Cytotoxicity of Endoperoxides from the Caribbean Sponge Plakortis halichondrioides towards Sensitive and Multidrug-Resistant Leukemia Cells: Acids vs. Esters Activity Evaluation
Abstract
:1. Introduction
2. Results
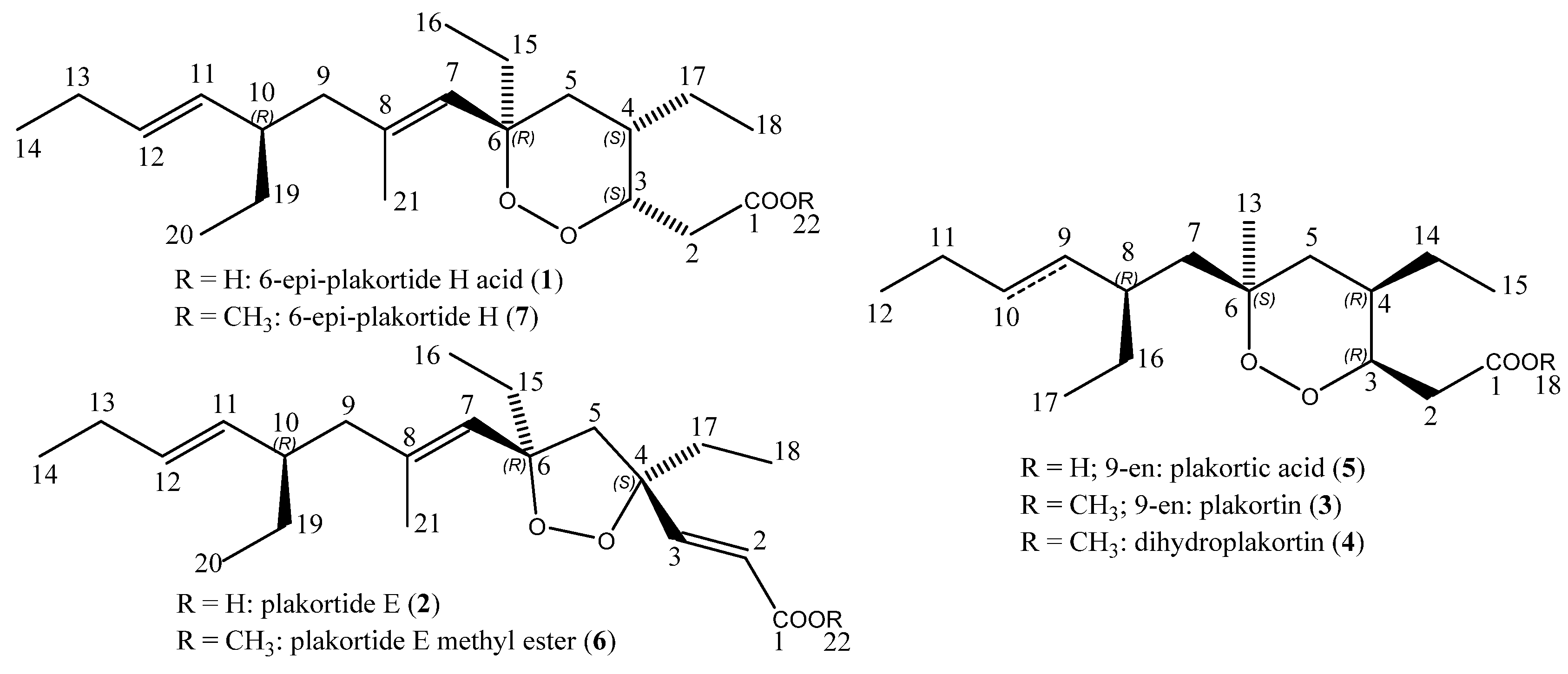
2.1. Isolation, Semi-Syntheses, and Identification of 6-Epi-Plakortide H Acid (1) and Its Methyl Ester 6-Epi-Plakortide H (7)
2.2. Isolation and Identification of Plakortide E (2), Plakortin (3), and Dihydroplakortin (4)
2.3. Semi-Synthesis of Plakortic Acid (5) and Plakortide E Methyl Ester (6)
2.4. Cytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. 6-Epi-plakortide H acid (1), [[(3S,4S,6R)-4,6-Diethyl-6-((1E,5E)-4-(R)-ethyl-2-methyl-octa-1,5-dienyl)-[1,2]dioxan-3-yl]-acetic acid]
4.2. Cytotoxicity Assays
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DCC | dicyclohexylcarbodiimide |
| DMAP | 4-dimethylamino pyridine |
| MDR | multi-drug resistant |
| NMR | nuclear magnetic resonance |
| RP-HPLC | reversed phase high performance liquid chromatography |
| SAR | structure-activity relationship |
| TLC | thin layer chromatography |
References
- Lucas, R.; Casapullo, A.; Ciasullo, L.; Gomez Paloma, L.; Payà, M. Cycloamphilectenes, a new type of potent marine diterpenes: Inhibition of nitric oxide production in murine macrophages. Life Sci. 2003, 72, 2543–2552. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Cirino, G.; De Gruttola, L.; Roviezzo, F. Tedanol: A potent anti-inflammatory ent-pimarane diterpene from the Caribbean sponge Tedania ignis. Bioorg. Med. Chem. 2009, 17, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Teta, R.; Irollo, E.; Della Sala, G.; Pirozzi, G.; Mangoni, A.; Costantino, V. Smenamides A and B, chlorinated peptide/polyketide hybrids containing a dolapyrrolidinone unit from the Caribbean sponge Smenospongia aurea. Evaluation of their role as leads in antitumor drug research. Mar. Drugs 2013, 11, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Miceli, R.; Ceccarelli, L.; Della Sala, G.; Irollo, E.; Mangoni, A.; Teta, R.; Pirozzi, G.; Costantino, V. Isolation and assessing the anti-proliferative activity in vitro of smenothiazole A and B, chlorinated thiazole-containing peptide/polyketides from the Caribbean sponge Smenospongia aurea. Mar. Drugs 2015, 13, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from sponges. 20. J-coupling analysis for stereochemical assignments in furanosides: Structure elucidation of vesparioside B, a glycosphingolipid from the marine sponge Spheciospongia vesparia. J. Org. Chem. 2008, 73, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Ectyoceramide, the first natural hexofuranosylceramide from the marine sponge Ectyoplasia ferox. Eur. J. Org. Chem. 2003, 2003, 1433–1437. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efferth, T.; Zacchino, S.; Georgiev, M.I.; Liu, L.; Wagner, H.; Panossian, A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine 2015, 22, A1–A3. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Rücker, G.; Falkenberg, M.; Manns, D.; Olbrich, A.; Fabry, U.; Osieka, R. Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs. Arzneimittelforschung 1996, 46, 196–200. [Google Scholar] [PubMed]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.G.; Dieckmann, D.; Efferth, T.; Schultz, E.S.; Funk, J.O.; Baur, A.; Schuler, G. Artesunate in the treatment of metastatic uveal melanoma—First experiences. Oncol. Rep. 2005, 14, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Gdynia, G.; Roth, W.; Bonavida, B.; Efferth, T. Combination treatment of malignant B-cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int. J. Oncol. 2009, 35, 149–158. [Google Scholar] [PubMed]
- Jansen, F.H.; Adoubi, I.; Kouassi Comoe, J.C.; DE Cnodder, T.; Jansen, N.; Tschulakow, A.; Efferth, T. First study of oral artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor markers. Anticancer Res. 2011, 31, 4417–4422. [Google Scholar] [PubMed]
- Rutteman, G.R.; Erich, S.A.; Mol, J.A.; Spee, B.; Grinwis, G.C.; Fleckenstein, L.; London, C.A.; Efferth, T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer Res. 2013, 33, 1819–1827. [Google Scholar] [PubMed]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Olbrich, A.; Sauerbrey, A.; Ross, D.D.; Gebhart, E.; Neugebauer, M. Activity of ascaridol from the anthelmintic herb Chenopodium anthelminticum L. against sensitive and multidrug-resistant tumor cells. Anticancer Res. 2002, 22, 4221–4224. [Google Scholar] [PubMed]
- Abbasi, R.; Efferth, T.; Kuhmann, C.; Opatz, T.; Hao, X.; Popanda, O.; Schmezer, P. The endoperoxide ascaridol shows strong differential cytotoxicity in nucleotide excision repair-deficient cells. Toxicol. Appl. Pharmacol. 2012, 259, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Varoglu, M.; Peters, B.M.; Crews, P. The structures and cytotoxic properties of polyketide peroxides from a Plakortis sponge. J. Nat. Prod. 1995, 58, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Valeriote, F.A.; Tenney, K.; Media, J.; Pietraszkiewicz, H.; Edelstein, M.; Johnson, T.A.; Amagata, T.; Crews, P. Discovery and development of anticancer agents from marine sponges: Perspectives based on a chemistry-experimental therapeutics collaborative program. J. Exp. Ther. Oncol. 2012, 10, 119–134. [Google Scholar] [PubMed]
- Rubush, D.M.; Morges, M.A.; Rose, B.J.; Thamm, D.H.; Rovis, T. An asymmetric synthesis of 1,2,4-trioxane anticancer agents via desymmetrization of peroxyquinols through a brønsted acid catalysis cascade. J. Am. Chem. Soc. 2012, 134, 13554–13557. [Google Scholar] [CrossRef] [PubMed]
- Opsenica, D.; Angelovski, G.; Pocsfalvi, G.; Juranić, Z.; Žižak, Ž.; Kyle, D.; Milhous, W.K.; Šolaja, B.A. Antimalarial and antiproliferative evaluation of bis-steroidal tetraoxanes. Bioorg. Med. Chem. 2003, 11, 2761–2768. [Google Scholar] [CrossRef]
- Parrish, J.D.; Ischay, M.A.; Lu, Z.; Guo, S.; Peters, N.R.; Yoon, T.P. Endoperoxide synthesis by photocatalytic aerobic [2+2+2] cycloadditions. Org. Lett. 2012, 14, 1640–1643. [Google Scholar] [CrossRef]
- Van Huijsduijnen, R.H.; Guy, R.K.; Chibale, K.; Haynes, R.K.; Peitz, I.; Kelter, G.; Phillips, M.A.; Vennerstrom, J.L.; Yuthavong, Y.; Wells, T.N.C. Anticancer properties of distinct antimalarial drug classes. PLoS ONE 2013, 8, e82962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In Vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Terzić, N.; Opsenica, D.; Milić, D.; Tinant, B.; Smith, K.S.; Milhous, W.K.; Šolaja, B.A. Deoxycholic acid-derived tetraoxane antimalarials and antiproliferatives. J. Med. Chem. 2007, 50, 5118–5127. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; Perkins, M.V. Structural diversity and chemical synthesis of peroxide and peroxide-derived polyketide metabolites from marine sponges. Nat. Prod. Rep. 2016, 33, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Hochmuth, T.; Teta, R.; Costantino, V.; Mangoni, A. Polyketide synthases in the microbiome of the marine sponge Plakortis halichondrioides: A metagenomic update. Mar. Drugs 2014, 12, 5425–5440. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Alarif, W.M.; Basaif, S.S.; Abo-Elkarm, M.; Hamann, M.T.; Wahba, A.E.; Ayyad, S.N. New cytotoxic cyclic peroxide acids from Plakortis sp. marine sponge. ARKIVOC 2015, 2015, 164–175. [Google Scholar] [PubMed]
- Jimenez, M.D.; Garzon, S.P.; Rodriguez, A.D. Plakortides M and N, bioactive polyketide endoperoxides from the Caribbean marine sponge Plakortis halichondrioides. J. Nat. Prod. 2003, 66, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Rudi, A.; Kashman, Y. Three new cytotoxic metabolites from the marine sponge Plakortis halichondrioides. J. Nat. Prod. 1993, 56, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Oli, S.; Abdelmohsen, U.R.; Hentschel, U.; Schirmeister, T. Identification of plakortide E from the Caribbean sponge Plakortis halichondroides as a trypanocidal protease inhibitor using bioactivity-guided fractionation. Mar. Drugs 2014, 12, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Carte, B.; Johnson, R.K.; Lahouratate, P. Plakortides, novel cyclic peroxides from the sponge Plakortis halichondrioides: activators of cardiac SR-Ca2+-pumping ATPase. J. Nat. Prod. 1996, 59, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Pinto, F.D.C.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic plakortides from the Brazilian marine sponge Plakortis angulospiculatus. J. Nat. Prod. 2015, 78, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an antibiotic from Plakortis halichondrioides. J. Org. Chem. 1978, 43, 3454–3457. [Google Scholar] [CrossRef]
- Kossuga, M.H.; Nascimento, A.M.; Reimão, J.Q.; Tempone, A.G.; Taniwaki, N.N.; Veloso, K.; Ferreira, A.G.; Cavalcanti, B.C.; Pessoa, C.; Moraes, M.O.; et al. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J. Nat. Prod. 2008, 71, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Persico, M.; Yang, F.; Lin, H.-W.; Guo, Y.W.; Basilico, N.; Parapini, S.; Taramelli, D.; Taglialatela-Scafati, O.; Fattorusso, C. Endoperoxide polyketides from a Chinese Plakortis simplex: Further evidence of the impact of stereochemistry on antimalarial activity of simple 1,2-dioxanes. Bioorg. Med. Chem. 2014, 22, 4572–4580. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Parapini, S.; Campagnuolo, C.; Basilico, N.; Taglialatela-Scafati, O.; Taramelli, D. Activity against Plasmodium falciparum of cycloperoxide compounds obtained from the sponge Plakortis simplex. J. Antimicrob. Chemother. 2002, 50, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Munro, M.H.G. (Eds.) Dictionary of Marine Natural Products; Chapman & Hall/CRC: London, UK, 2015.
- Phillipson, D.W.; Rinehart, K.L. Antifungal peroxide-containing acids from two Caribbean sponges. J. Am. Chem. Soc. 1983, 105, 7735–7736. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Bean, M.F.; Carte, B.K.; Westley, J.W.; Johnson, R.K.; Lahouratate, P. The plakortones, novel bicyclic lactones from the sponge Plakortis halichondrioides: Activators of cardiac SR-Ca2+-pumping ATPase. Tetrahedron 1996, 52, 377–394. [Google Scholar] [CrossRef]
- Sun, X.Y.; Tian, X.Y.; Li, Z.W.; Peng, X.S.; Wong, H.N. Total synthesis of plakortide E and biomimetic synthesis of plakortone B. Chemistry 2011, 17, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- The Sponge Guide. Available online: http://www.spongeguide.org (accessed on 7 July 2013).
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, A.; Gekeler, V.; Neumann, M.; Frese, G.; Handgretinger, R.; Kardos, G.; Diddens, H.; Niethammer, D. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990, 50, 6793–6799. [Google Scholar] [PubMed]
- Brugger, D.; Herbart, H.; Gekeler, V.; Seitz, G.; Liu, C.; Klingebiel, T.; Orlikowsky, T.; Einsele, H.; Denzlinger, C.; Bader, P.; et al. Functional analysis of P-glycoprotein and multidrug resistance associated protein related multidrug resistance in AML-blasts. Leuk. Res. 1999, 23, 467–475. [Google Scholar] [CrossRef]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Ngameni, B.; Wiench, B.; Krusche, B.; Horwedel, C.; Ngadjui, B.T.; Efferth, T. Cytotoxicity and mode of action of four naturally occurring flavonoids from the genus Dorstenia: Gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011, 77, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Alsalim, T.; Saeed, B.; Saeed, M.E.; Kadioglu, O.; Abbo, H.S.; Titinchi, S.J.; Efferth, T. Modulation of P-glycoprotein activity by novel synthetic curcumin derivatives in sensitive and multidrug-resistant T-cell acute lymphoblastic leukemia cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.W.; Yarbrough, J.W. Trends in structure-toxicity relationships for carbonyl-containing α,β-unsaturated compounds. SAR QSAR Environ. Res. 2004, 15, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, D.J.; Hamann, M.T. Isolation and biological evaluation of filiformin, plakortide F, and plakortone G from the Caribbean sponge Plakortis sp. J. Nat. Prod. 2001, 64, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, C.; Campiani, G.; Catalanotti, B.; Persico, M.; Basilico, N.; Parapini, S.; Taramelli, D.; Campagnuolo, C.; Fattorusso, E.; Romano, A.; et al. Endoperoxide derivatives from marine organisms: 1,2-dioxanes of the plakortin family as novel antimalarial agents. J. Med. Chem. 2006, 49, 7088–7094. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Munro, M.H.G. (Eds.) Dictionary of Marine Natural Products; Chapman & Hall/CRC: New York, NY, USA, 2008.
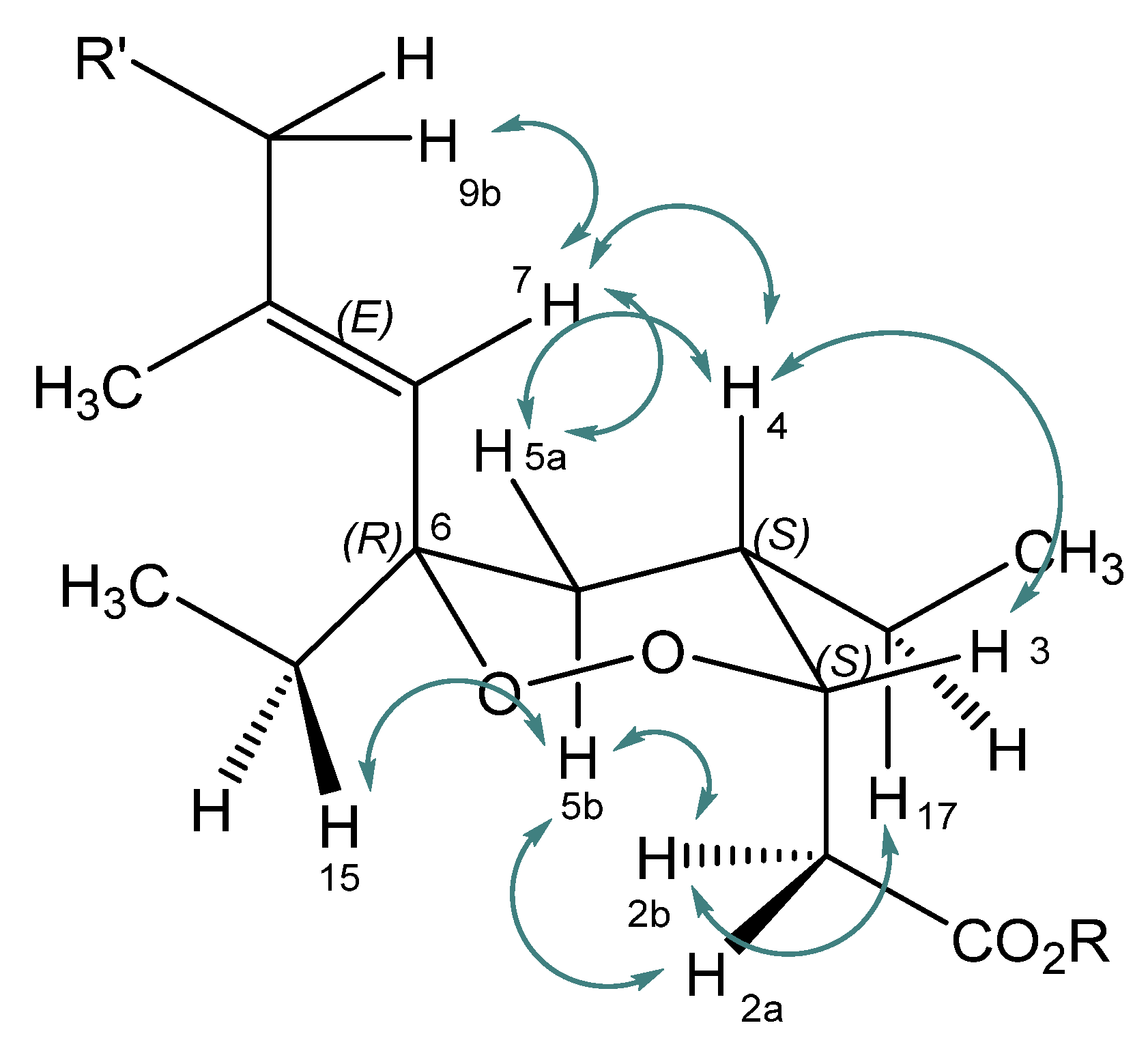
| Position | δC | Mult | δH | Mult | J in Hz | NOESY |
|---|---|---|---|---|---|---|
| 1 | 177.06 | C | ||||
| 2 | 31.31 | CH2 | 3.07 (2a) | dd | 15.9, 9.6 | 2b, 5b |
| 2.41 (2b) | dd | 15.9, 3.4 | 2a, 2b, 17 | |||
| 3 | 78.64 | CH | 4.44 | ddd | 3.3, 5.2, 9.5 | 2a, 2b, 4 |
| 4 | 35.37 | CH | 2.09 a | 3, 5a, 7 | ||
| 5 | 35.52 | CH2 | 1.61 a (5a) | m | 4, 7, 5b | |
| 1.26 a (5b) | 2a, 2b, 5a, 15 | |||||
| 6 | 84.49 | C | ||||
| 7 | 127.13 | CH | 5.12 | s | 4, 5a, 9b | |
| 8 | 137.58 | C | ||||
| 9 | 47.60 | CH2 | 2.06 a–1.94 a | 7 | ||
| 10 | 42.60 | CH | 2.02 a | |||
| 11 | 133.14 | CH | 5.09 | dd | 15.1 | |
| 12 | 131.89 | CH | 5.35 | dt | 15.1, 6.2, 6.2 | |
| 13 | 25.77 | CH2 | 1.97 a | |||
| 14 | 14.15 | CH3 | 0.98 | t | 7.4 | |
| 15 | 32.58 | CH2 | 1.55 | m | 5b | |
| 16 | 7.78 | CH3 | 0.86 | t | 7.4 | |
| 17 | 25.12 | CH2 | 1.16 a | 2b | ||
| 18 | 11.12 | CH3 | 0.92 | t | 7.6 | |
| 19 | 28.05 | CH2 | 1.39 | |||
| 1.17 a | m | |||||
| 20 | 11.78 | CH3 | 0.84 | t | 7.4 | |
| 21 | 17.04 | CH3 | 1.70 | s |
| Compound | CCRF-CEM IC50 [µM] | CEM/ADR5000 IC50 [µM] | Resistance Ratio |
|---|---|---|---|
| 6-epi-Plakortide H acid (1) | 0.18 ± 0.003 | 0.36 ± 0.01 | 2.00 |
| Plakortide E (2) | 1.90 ± 0.09 | 4.30 ± 0.1 | 2.26 |
| Plakortin (3) | 1.97 ± 0.06 | 2.26 ± 0.08 | 1.15 |
| Dihydroplakortin (4) | 1.13 ± 0.11 | 1.85 ± 0.13 | 1.64 |
| Plakortic acid (5) | 0.19 ± 0.004 | 0.24 ± 0.009 | 1.26 |
| Plakortide E methyl ester (6) | NI 1 | NI 1 | N/A |
| 6-epi-Plakortide H (7) | NI 1 | NI 1 | N/A |
| Doxorubicin * | 0.012 ± 0.002 | 12.2 ± 54.2 | 1,036 |
| Epirubicin * | 0.022 ± 0.003 | 10.50 ± 3.90 | 484 |
| Vincristine * | 0.002 ± 0.0001 | 1.04 ± 0.15 | 613 |
| Docetaxel * | 0.0004 ± 0.0001 | 0.18 ± 0.02 | 438 |
| Paclitaxel * | 0.004 ± 0.0004 | 0.741 ± 0.137 | 200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirmeister, T.; Oli, S.; Wu, H.; Della Sala, G.; Costantino, V.; Seo, E.-J.; Efferth, T. Cytotoxicity of Endoperoxides from the Caribbean Sponge Plakortis halichondrioides towards Sensitive and Multidrug-Resistant Leukemia Cells: Acids vs. Esters Activity Evaluation. Mar. Drugs 2017, 15, 63. https://doi.org/10.3390/md15030063
Schirmeister T, Oli S, Wu H, Della Sala G, Costantino V, Seo E-J, Efferth T. Cytotoxicity of Endoperoxides from the Caribbean Sponge Plakortis halichondrioides towards Sensitive and Multidrug-Resistant Leukemia Cells: Acids vs. Esters Activity Evaluation. Marine Drugs. 2017; 15(3):63. https://doi.org/10.3390/md15030063
Chicago/Turabian StyleSchirmeister, Tanja, Swarna Oli, Hongmei Wu, Gerardo Della Sala, Valeria Costantino, Ean-Jeong Seo, and Thomas Efferth. 2017. "Cytotoxicity of Endoperoxides from the Caribbean Sponge Plakortis halichondrioides towards Sensitive and Multidrug-Resistant Leukemia Cells: Acids vs. Esters Activity Evaluation" Marine Drugs 15, no. 3: 63. https://doi.org/10.3390/md15030063






