumn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | t | p | t | p | t | p | t | p | |||
| Dongdae-Aenggang | 1.509 | 0.063 | 2.296 | 0.008 | 2.5082 | 0.001 | 2.213 | 0.001 | |||
| Dongdae | Aenggang | ||||||||||
| Season | t | P | t | P | |||||||
| Winter-Spring | 1.177 | 0.221 | 1.012 | 0.374 | |||||||
| Winter-Summer | 1.648 | 0.011 | 1.764 | 0.008 | |||||||
| Winter-Autumn | 1.245 | 0.151 | 2.878 | 0.002 | |||||||
| Spring-Summer | 1.717 | 0.012 | 1.603 | 0.026 | |||||||
| Spring-Autumn | 1.214 | 0.175 | 2.793 | 0.001 | |||||||
| Summer-Autumn | 1.045 | 0.373 | 2.492 | 0.001 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.M.; Kwak, S.N.; Riedel, R. Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea. Diversity 2020, 12, 89. https://doi.org/10.3390/d12030089
Park JM, Kwak SN, Riedel R. Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea. Diversity. 2020; 12(3):89. https://doi.org/10.3390/d12030089
Chicago/Turabian StylePark, Joo Myun, Seok Nam Kwak, and Ralf Riedel. 2020. "Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea" Diversity 12, no. 3: 89. https://doi.org/10.3390/d12030089
APA StylePark, J. M., Kwak, S. N., & Riedel, R. (2020). Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea. Diversity, 12(3), 89. https://doi.org/10.3390/d12030089






