Diversity of Seagrass-Associated Decapod Crustaceans in a Tropical Reef Lagoon Prior to Large Environmental Changes: A Baseline Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Epifauna Sampling
2.3. Data Analyses
3. Results
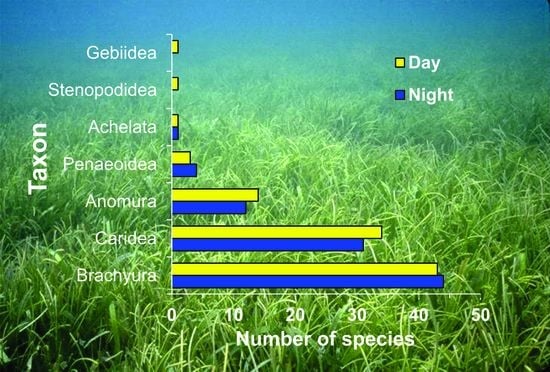
3.1. Decapod Species and Abundance
3.2. Ecological Indices
3.3. Community Composition
3.4. Changes in Density of the 10 Most Abundant Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Summer | Winter | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Species | Day | Night | Day | Night | Total | % | Cumulative % |
| 1 | Latreutes fucorum | 1998 | 5504 | 949 | 2554 | 11005 | 20.68 | 20.68 |
| 2 | Cuapetes americanus | 1209 | 4764 | 286 | 1375 | 7634 | 14.35 | 35.03 |
| 3 | Thor manningi | 519 | 4073 | 200 | 1435 | 6227 | 11.70 | 46.73 |
| 4 | Pagurus annulipes | 488 | 3893 | 507 | 767 | 5655 | 10.63 | 57.36 |
| 5 | Pagurus brevidactylus | 308 | 2509 | 400 | 1976 | 5193 | 9.76 | 67.12 |
| 6 | Clibanarius tricolor | 185 | 478 | 171 | 3807 | 4641 | 8.72 | 75.84 |
| 7 | Thor dobkini | 166 | 1671 | 49 | 314 | 2200 | 4.13 | 79.97 |
| 8 | Alpheus normanni | 15 | 911 | 8 | 1103 | 2037 | 3.83 | 83.80 |
| 9 | Tozeuma carolinense | 436 | 348 | 467 | 277 | 1528 | 2.87 | 86.67 |
| 10 | Processa bermudensis | 4 | 693 | 6 | 452 | 1155 | 2.17 | 88.84 |
| 11 | Leander tenuicornis | 5 | 82 | 33 | 537 | 657 | 1.23 | 90.08 |
| 12 | Processa fimbriata | 7 | 422 | 14 | 169 | 612 | 1.15 | 91.23 |
| 13 | Ancylomenes pedersoni | 198 | 171 | 62 | 104 | 535 | 1.01 | 92.23 |
| 14 | Sicyonia laevigata | 8 | 85 | 11 | 380 | 484 | 0.91 | 93.14 |
| 15 | Sicyonia parri | 1 | 84 | 3 | 355 | 443 | 0.83 | 93.98 |
| 16 | Trachycaris restrictus | 20 | 148 | 45 | 122 | 335 | 0.63 | 94.61 |
| 17 | Metapenaeopsis goodei | 0 | 65 | 20 | 208 | 293 | 0.55 | 95.16 |
| 18 | Portunus sp. | 2 | 74 | 19 | 135 | 230 | 0.43 | 95.59 |
| 19 | Mithraculus forceps | 80 | 42 | 31 | 27 | 180 | 0.34 | 95.93 |
| 20 | Mithraculus sculptus | 23 | 61 | 54 | 40 | 178 | 0.33 | 96.26 |
| 21 | Paguristes tortugae | 3 | 58 | 1 | 80 | 142 | 0.27 | 96.53 |
| 22 | Nikoides schmitti | 0 | 8 | 6 | 124 | 138 | 0.26 | 96.79 |
| 23 | Mithrax sp. 1 | 35 | 54 | 27 | 21 | 137 | 0.26 | 97.05 |
| 24 | Pitho aculeata | 9 | 67 | 17 | 38 | 131 | 0.25 | 97.29 |
| 25 | Panopeus occidentalis | 48 | 24 | 25 | 13 | 110 | 0.21 | 97.50 |
| 26 | Mithraculus coryphe | 3 | 5 | 69 | 23 | 100 | 0.19 | 97.69 |
| 27 | Chorinus heros | 0 | 27 | 8 | 63 | 98 | 0.18 | 97.87 |
| 28 | Latreutes parvulus | 6 | 78 | 2 | 11 | 97 | 0.18 | 98.05 |
| 29 | Achelous ordwayi | 1 | 32 | 5 | 52 | 90 | 0.17 | 98.22 |
| 30 | Pagurus sp. 1 | 0 | 43 | 10 | 29 | 82 | 0.15 | 98.38 |
| 31 | Panulirus argus | 0 | 25 | 6 | 45 | 76 | 0.14 | 98.52 |
| 32 | Calcinus tibicen | 0 | 1 | 6 | 61 | 68 | 0.13 | 98.65 |
| 33 | Pagurus sp. 2 | 0 | 26 | 16 | 24 | 66 | 0.12 | 98.77 |
| 34 | Mithrax pleuracanthus | 8 | 10 | 18 | 21 | 57 | 0.11 | 98.88 |
| 35 | Dardanus venosus | 0 | 13 | 1 | 39 | 53 | 0.10 | 98.98 |
| 36 | Podochela macrodera | 10 | 9 | 13 | 20 | 52 | 0.10 | 99.08 |
| 37 | Omalacantha bicornuta | 9 | 19 | 8 | 15 | 51 | 0.10 | 99.171 |
| 38 | Hippolyte zostericola | 18 | 5 | 6 | 1 | 30 | 0.06 | 99.228 |
| 39 | Clibanarius sp. | 2 | 6 | 1 | 21 | 30 | 0.06 | 99.284 |
| 40 | Paguristes puncticeps | 1 | 5 | 2 | 19 | 27 | 0.05 | 99.335 |
| 41 | Pitho sp. | 1 | 22 | 0 | 2 | 25 | 0.05 | 99.382 |
| 42 | Macrocoeloma diplacanthum | 2 | 7 | 7 | 8 | 24 | 0.05 | 99.427 |
| 43 | Gnathophyllum americanum | 7 | 8 | 2 | 6 | 23 | 0.04 | 99.470 |
| 44 | Micropanope nuttingi | 12 | 2 | 2 | 5 | 21 | 0.04 | 99.509 |
| 45 | Thor amboinensis | 0 | 0 | 14 | 4 | 18 | 0.03 | 99.543 |
| 46 | Macrocoeloma subparelellum | 0 | 0 | 7 | 11 | 18 | 0.03 | 99.577 |
| 47 | Eurypanopeus dissimilis | 1 | 2 | 7 | 5 | 15 | 0.03 | 99.605 |
| 48 | Neopanope packardii | 9 | 3 | 0 | 2 | 14 | 0.03 | 99.632 |
| 49 | Thersandrus compressus | 4 | 1 | 1 | 5 | 11 | 0.02 | 99.652 |
| 50 | Moreiradromia antillensis | 3 | 1 | 2 | 4 | 10 | 0.02 | 99.671 |
| 51 | Calappa sulcata | 1 | 0 | 1 | 8 | 10 | 0.02 | 99.690 |
| 52 | Teleophrys sp. | 3 | 1 | 4 | 1 | 9 | 0.02 | 99.707 |
| 53 | Alpheus peasei | 1 | 1 | 0 | 7 | 9 | 0.02 | 99.724 |
| 54 | Xanthoid G | 4 | 3 | 0 | 0 | 7 | 0.013 | 99.737 |
| 55 | Petrolisthes galatinus | 0 | 2 | 0 | 5 | 7 | 0.013 | 99.750 |
| 56 | Cyclozodium angustum | 0 | 0 | 1 | 6 | 7 | 0.013 | 99.763 |
| 57 | Alpheus armatus | 1 | 0 | 1 | 5 | 7 | 0.013 | 99.776 |
| 58 | Hippolyte obliquimanus | 0 | 0 | 3 | 3 | 6 | 0.011 | 99.788 |
| 59 | Tuleariocaris neglecta | 0 | 0 | 2 | 3 | 5 | 0.009 | 99.797 |
| 60 | Panopeus herbsti | 0 | 0 | 2 | 3 | 5 | 0.009 | 99.806 |
| 61 | Mithrax sp. 2 | 2 | 2 | 0 | 1 | 5 | 0.009 | 99.816 |
| 62 | Xanthoid H | 2 | 2 | 0 | 0 | 4 | 0.008 | 99.823 |
| 63 | Xanthoid B | 3 | 0 | 0 | 1 | 4 | 0.008 | 99.831 |
| 64 | Macrocoeloma laevigatum | 1 | 2 | 1 | 0 | 4 | 0.008 | 99.838 |
| 65 | Alpheus sp. 1 | 0 | 2 | 0 | 2 | 4 | 0.008 | 99.846 |
| 66 | Xanthoid L | 2 | 0 | 0 | 1 | 3 | 0.006 | 99.852 |
| 67 | Sicyonia brevirostris | 0 | 0 | 0 | 3 | 3 | 0.006 | 99.857 |
| 68 | Sicyonia stimpsoni | 0 | 0 | 1 | 2 | 3 | 0.006 | 99.863 |
| 69 | Panopeus sp. 1 | 0 | 3 | 0 | 0 | 3 | 0.006 | 99.868 |
| 70 | Paguristes sp. 1 | 0 | 1 | 1 | 1 | 3 | 0.006 | 99.874 |
| 71 | Hexapanopeus angustifrons | 0 | 0 | 1 | 2 | 3 | 0.006 | 99.880 |
| 72 | Euryplax nitida | 0 | 0 | 0 | 3 | 3 | 0.006 | 99.885 |
| 73 | Epialtus longirostris | 1 | 0 | 0 | 2 | 3 | 0.006 | 99.891 |
| 74 | Diogenid A | 0 | 2 | 0 | 1 | 3 | 0.006 | 99.897 |
| 75 | Alpheus armillatus | 1 | 0 | 0 | 2 | 3 | 0.006 | 99.902 |
| 76 | Xanthoid F | 0 | 2 | 0 | 0 | 2 | 0.004 | 99.906 |
| 77 | Xanthoid A | 1 | 0 | 1 | 0 | 2 | 0.004 | 99.910 |
| 78 | Xanthoid M | 2 | 0 | 0 | 0 | 2 | 0.004 | 99.914 |
| 79 | Xanthoid I | 1 | 1 | 0 | 0 | 2 | 0.004 | 99.917 |
| 80 | Thoe puella | 2 | 0 | 0 | 0 | 2 | 0.004 | 99.921 |
| 81 | Hippolyte sp. | 0 | 0 | 1 | 1 | 2 | 0.004 | 99.925 |
| 82 | Anomuran A | 0 | 1 | 0 | 1 | 2 | 0.004 | 99.929 |
| 83 | Alpheus sp. 3 | 0 | 2 | 0 | 0 | 2 | 0.004 | 99.932 |
| 84 | Xanthoid E | 0 | 1 | 0 | 0 | 1 | 0.002 | 99.934 |
| 85 | Xanthoid D | 0 | 1 | 0 | 0 | 1 | 0.002 | 99.936 |
| 86 | Xanthoid C | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.938 |
| 87 | Xanthoid N | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.940 |
| 88 | Xanthoid K | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.942 |
| 89 | Xanthoid J | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.944 |
| 90 | Upogebia affinis | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.945 |
| 91 | Synalpheus fritzmülleri | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.947 |
| 92 | Synalpheus sp. 3 | 0 | 0 | 1 | 0 | 1 | 0.002 | 99.949 |
| 93 | Synalpheus sp. 2 | 0 | 0 | 1 | 0 | 1 | 0.002 | 99.951 |
| 94 | Synalpheus sp. 1 | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.953 |
| 95 | Stenorhynchus seticornis | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.955 |
| 96 | Stenopus hispidus | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.957 |
| 97 | Speloeophorus pontifer | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.959 |
| 98 | Porcellanid A | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.961 |
| 99 | Pilumnus sp. 2 | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.962 |
| 100 | Pilumnus sp. 1 | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.964 |
| 101 | Pachygrapsus gracilis | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.966 |
| 102 | Periclimenes iridiscens | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.968 |
| 103 | Mithrax sp. 3 | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.970 |
| 104 | Majoid A | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.972 |
| 105 | Macrocoeloma trispinosum | 0 | 1 | 0 | 0 | 1 | 0.002 | 99.974 |
| 106 | Lysmata anchisteus | 0 | 0 | 1 | 0 | 1 | 0.002 | 99.976 |
| 107 | Lobopilumnus agassizii | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.977 |
| 108 | Latreutes inermis | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.979 |
| 109 | Gnathophyllum sp. | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.981 |
| 110 | Discias atlanticus | 0 | 1 | 0 | 0 | 1 | 0.002 | 99.983 |
| 111 | Diogenid B | 0 | 1 | 0 | 0 | 1 | 0.002 | 99.985 |
| 112 | Caridean B | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.987 |
| 113 | Caridean A | 0 | 0 | 1 | 0 | 1 | 0.002 | 99.989 |
| 114 | Alpheus sp. 4 | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.991 |
| 115 | Alpheus sp. 2 | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.992 |
| 116 | Alpheopsis trispinosus | 1 | 0 | 0 | 0 | 1 | 0.002 | 99.994 |
| 117 | Alpheopsis trigonus | 0 | 0 | 0 | 1 | 1 | 0.002 | 99.996 |
| 118 | Acanthonyx petiverii | 0 | 0 | 1 | 0 | 1 | 0.002 | 99.998 |
| 119 | Alpheus websteri | 0 | 0 | 0 | 1 | 1 | 0.002 | 100 |
References
- Gore, R.H.; Gallaher, E.E.; Scotto, L.E.; Wilson, K.A. Studies on decapods of the Indian River region off Florida. XI. Community composition, structure, biomass and species-area relationship of seagrass and drift algae-associated macrocrustaceans. Est. Coast. Shelf Sci. 1981, 12, 458–503. [Google Scholar]
- Jackson, E.L.; Rowden, A.A.; Attrill, M.J.; Bossey, S.J.; Jones, M.B. The importance of seagrass beds as a habitat for fishery species. Oceanogr. Mar. Biol. Annu. Rev. 2001, 39, 269–303. [Google Scholar]
- Hay, K.H.; Hays, G.C.; Orth, R. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Middelburgi, D.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Macreadie, P.; Baird, M.; Trevathan-Tackett, S.; Larkum, A.; Ralph, P. Quantifying and modelling the carbon sequestration capacity of seagrass meadows—A critical assessment. Mar. Pollut. Bull. 2014, 83, 430–439. [Google Scholar] [CrossRef]
- Clarke, D.; York, P.H.; Rasheed, M.A.; Northfield, T.D. Does biodiversity–ecosystem function literature neglect tropical ecosystems? Trends Ecol. Evol. 2017, 32, 320–323. [Google Scholar] [CrossRef]
- York, P.H.; Hyndes, G.; Bishop, M.J.; Barnes, R. Faunal Assemblages of Seagrass Ecosystems. In Seagrasses of Australia; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 541–588. [Google Scholar]
- Heck, K.L. Comparative species richness, composition, and abundance of invertebrates in Caribbean seagrass (Thalassia testudinum) meadows (Panamá). Mar. Biol. 1977, 41, 335–348. [Google Scholar] [CrossRef]
- Greening, H.; Livingston, R. Diel variation in the structure of seagrass-associated epibenthic macroinvertebrate communities. Mar. Ecol. Prog. Ser. 1982, 7, 147–156. [Google Scholar] [CrossRef]
- Lewis, F.G. Crustacean epifauna of seagrass and macroalgae in Apalachee Bay, Florida, USA. Mar. Biol. 1987, 94, 219–229. [Google Scholar] [CrossRef]
- Knowles, L.L.; Bell, S.S. The influence of habitat structure in faunal-habitats associations in a Tampa Bay seagrass system, Florida. Bull. Mar. Sci. 1998, 62, 781–794. [Google Scholar]
- Heck, K.L.; Orth, R.J. Structural components of eelgrass (Zostera marina) meadows in the lower Chesapeake Bay: Decapod Crustacea. Estuaries 1980, 3, 289. [Google Scholar] [CrossRef]
- Bauer, R.T. Diel and seasonal variation in species composition and abundance of the caridean shrimps (Crustacea, Decapoda) from seagrass meadows on the north coast of Puerto Rico. Bull. Mar. Sci. 1985, 36, 150–162. [Google Scholar]
- Holmquist, J.; Powell, G.V.N.; Sogard, S.M. Decapod and stomatopod assemblages on a system of seagrass-covered mud banks in Florida Bay. Mar. Biol. 1989, 100, 473–483. [Google Scholar] [CrossRef]
- Ramírez, Á.M.; Urra, J.; Marina, P.; Rueda, J.L.; Raso, J.E.G. Crustacean decapod assemblages associated with fragmented Posidonia oceanica meadows in the Alboran Sea (Western Mediterranean Sea): Composition, temporal dynamics and influence of meadow structure. Mar. Ecol. 2015, 37, 344–358. [Google Scholar] [CrossRef]
- Edgar, G.J.; Shaw, C. The production and trophic ecology of shallow-water fish assemblages in southern Australia II. Diets of fishes and trophic relationships between fishes and benthos at Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 1995, 194, 83–106. [Google Scholar] [CrossRef]
- Zupo, V.; Nelson, W.G. Factors influencing the association patterns of Hippolyte zostericola and Palaemonetes intermedius (Decapoda: Natantia) with seagrasses of the Indian River Lagoon, Florida. Mar. Biol. 1999, 134, 181–190. [Google Scholar] [CrossRef]
- Boada, J.; Pagès, J.F.; Gera, A.; MacPherson, E.; Santana, Y.; Romero, J.; Alcoverro, T. The richness of small pockets: Decapod species peak in small seagrass patches where fish predators are absent. Mar. Environ. Res. 2018, 142, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mariño, J.; Mendoza, M.D.; Sánchez, B.L. Composition and abundance of decapod crustaceans in mixed seagrass meadows in the Paraguaná Peninsula, Venezuela. Iheringia Série Zool. 2018, 108, 2018004. [Google Scholar] [CrossRef]
- Holmquist, J.G.; Powell, G.V.N.; Sogard, S.M. Decapod and stomatopod communities of seagrass-covered mud banks in Florida Bay: Inter-and intra-bank heterogeneity with special reference to isolated subenvironments. Bull. Mar. Sci. 1989, 44, 251–262. [Google Scholar]
- Raso, J.E.G.; Martin, M.J.; Diaz, V.; Cobos, V.; Manjón-Cabeza, M.E. Diel and seasonal changes in the structure of a decapod (Crustacea: Decapoda) community of Cymodocea nodosa from Southeastern Spain (West Mediterranean Sea). Hydrobiologia 2006, 557, 59–68. [Google Scholar] [CrossRef]
- Bauer, R.T. Hermit crab fauna from seagrass meadows in Puerto Rico: Species composition, diel and seasonal variation in abundance. J. Crust. Biol. 1985, 5, 249–257. [Google Scholar] [CrossRef]
- Bauer, R.T. Penaeoid shrimp fauna from tropical seagrass meadows: Species composition, diurnal and seasonal variation in abundance. Proc. Biol. Soc. Wash. 1985, 98, 177–190. [Google Scholar]
- Barba Macías, E. Faunistic analysis of the caridean shrimps inhabiting seagrasses along the NW coast of the Gulf of Mexico and Caribbean Sea. Rev. Biol. Trop. 2012, 60, 1161–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrivillaga, A.; Baltz, D.M. Comparison of fishes and macroinvertebrates on seagrass and bare-sand sites on Guatemala’s Atlantic coast. Bull. Mar. Sci. 1999, 65, 301–319. [Google Scholar]
- Chace, F.A. The shrimps of the Smithsonian-Bredin Caribbean Expeditions with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithson. Contrib. Zool. 1972, 1–179. [Google Scholar] [CrossRef]
- Markham, J.C.; Donath-Hernández, F.E.; Villalobos-Hiriart, J.L.; Cantú Díaz-Barriga, A. Notes on the shallow-water marine Crustacea of the Caribbean coast of Quintana Roo, México. An. Inst. Biol. Univ. Nal. Autón. México Ser. Zool. 1990, 61, 405–446. [Google Scholar]
- Román-Contreras, R.; Martínez-Mayén, M. Shallow water hippolytid shrimps (Crustacea: Decapoda: Caridea) from the Mexican Caribbean coast. Hidrobiológica 2009, 19, 119–128. [Google Scholar]
- Román-Contreras, R.; Martínez-Mayén, M. Palaemonidae (Crustacea: Decapoda: Caridea) de las aguas someras de Quintana Roo, Caribe mexicano. Rev. Mex. Biodivers. 2010, 81, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Briones-Fourzán, P.; Lozano-Álvarez, E. The importance of Lobophora variegata (Phaeophyta: Dictyotales) as a habitat for small juveniles of Panulirus argus (Decapoda: Palinuridae) in a tropical reef lagoon. Bull. Mar. Sci. 2001, 68, 207–219. [Google Scholar]
- Briones-Fourzán, P.; Pérez-Ortiz, M.; Negrete-Soto, F.; Barradas-Ortiz, C.; Lozano-Álvarez, E. Ecological traits of Caribbean sea anemones and symbiotic crustaceans. Mar. Ecol. Prog. Ser. 2012, 470, 55–68. [Google Scholar] [CrossRef]
- Ruiz-Rentería, F.; van Tussenbroek, B.I.; Jordán-Dahlgren, E. Puerto Morelos, Quintana Roo, México. In CARICOMP–Caribbean Coral Reef, Seagrass, and Mangrove Sites; Kjerve, B., Ed.; UNESCO: Paris, France, 1998; pp. 56–66. [Google Scholar]
- Rodríguez-Martínez, R.; Ruíz-Rentería, F.; Van Tussenbroek, B.; Barba-Santos, G.; Escalante-Mancera, E.; Jordán-Garza, G.; Jordán-Dahlgren, E. Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Rev. Biol. Trop. 2010, 58, 23–43. [Google Scholar] [PubMed]
- Cortés, J.; Oxenford, H.A.; van Tussenbroek, B.I.; Jordán-Dahlgren, E.; Cróquer, A.; Bastidas, C.; Ogden, J.C. The CARICOMP network of Caribbean marine maboratories (1985–2007): History, key findings, and lessons learned. Front. Mar. Sci. 2019, 5, 519. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I. Dynamics of seagrasses and associated algae in coral reef lagoons. Hidrobiológica 2011, 21, 293–310. [Google Scholar]
- van Tussenbroek, B.I.; Cortes, J.; Collin, R.; Fonseca, A.C.; Gayle, P.M.H.; Guzman, H.M.; Jácome, G.E.; Juman, R.; Koltes, K.H.; Oxenford, H.A.; et al. Caribbean-wide, long-term study of seagrass beds reveals local variations, shifts in community structure and occasional collapse. PLoS ONE 2014, 9, e90600. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Martínez, R. Community involvement in marine protected areas: The case of Puerto Morelos reef, México. J. Environ. Manag. 2008, 88, 1151–1160. [Google Scholar] [CrossRef]
- Estrada-Olivo, J.J. Riqueza Específica y Abundancia de la Macrofauna Béntica Asociada a Pastizales Marinos en la Laguna Arrecifal de Puerto Morelos, Quintana Roo, México. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 1999. [Google Scholar]
- Monroy-Velázquez, L.V. Variaciones en la Composición y Abundancia de la Fauna de Decápodos Asociados a Pastizales Marinos en el Caribe Mexicano. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2000. [Google Scholar]
- Instituto Nacional de Ecología. Programa de Manejo Parque Nacional Arrecife de Puerto Morelos; Secretaría del Medio Ambiente, Recursos Naturales y Pesca: Cd. de México, Mexico, 2000. [Google Scholar]
- Briones-Fourzán, P.; Castañeda-Fernández de Lara, V.; Lozano-Álvarez, E.; Estrada-Olivo, J. Feeding ecology of the three juvenile phases of the spiny lobster Panulirus argus in a tropical reef lagoon. Mar. Biol. 2003, 142, 855–865. [Google Scholar] [CrossRef]
- Murray, G. Constructing paradise: The impacts of big tourism in the Mexican coastal zone. Coast. Manag. 2007, 35, 339–355. [Google Scholar] [CrossRef]
- Suchley, A.; Alvarez-Filip, L. Local human activities limit marine protection efficacy on Caribbean coral reefs. Conserv. Lett. 2018, 11, e12571. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Hernández-Arana, H.; Rodríguez-Martínez, R.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; Van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [Green Version]
- Coronado, C.; Candela, J.; Iglesias-Prieto, R.; Sheinbaum, J.; Lopez, M.; Ocampo-Torres, F.J. On the circulation in the Puerto Morelos fringing reef lagoon. Coral Reefs 2007, 26, 149–163. [Google Scholar] [CrossRef]
- Merino, M.; Otero, L. Atlas Ambiental Costero, Puerto Morelos-Quintana Roo; Centro de Investigaciones de Quintana Roo: Chetumal, Mexico, 1990. [Google Scholar]
- van Tussenbroek, B.I. Thalassia testudinum leaf dynamics in a Mexican Caribbean coral reef lagoon. Mar. Boil. 1995, 122, 33–40. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I. Above- and below-ground biomass and production by Thalassia testudinum in a tropical reef lagoon. Aquat. Bot. 1998, 61, 69–82. [Google Scholar] [CrossRef]
- Rathbun, M.J. The spider crabs of America. Bull. United States Natl. Mus. 1925, 129, 1–613. [Google Scholar] [CrossRef]
- Rathbun, M.J. The cancroid crabs of America of the families Euryalidae, Portunidae, Atelecyclidae, Cancridae, and Xanthidae. Bull. United States Natl. Mus. 1930, 152, 1–609. [Google Scholar] [CrossRef]
- Provenzano, A.J., Jr. The shallow water hermit crabs of Florida. Bull. Mar. Sci. 1959, 9, 349–420. [Google Scholar]
- Felder, D.L. An Annotated Key to Crabs and Lobsters (Decapoda, Reptantia) from Coastal Waters of the Northwestern Gulf of Mexico; Louisiana St. University Publication No. LSU-SG-73-02: Baton Rouge, LA, USA, 1973. [Google Scholar]
- Rodríguez, G. Los Crustáceos Decápodos de Venezuela; Instituto Venezolano de Investigaciones Científicas: Caracas, Venezuela, 1980. [Google Scholar]
- Williams, A.B. Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida; Smithsonian Institution Press: Washington, DC, USA, 1984. [Google Scholar]
- Abele, L.G.; Kim, W. An Illustrated Guide to the Marine Decapod Crustaceans of Florida; Florida State University: Tallahassee, FL, USA, 1986. [Google Scholar]
- Humann, P. Reef Creature Identification: Florida, Caribbean, Bahamas; New World Publications: Jacksonville, FL, USA, 1992. [Google Scholar]
- Hernández-Aguilera, J.L.; Toral-Almazán, R.E.; Ruiz-Nilo, J.A. Especies Catalogadas de Crustáceos Estomatópodos y Decápodos para el Golfo de México, Río Bravo, Tamps., a Progreso, Yuc.; Secretaría de Marina y CONABIO: Cd. de México, México, 1996. [Google Scholar]
- Warwick, R.; Dashfield, S.; Somerfield, P. The integral structure of a benthic infaunal assemblage. J. Exp. Mar. Biol. Ecol. 2006, 330, 12–18. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R.; Somerfield, P.; Gorley, R.N. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 2008, 366, 56–69. [Google Scholar] [CrossRef]
- Magurran, A.E.; Dornelas, M. Biological diversity in a changing world. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3593–3597. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Mueller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, B.R.; Johnson, M.W.; Cammarata, K.; Smee, D.L. Changes in seagrass species composition in Northwestern Gulf of Mexico estuaries: Effects on associated seagrass fauna. PLoS ONE 2014, 9, e107751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azovsky, A.I. Species–area and species–sampling effort relationships: Disentangling the effects. Ecography 2011, 34, 18–30. [Google Scholar] [CrossRef]
- Heck, K.L., Jr. Some determinants of the composition and abundance of motile macro-invertebrates species in tropical and temperate turtlegrass (Thalassia testudinum) meadows. J. Biogeogr. 1979, 6, 183–197. [Google Scholar] [CrossRef]
- Lewis, F. Distribution of macrobenthic crustaceans associated with Thalassia, Halodule and bare sand substrata. Mar. Ecol. Prog. Ser. 1984, 19, 101–113. [Google Scholar] [CrossRef]
- Stoner, A.W.; Lewis, F. The influence of quantitative and qualitative aspects of habitat complexity in tropical sea-grass meadows. J. Exp. Mar. Biol. Ecol. 1985, 94, 19–40. [Google Scholar] [CrossRef]
- Virnstein, R.W.; Howard, R.K. Motile epifauna of marine macrophytes in the Indian River Lagoon, Florida. II. Comparisons between drift algae and three species of seagrasses. Bull. Mar. Sci. 1987, 41, 13–26. [Google Scholar]
- Leopardas, V.; Uy, W.; Nakaoka, M. Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: Variation among vegetation dominated by different seagrass species. J. Exp. Mar. Biol. Ecol. 2014, 457, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huang, Y.; Arbi, U.Y.; Lin, H.; Azkab, M.H.; Wang, J.; He, X.; Mou, J.; Liu, K.; Zhang, S. An ecological survey of the abundance and diversity of benthic macrofauna in Indonesian multispecific seagrass beds. Acta Oceanol. Sin. 2018, 37, 82–89. [Google Scholar] [CrossRef]
- Parker, J.D.; Duffy, J.; Orth, R. Plant species diversity and composition: Experimental effects on marine epifaunal assemblages. Mar. Ecol. Prog. Ser. 2001, 224, 55–67. [Google Scholar] [CrossRef]
- De Grave, S.; Livingston, D.; Speight, M. Diel variation in sea grass dwelling shrimp: When to sample at night? J. Mar. Biol. Assoc. U. K. 2006, 86, 1421–1422. [Google Scholar] [CrossRef] [Green Version]
- Young, D.K.; Young, M.W. Macrobenthic invertebrates in bare sand and seagrass (Thalassia testudinum) at Carrie Bow Cay, Belize. In The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, 1: Structure and Communities; Rützler, K., Macintyre, I.G., Eds.; Smithsonian Contributions Marine Science 12: Washington, DC, USA, 1982; pp. 115–126. [Google Scholar]
- Boström, C.; Jackson, E.; Simenstad, C.A. Seagrass landscapes and their effects on associated fauna: A review. Estuar. Coast. Shelf Sci. 2006, 68, 383–403. [Google Scholar] [CrossRef]
- Tanner, J. Landscape ecology of interactions between seagrass and mobile epifauna: The matrix matters. Estuar. Coast. Shelf Sci. 2006, 68, 404–412. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Etherington, L.L.; Elis, W.E. Organism response to habitat patchiness: Species and habitat-dependent recruitment of decapod crustaceans. J. Exp. Mar. Biol. Ecol. 1998, 223, 111–132. [Google Scholar] [CrossRef]
- Healey, D.; Hovel, K.A. Seagrass bed patchiness: Effects on epifaunal communities in San Diego Bay, USA. J. Exp. Mar. Biol. Ecol. 2004, 313, 155–174. [Google Scholar] [CrossRef]
- Johnson, M.; Heck, K.L., Jr. Effects of habitat fragmentation per se on decapods and fishes inhabiting seagrass meadows in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2006, 306, 233–246. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Marion, S.R.; Lombana, A.V.; Orth, R.J. Faunal communities are invariant to fragmentation in experimental seagrass landscapes. PLoS ONE 2016, 11, e0156550. [Google Scholar] [CrossRef]
- Howard, R.K. Diel variation in the abundance of epifauna associated with seagrasses of the Indian River, Florida, USA. Mar. Biol. 1987, 96, 137–142. [Google Scholar] [CrossRef]
- Bedini, R.; Pertusati, M.; Batistini, F.; Piazzi, L. Spatial and temporal variation of motile macro-invertebrate assemblages associated with Posidonia oceanica meadows. Acta Adriat. 2011, 52, 201–214. [Google Scholar]
- Bauer, R.T. Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. J. Exp. Mar. Biol. Ecol. 1989, 127, 175–187. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Unsworth, R.K.F. Long-term climate-associated dynamics of a tropical seagrass meadow: Implications for the future. Mar. Ecol. Prog. Ser. 2011, 422, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.O.; Lirman, D.; Pittman, S.J.; Serafy, J. Spatial patterns of seagrasses and salinity regimes interact to structure marine faunal assemblages in a subtropical bay. Mar. Ecol. Prog. Ser. 2018, 594, 21–38. [Google Scholar] [CrossRef]
- Carruthers, T.; van Tussenbroek, B.I.; Dennison, W. Influence of submarine springs and wastewater on nutrient dynamics of Caribbean seagrass meadows. Estuar. Coast. Shelf Sci. 2005, 64, 191–199. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Jordán-Dahlgren, E. Afluencia masiva de sargazo pelágico a la costa del Caribe mexicano (2014–2015). In Florecimientos Algales Nocivos en México; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortiz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, México, 2016; pp. 352–365. [Google Scholar]
- Rodríguez-Martínez, R.; Medina-Valmaseda, A.; Blanchon, P.; Monroy-Velázquez, L.; Almazán-Becerril, A.; Delgado-Pech, B.; Vásquez-Yeomans, L.; Francisco, V.; García-Rivas, M. Faunal mortality associated with massive beaching and decomposition of pelagic Sargassum. Mar. Pollut. Bull. 2019, 146, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.A.; Wright, A.; Ayvazian, S.G.; Finn, J.T.; Golden, H.; Merson, R.R.; Harrison, J.A. Nitrogen loading alters seagrass ecosystem structure and support of higher trophic levels. Aquat. Conserv. Mar. Freshw. Ecosyst. 2002, 12, 193–212. [Google Scholar] [CrossRef]
- Seitz, R.D.; Lewis, C.J.E. Loss of seagrass results in changes to benthic infaunal community structure and decreased secondary production. Bull. Mar. Sci. 2018, 94, 1273–1292. [Google Scholar] [CrossRef]
- Githaiga, M.N.; Frouws, A.M.; Kairo, J.G.; Huxham, M. Seagrass removal leads to rapid changes in fauna and loss of carbon. Front. Ecol. Evol. 2019, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Matheson, R.E.; Camp, D.K.; Sogard, S.M.; Bjorgo, K.A. Changes in seagrass-associated fish and crustacean communities on Florida Bay mud banks: The effects of recent ecosystem changes? Estuaries 1999, 22, 534. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Coll, M.; Lotze, H.K. Regional-scale differences in eutrophication effects on eelgrass-associated (Zostera marina) macrofauna. Estuar. Coasts 2017, 40, 1096–1112. [Google Scholar] [CrossRef]
- Blake, R.; Duffy, J.E.; Richardson, J.P. Patterns of seagrass community response to local shoreline development. Estuar. Coasts 2014, 37, 1549–1561. [Google Scholar] [CrossRef]




| Ecological Index | Effect | DF | MS | F | p |
|---|---|---|---|---|---|
| S | Intercept | 1 | 29568.05 | 380.541 | <0.001 |
| Season | 1 | 120.05 | 1.545 | 0.232 | |
| Time of Day | 1 | 661.25 | 8.510 | 0.010 | |
| Season × TD | 1 | 26.45 | 0.340 | 0.568 | |
| Error | 16 | 77.7 | |||
| N | Intercept | 1 | 141570526 | 47.343 | <0.001 |
| Season | 1 | 7129374 | 2.384 | 0.142 | |
| Time of Day | 1 | 58150730 | 19.447 | <0.001 | |
| Season × TD | 1 | 2759502 | 0.923 | 0.351 | |
| Error | 16 | 2990285 | |||
| H’ | Intercept | 1 | 96.181 | 563.640 | <0.001 |
| Season | 1 | 0.580 | 3.397 | 0.084 | |
| Time of Day | 1 | 0.034 | 0.197 | 0.663 | |
| Season × TD | 1 | 0.064 | 0.373 | 0.550 | |
| Error | 16 | 0.171 | |||
| J’ | Intercept | 1 | 7.346 | 812.897 | <0.001 |
| Season | 1 | 0.026 | 2.913 | 0.107 | |
| Time of Day | 1 | 0.006 | 0.519 | 0.482 | |
| Season × TD | 1 | 0.008 | 0.939 | 0.347 | |
| Error | 16 | 0.009 | |||
| 1-lambda | Intercept | 1 | 13.016 | 1014.027 | <0.001 |
| Season | 1 | 0.0084 | 0.656 | 0.430 | |
| Time of Day | 1 | 0.0004 | 0.029 | 0.867 | |
| Season × TD | 1 | 0.0188 | 1.470 | 0.243 | |
| Error | 16 | 0.0128 |
| Summer | Winter | |||
|---|---|---|---|---|
| Species | Day | Night | Day | Night |
| Latreutes fucorum | 1.168 (0.495) ab | 3.219 (1.609) b | 0.555 (0.500) a | 1.494 (0.385) ab |
| Cuapetes americanus | 0.707 (0.705) ab | 2.786 (2.021) b | 0.167 (0.110) a | 0.804 (0.844) ab |
| Thor manningi | 0.304 (0.277) ab | 2.382 (2.027) b | 0.117 (0.082) a | 0.839 (0.639) ab |
| Pagurus annulipes | 0.285 (0.158) a | 2.277 (1.405) b | 0.296 (0.258) a | 0.449 (0.338) a |
| Pagurus brevidactylus | 0.180 (0.152) a | 1.467 (0.940) b | 0.234 (0.182) a | 1.156 (1.109) b |
| Clibanarius tricolor | 0.108 (0.179) a | 0.280 (0.233) ab | 0.100 (0.168) a | 2.226 (3.912) b |
| Thor dobkini | 0.097 (0.065) a | 0.977 (0.817) b | 0.029 (0.024) a | 0.184 (0.160) ab |
| Alpheus normanni | 0.009 (0.009) a | 0.533 (0.364) ab | 0.005 (0.007) a | 0.645 (0.619) b |
| Tozeuma carolinense | 0.255 (0.107) | 0.204 (0.117) | 0.273 (0.179) | 0.162 (0.157) |
| Processa bermudensis | 0.002 (0.003) a | 0.405 (0.185) b | 0.004 (0.005) a | 0.264 (0.209) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briones-Fourzán, P.; Monroy-Velázquez, L.V.; Estrada-Olivo, J.; Lozano-Álvarez, E. Diversity of Seagrass-Associated Decapod Crustaceans in a Tropical Reef Lagoon Prior to Large Environmental Changes: A Baseline Study. Diversity 2020, 12, 205. https://doi.org/10.3390/d12050205
Briones-Fourzán P, Monroy-Velázquez LV, Estrada-Olivo J, Lozano-Álvarez E. Diversity of Seagrass-Associated Decapod Crustaceans in a Tropical Reef Lagoon Prior to Large Environmental Changes: A Baseline Study. Diversity. 2020; 12(5):205. https://doi.org/10.3390/d12050205
Chicago/Turabian StyleBriones-Fourzán, Patricia, Luz Verónica Monroy-Velázquez, Jaime Estrada-Olivo, and Enrique Lozano-Álvarez. 2020. "Diversity of Seagrass-Associated Decapod Crustaceans in a Tropical Reef Lagoon Prior to Large Environmental Changes: A Baseline Study" Diversity 12, no. 5: 205. https://doi.org/10.3390/d12050205