Optimization and Application of Reflective LSPR Optical Fiber Biosensors Based on Silver Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Silver Nanoparticles
2.3. Preparation of Ag NP-Based LSPR Sensor Probes
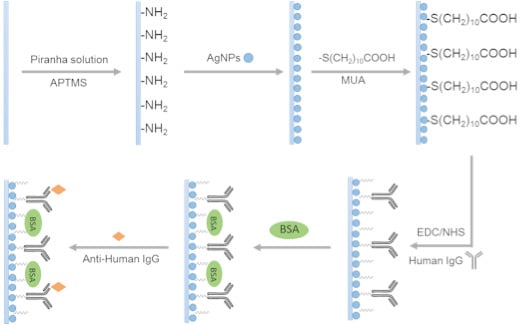
2.4. Functionalization of Sensor Probes

2.5. Monitoring of the Antigen-Antibody Interaction
2.6. Optical Setup and Data Processing

3. Results and Discussion
3.1. Characterization of the Sensor Probe

| σSO | SRI | rRI | |
|---|---|---|---|
| Wavelength shift | 0.35 nm | 387 nm/RIU | 9.04 × 10−4 RIU |
| Reflectivity change | 0.75% | 160%/RIU | 4.68 × 10−3 RIU |
3.2. Optimization of Sensor Performance


3.3. Stability of the Sensor Probe

3.4. Biosensor Applications (the Functionalization and the Bimolecular Interaction Monitoring of the Sensor Probe)

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, T.; Lee, S.Y.; Song, E.Y.; Chun, H.; Lee, B. Plasmonic nanostructures for nano-scale bio-sensing. Sensors 2011, 11, 10907–10929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Hu, A.; Zhang, T.; Lei, W.; Xue, X.-J.; Zhou, Y.; Duley, W.W. Self-assembly of large-scale and ultrathin silver nanoplate films with tunable plasmon resonance properties. ACS Nano 2011, 5, 9082–9092. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Smith, D.R.; Schultz, S. Local refractive index dependence of plasmon resonance spectra from individual nanoparticles. Nano Lett. 2003, 3, 485–491. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical fiber LSPR biosensor prepared by gold nanoparticle assembly on polyelectrolyte multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; D’Agostino, G.; Dona, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized surface plasmon resonance with five-branched gold nanostars in a plastic optical fiber for bio-chemical sensor implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, H.; Wang, W.; Gao, P.; Yao, J. Ethanol vapor sensing properties of triangular silver nanostructures based on localized surface plasmon resonance. Sensors 2011, 11, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, Y.J.; Zhang, X.Y.; Wu, J.Y. Synthesis of silver nanostructures by multistep methods. Sensors 2014, 14, 5860–5889. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Zou, S.; Schatz, G.C.; Duyne, R.P.V. A nanoscale optical biosensor: The long range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2003, 108, 109–116. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Luo, P.; Jin, J.; Xing, J.; Wang, Z.; Wong, S.T. Fabrication of localized surface plasmon resonance fiber probes using ionic self-assembled gold nanoparticles. Sensors 2010, 10, 6477–6487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jin, S.M.; Lee, S.K.; Kim, M.G.; Shin, Y.B. Detection of biomolecular binding through enhancement of localized surface plasmon resonance (LSPR) by gold nanoparticles. Sensors 2009, 9, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tu, M.H.; Sun, T.; Grattan, K.T.V. Wavelength-based localized surface plasmon resonance optical fiber biosensor. Sens. Actuators B Chem. 2013, 181, 611–619. [Google Scholar] [CrossRef]
- Mishra, S.K.; Gupta, B.D. Surface plasmon resonance based fiber optic sensor for the detection of CrO42− using Ag/ITO/hydrogel layers. Anal. Methods 2014, 6, 5191–5197. [Google Scholar] [CrossRef]
- Fan, M.; Thompson, M.; Andrade, M.L.; Brolo, A.G. Silver nanoparticles on a plastic platform for localized surface plasmon resonance biosensing. Anal. Chem. 2010, 82, 6350–6352. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, T.; Zhao, X.; Li, X.; Tan, W. The optical property of core-shell nanosensors and detection of atrazine based on localized surface plasmon resonance (LSPR) sensing. Sensors 2014, 14, 13273–13284. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Lin, S.S.; Lin, T.J.; Du, J.K. Detection of hydrofluoric acid by a SiO2 sol-gel coating fiber-optic probe based on reflection-based localized surface plasmon resonance. Sensors 2011, 11, 1907–1923. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, S.W.; Jung, J.A.; Ahn, J.; Kim, M.G.; Shin, Y.B. Signal amplification by enzymatic reaction in an immunosensor based on localized surface plasmon resonance (LSPR). Sensors 2010, 10, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gupta, B.D. Fiber optic sensor based on surface plasmon resonance with nanoparticle films. Photon. Nanostr. Fundam. Appl. 2005, 3, 30–37. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensor based on surface plasmon resonance with Ag-Au alloy nanoparticle films. Nanotechnology 2006, 7, 124–131. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Chau, L.-K. Colloidal gold-modified optical fiber for chemical and biochemical sensing. Anal. Chem. 2003, 75, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. LSPR optical fibre sensors based on hollow gold nanostructures. Sens. Actuators B Chem. 2014, 191, 37–44. [Google Scholar] [CrossRef]
- Tu, H.; Sun, T.; Grattan, K.T. SPR-based optical fiber sensors using gold–silver alloy particles as the active sensing material. IEEE Sens. J. 2013, 13, 2192–2199. [Google Scholar] [CrossRef]
- Sciacca, B.; Monro, T.M. Dip biosensor based on localized surface plasmon resonance at the tip of an optical fiber. Langmuir 2014, 30, 946–54. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Mendoza, J.G.; Padilla-Vivanco, A.; Toxqui-Quitl, C.; Zaca-Moran, P.; Villegas-Hernandez, D.; Chavez, F. Optical fiber sensor based on localized surface plasmon resonance using silver nanoparticles photodeposited on the optical fiber end. Sensors 2014, 14, 18701–18710. [Google Scholar] [CrossRef] [PubMed]
- Chau, L.-K.; Lin, Y.-F.; Cheng, S.-F.; Lin, T.-J. Fiber-optic chemical and biochemical probes based on localized surface plasmon resonance. Sens. Actuators B Chem. 2006, 113, 100–105. [Google Scholar] [CrossRef]
- Consales, M.; Ricciardi, A.; Crescitelli, A.; Esposito, E.; Cutolo, A.; Cusano, A. Lab-on-fiber technology: Toward multifunctional optical nanoprobes. ACS Nano 2012, 6, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zou, Y.; Mo, Y.; Guo, J.; Lindquist, R.G. E-beam patterned gold nanodot arrays on optical fiber tips for localized surface plasmon resonance biochemical sensing. Sensors 2010, 10, 9397–9406. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Handa, Y.; Kajikawa, K. Optical fiber affinity biosensor based on localized surface plasmon resonance. Appl. Phys. Lett. 2004, 85, 4231–4233. [Google Scholar] [CrossRef]
- Sai, V.V.; Kundu, T.; Mukherji, S. Novel U-bent fiber optic probe for localized surface plasmon resonance based biosensor. Biosens. Bioelectron. 2009, 24, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Arora, V.; Sapra, S.; Gupta, B.D. Localized surface plasmon resonance-based fiber optic U-shaped biosensor for the detection of blood glucose. Plasmonics 2011, 7, 261–268. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Mukherji, S. Gold nanoparticle coated U-bend fibre optic probe for localized surface plasmon resonance based detection of explosive vapours. Sens. Actuators B Chem. 2014, 192, 804–811. [Google Scholar] [CrossRef]
- Jorgenson, R.C.; Yee, S.S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B Chem. 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Sagle, L.B.; Ruvuna, L.K.; Ruemmele, J.A.; Van Duyne, R.P. Advances in localized surface plasmon resonance spectroscopy biosensing. Nanomedicine 2011, 6, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Bharadwaj, R.; Mukherji, S.; Kundu, T. Study of localized surface-plasmon-resonance-based optical fiber sensor. Appl. Opt. 2011, 50, E138–E144. [Google Scholar] [CrossRef]
- Dutta, R.; Singh, B.P.; Kundu, T. Plasmonic Coupling Effect on spectral response of silver nanoparticles immobilized on an optical fiber sensor. J. Phys. Chem. C 2013, 117, 17167–17176. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Lu, C.-J. Surface modification on silver nanoparticles for enhancing vapor selectivity of localized surface plasmon resonance sensors. Sens. Actuators B Chem. 2009, 135, 492–498. [Google Scholar] [CrossRef]
- Cao, J.; Galbraith, E.K.; Sun, T.; Grattan, K.T. Cross-comparison of surface plasmon resonance-based optical fiber sensors with different coating structures. IEEE Sens. J. 2012, 12, 2355–2361. [Google Scholar] [CrossRef]
- Lin, T.J.; Chung, M.F. Detection of cadmium by a fiber-optic biosensor based on localized surface plasmon resonance. Biosens. Bioelectron. 2009, 24, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, M.A. Noble metal nanoparticle pairs: Effect of medium for enhanced nanosensing. Nano Lett. 2008, 8, 4347–4352. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Shi, S.; Su, R.; Qi, W.; Huang, R.; Wang, M.; Wang, L.; He, Z. Optimization and Application of Reflective LSPR Optical Fiber Biosensors Based on Silver Nanoparticles. Sensors 2015, 15, 12205-12217. https://doi.org/10.3390/s150612205
Chen J, Shi S, Su R, Qi W, Huang R, Wang M, Wang L, He Z. Optimization and Application of Reflective LSPR Optical Fiber Biosensors Based on Silver Nanoparticles. Sensors. 2015; 15(6):12205-12217. https://doi.org/10.3390/s150612205
Chicago/Turabian StyleChen, Jiangping, Se Shi, Rongxin Su, Wei Qi, Renliang Huang, Mengfan Wang, Libing Wang, and Zhimin He. 2015. "Optimization and Application of Reflective LSPR Optical Fiber Biosensors Based on Silver Nanoparticles" Sensors 15, no. 6: 12205-12217. https://doi.org/10.3390/s150612205