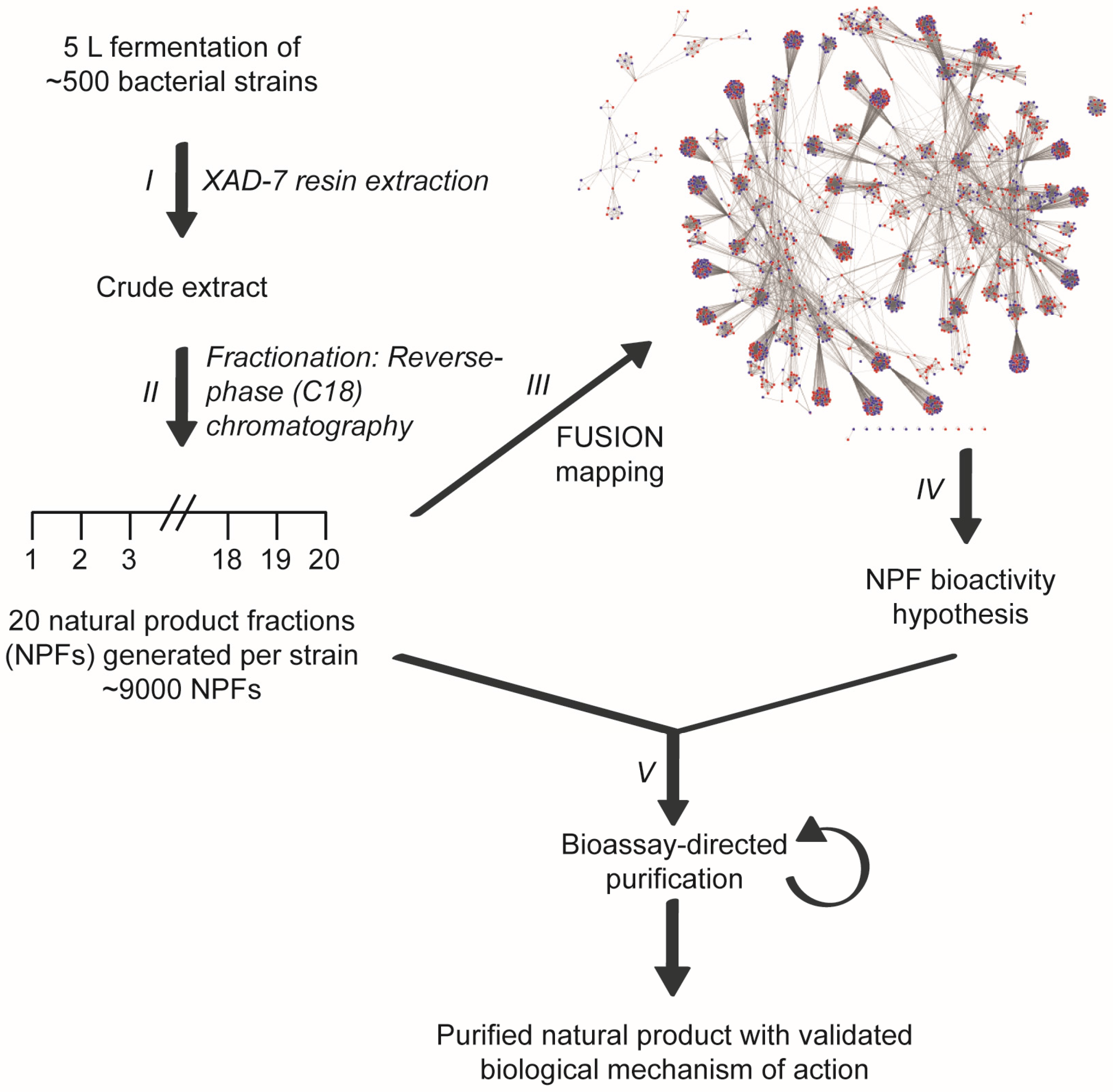
2. Results
AKT signaling is central to a large number of functional cellular processes and is frequently dysregulated in diseases such as cancer [
3,
4,
5,
6,
7,
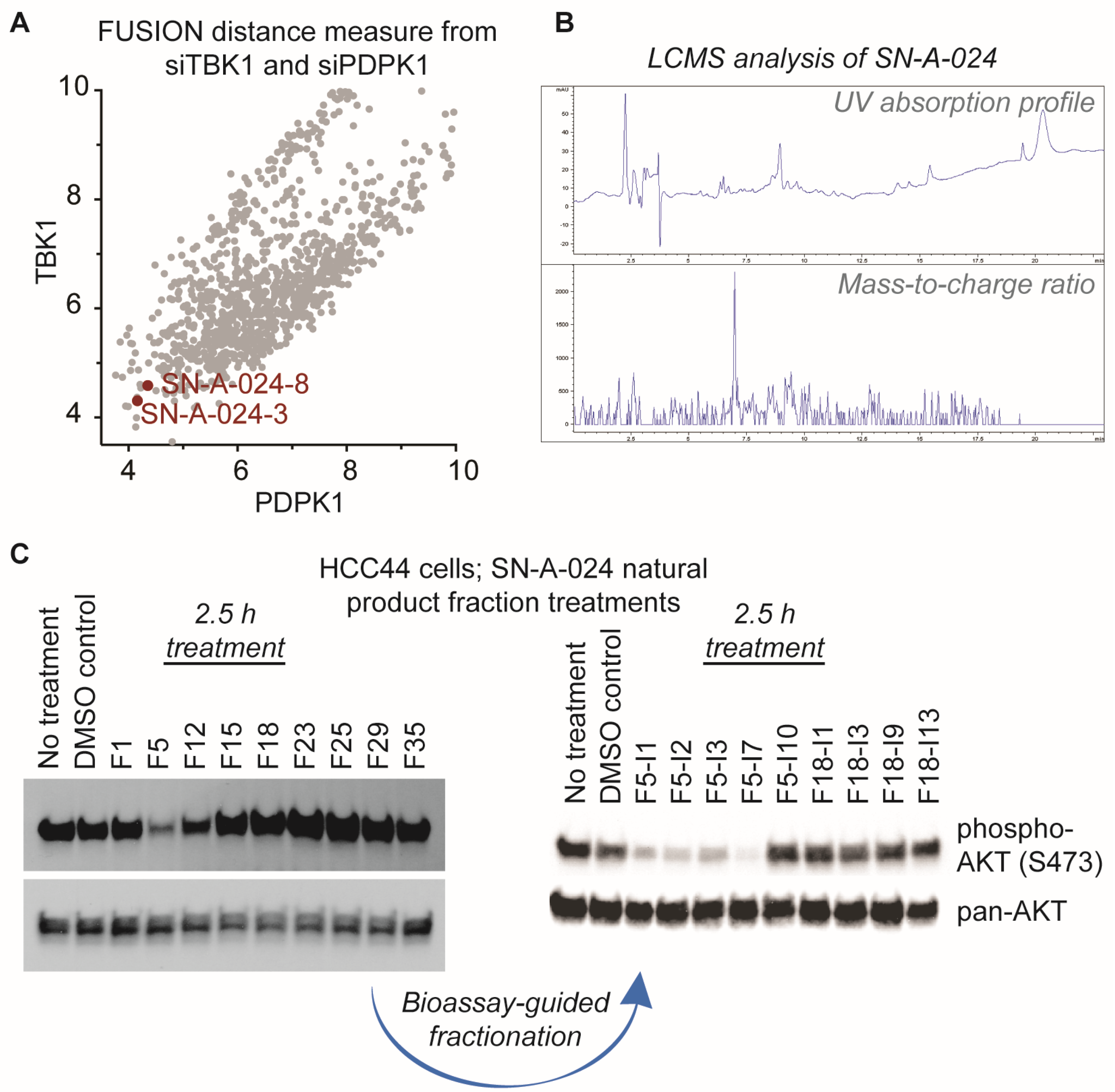
8]. With the overall goal of identifying natural products that target this signaling pathway, we evaluated the FUSION map for known perturbagens of AKT signaling; specifically, we assessed the intersection of the siRNA clades generated by siTBK1 and siPDPK1 (TANK-binding kinase 1 and 3-phosphoinositide dependent protein kinase 1, respectively) (
Figure 2A). This approach was chosen because it has been established that TBK1 and PDPK1 (also known as PDK1) have direct regulatory roles in AKT activation [
9,
10,
11]. We hypothesized that natural product fractions sharing similar, relatively small distance measures from both kinases within the FUSION map would perturb AKT signaling in a manner phenotypically similar to the known siRNAs. From the siTBK1-siPDK1 intersection, we identified two natural product fractions, SN-A-024-8 and SN-A-024-3, produced by the same strain of
Streptomyces coeruleoaurantiacus. The strain (SN-A-024) was originally isolated from sediment samples obtained in West Plana Cay, Bahamas, and the fractions of secondary metabolites generated by the microbes were found to be chemically complex (
Figure 2B). To test the FUSION-derived hypothesis that SN-A-024 produced small molecule modulators of AKT signaling, we cultured and re-fractionated the strain for biological testing. The resulting fractions were tested in HCC44 non-small cell lung cancer cells for their effect on AKT active site phosphorylation (serine 473) after 2.5 h of treatment. Of the nine fractions tested, one fraction (F5) was found to be unique in its ability to attenuate AKT phosphorylation at the S473 site (
Figure 2C). Sub-fractionation of the F5 fraction by automated liquid chromatography (ISCO) followed by biological testing of the subsequent fractions further resolved a subset of chemical fractions with robust activity against AKT phosphorylation.
This process of biological phenotype-guided fractionation was iterated until a fraction enriched in a single molecule was isolated. High-resolution mass spectrometry (ESI-MS) analysis of the natural product afforded a mass/charge ratio consistent with the empirical formula C
12H
17N
5O
4 (HRMS
m/z [M + H] 296.1358). Final structural elucidation was accomplished by coupling the high-resolution ESI-MS (HRMS) data with NMR analyses of the compound to reveal
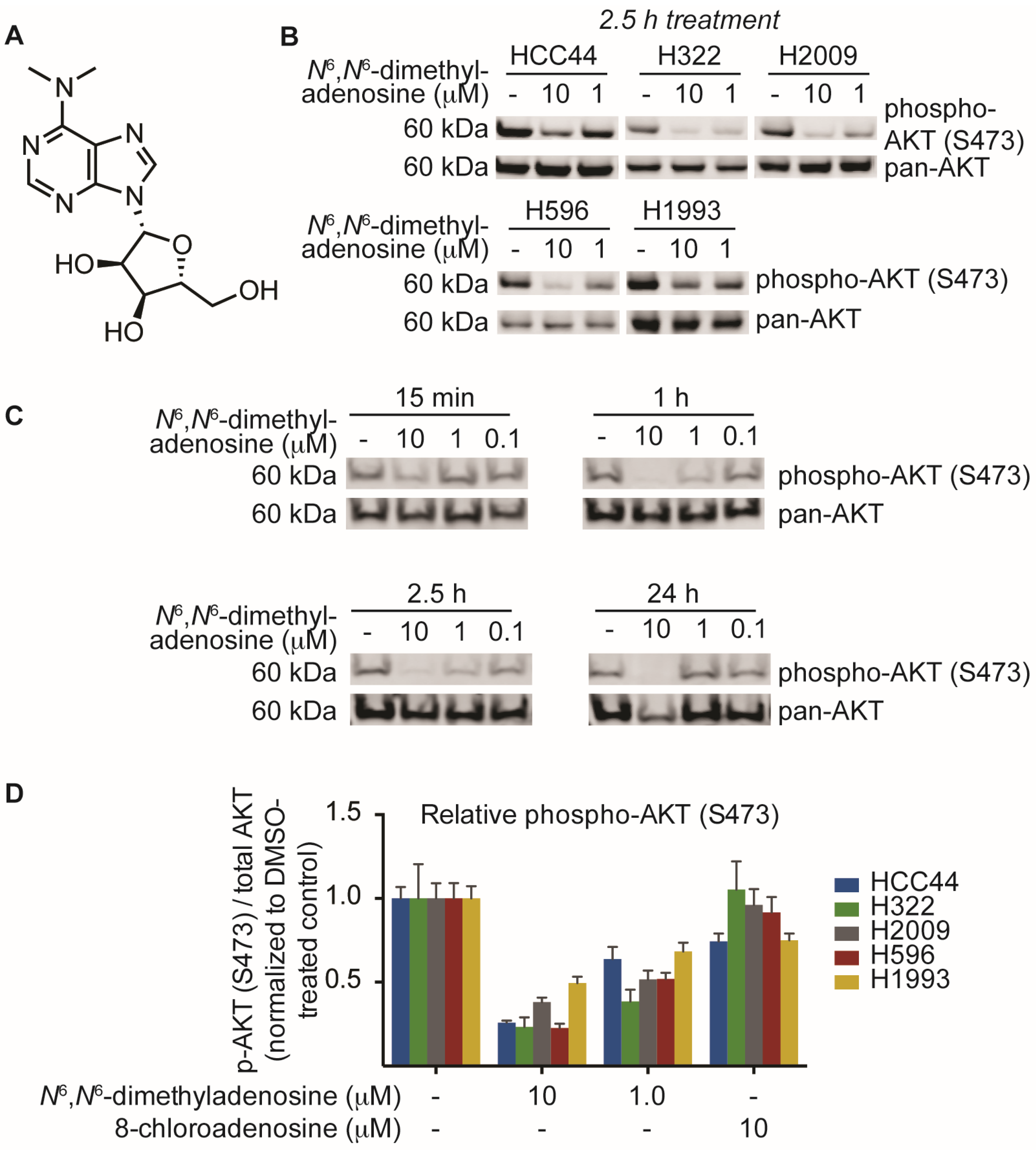
N6,
N6-dimethyladenosine as the major metabolite (
Figure 3A). After elucidation of the structure, we sought to validate our biological findings using a commercially available source of
N6,
N6-dimethyladenosine. Upon testing the small molecule against five non-small cell lung cancer cell lines, we found that commercial
N6,
N6-dimethyladenosine recapitulated SN-A-024’s effect on AKT signaling, attenuating S473 phosphorylation in all cell types tested at both 10 and 1 micromolar concentrations (
Figure 3B). A time course assessment of
N6,
N6-dimethyladenosine’s effect on phospho-AKT in HCC44 cells established that the small molecule rapidly blunted AKT signaling, with only 15 min of treatment reducing levels by approximately half (
Figure 3C).
Modified nucleoside analogs are commonly found in tRNA and rRNA in a variety of organisms and contribute to the structural diversity of RNA [
12,
13,
14,
15]. The nucleoside analog
N6,
N6-dimethyladenosine has been well-studied in the context of rRNA; the 3’-end of many species’ rRNA sequences contains two adjacent, dimethylated adenosine residues that participate in rRNA stability [
16,
17,
18]. The protein complex responsible for the methylation of adenosine to form the
N6,
N6-dimethyl variant has not been fully characterized. The METTL3-METTL14 methyltransferase complex has been identified in mammalian cells as a biosynthetic mechanism by which the monomethylated variant
N6-methyladenosine can be generated; interestingly though, the enzymes responsible for monomethylation do not appear to facilitate dimethylation events at the
N6 position, suggesting functional distinctions in the methyltransferase components [
19,
20].
Several nucleoside analogs have been employed for therapeutic intervention in the clinic; vidarabine, a synthetic ribosyl-modified adenosine analog based upon isolates from a marine organism, has been used extensively as an antiviral therapeutic while 8-chloroadenosine and 8-aminoadenosine have been used as anti-cancer agents [
21,
22,
23,
24]. Despite the structural similarity between 8-chloroadenosine and the natural product isolate
N6,
N6-dimethyladenosine, 8-chloroadenosine has been reported to
increase AKT S473 phosphorylation in the context of renal cell carcinoma [
25,
26]. We examined the possibility that the paradoxical differences observed between the two molecules may indicate lineage-specific sensitivity. Testing equimolar amounts of the two nucleoside analogs, we measured AKT phosphorylation by in-cell western in five lung cancer cell lines. The results indicated a clear separation in the activities of
N6,
N6-dimethyladenosine and 8-chloroadenosine:
N6,
N6-dimethyladenosine strongly inhibited AKT phosphorylation while 8-chloroadenosine elicited only a moderate effect in all cell lines tested (
Figure 3D). These data suggest that the two nucleoside analogs differ in their mechanisms of action to some extent and more broadly, the results may indicate that specificity of nucleoside analog-protein binding events is higher than was initially considered.
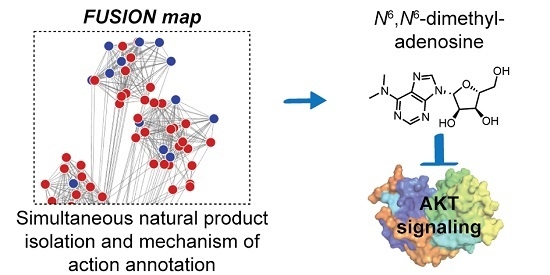
Implementing a FUSION-directed strategy for the selection of a complex natural product fraction allowed for the rapid identification of SN-A-024’s biologically active metabolite from a complex mixture of chemicals. With our initial FUSION hypothesis confirmed and a purified molecule in hand, we next aimed to assess whether additional signaling pathways were impacted by
N6,
N6-dimethyladenosine. To address this, the nucleoside analog was first tested at three different concentrations against HCC44 cells and an immunoblot assay was used to measure global tyrosine phosphorylation. After four hours of treatment, the three
N6,
N6-dimethyladenosine-treated samples showed no change in total tyrosine phosphorylation compared to the control-treated sample, suggesting that the natural product’s effect on AKT resulted from specific pathway perturbations instead of global tyrosine kinome deregulation (
Figure 4A). To further survey the effect of
N6,
N6-dimethyladenosine on other cellular signaling pathways, a reverse phase protein array (RPPA) experiment was designed and conducted using HCC44 cells (Supporting Information). Staurosporine, a broad-acting ATP-competitive kinase inhibitor, was included in the experiment to benchmark non-specific kinase inhibition effects in the natural product-treated samples. Notably, among the 304 antibodies tested in the RPPA panel, the largest
N6,
N6-dimethyladenosine-dependent change detected compared to control samples was AKT-S473 phosphorylation. Importantly, AKT-S473 phosphorylation was not inhibited by staurosporine. Globally, the RPPA experiment produced expected, dose-sensitive responses between the 10 micromolar and 1 micromolar
N6,
N6-dimethyladenosine-treated samples following normalization to the controls, while staurosporine displayed a distinct response (
Figure 4B). Closer comparison of the staurosporine- versus
N6,
N6-dimethyladenosine-treated samples provided insight into the selective biological space targeted by each molecule (
Figure 4C). Collectively, the RPPA results speak to the specificity of the marine-derived natural product and delineate its mechanism of action from those of promiscuous kinase effectors.
3. Discussion
The work presented herein describes the isolation and characterization of the marine-derived natural product N6,N6-dimethyladenosine, which was identified via an iterative fractionation process guided by the assessment of AKT phosphorylation at the S473 site. Nucleoside modifications can be found in all three domains of life and N6,N6-dimethyladenosine has specifically been studied in the context of rRNA function. Despite the ubiquitous presence of nucleoside modifications in single and multicellular organisms, we have only an inchoate understanding of their function at the present. The isolation and bioassay development process that led to the identification of N6,N6-dimethyladenosine was significantly expedited by first employing a FUSION-directed approach to generate a mechanism of action hypothesis. We initiated our study with the goal of identifying novel small molecules that could perturb the AKT signaling axis. Considering the dysregulated state of AKT signaling in many diseases, small molecule modulators targeting different pathway components are invaluable for expanding our basic understanding of disease mechanisms. Consistent with our initial hypothesis, we found that N6,N6-dimethyladenosine-treatment rapidly decreases the activity of AKT in a variety of non-small cell lung cancer cell lines. An RPPA panel consisting of 304 antibodies validated this finding and reinforced the biological basis for the connection made between SN-A-024 and siTBK1/siPDK1 in the FUSION map. The global perspective afforded by the RPPA experiment additionally suggested that the natural product’s mechanism of action exhibits some degree of specificity, despite its ostensibly ubiquitous scaffold. In all, this report details the successful implementation of the FUSION strategy to pair natural products with their biological modes of action. We report the effect of the natural product N6,N6-dimethyladenosine on AKT signaling and highlight the potential for this molecule as a tool for exploring new targetable biology surrounding AKT regulation. Given current efforts of the biological community to deploy natural product libraries in large-scale, cell-based phenotypic screens, we anticipate the FUSION platform will also have general utility for stratification of hits from these screens into biological “complementation groups” which will, in turn, allow for accelerated annotation of the biological modes-of-action of molecules with bioactivities of interest.
4. Materials and Methods
4.1. Collection and Phylogenetic Analysis of Strain SNA-024
The marine-derived bacterium strain SNA-024 was isolated from a sediment sample collected from West Plana Cay, Bahamas. Bacterial spores were collected via a stepwise centrifugation as follows: 2 grams of sediment was dried over 24 h in an incubator at 35 °C and the resulting sediment was added to 10 mL sH2O containing 0.05% Tween 20. After vigorous vortex for 10 min, the sediment was centrifuged at 18,000 rpm for 25 min (4 °C) and the resulting spore pellet was collected. The resuspended spore pellet (4 mL sH2O) was plated on an acidified Gause media, giving rise to individual colonies of SNA-024 after 2 weeks. Analysis of the 16S rRNA sequence of SNA-024 revealed 98% identity to Streptomyces coeruleoaurantiacus.
4.2. Cultivation and Extraction
Bacterium SNA-024 was cultured in 15 × 2.8 L Fernbach flasks each containing 1 L of seawater-based medium (10 grams starch, 4 grams yeast extract, 2 grams peptone, 1 gram CaCO3, 40 milligrams Fe2(SO4)3·4H2O, 100 milligrams KBr) and shaken at 200 rpm at 27 °C. After seven days of cultivation, sterilized XAD-7-HP resin (20 g/L) was added to absorb the organic products, and the culture and resin were shaken at 200 rpm for 2 h. The resin was filtered through cheesecloth, washed with deionized water, and eluted with acetone. The acetone-soluble fraction was dried in vacuo to yield 11.4 grams of extract.
4.3. Purification of a Fraction Enriched in N6,N6-Dimethyladenosine
Crude extract (11.4 grams) was separated into nine fractions by reverse phase chromatography (C18) using a stepwise gradient, with MeOH/H2O (20%–100%). The biologically active fraction (F5, 91.4 milligrams) was further purified using an automated reversed phase chromatography (ISCO, RediSep Rf Gold 30 grams C18, 35 mL/min) using a gradient solvent system from 10% to 100% MeOH:H2O over 25 min, and 30 fractions (F5-I1–F5-I30) were collected. Fractions were combined based on LC-MS profile similarity to a total of five fractions (F5-I1, F5-I3, F5-I5, F5-I7, and F5-I9). Biologically active F5-I7 (5.3 milligrams) was further purified by reverse-phase HPLC (Phenomenex Luna, C18, 250 × 10.0 mm, 5 μm, 2.5 mL/min) using a gradient solvent system, acetonitrile/water with 0.1% formic acid (10%–100%) over 20 min with five fractions collected. Fraction F5-I7-1 (~0.5 milligrams, tR = 7.0 min) was biologically active and used for structural characterization (HRMS and NMR), and biological assays. As described below, the inseparable mixture contained in fraction F5-I7-1 was analyzed by HRMS and NMR to determine the major component.
4.4. NMR Characterization
High-resolution ESI-MS (HRMS) analysis of the enriched fraction F5-I7-1 gave
m/z 296.1352 [M + H]⁺ for the major metabolite consistent with a molecular formula of C
12H
17N
5O
4 (calculated for C
12H
18N
5O
4, 296.1358).
1H-NMR data indicated that the major metabolite was a nucleoside in nature with two aromatic signals and signals diagnostic of a glucoside. Analysis of the molecular formula and the diagnostic NMR signals suggested a structure consistent with
N6,
N6-dimethyladenosine. Due to fraction F5-I7-1 material constraints, commercially available
N6,
N6-dimethyladenosine (ChemBridge Corporation, San Diego, CA, USA) was used to directly compare the
1H NMR.
1H-NMR comparison of commercial
N6,
N6-dimethyladenosine with the enriched fraction F5-I7-1 indicated that the major metabolite was
N6,
N6-dimethyladenosine (
Supplementary Table S1,
Supplementary Figure S1).
4.5. Reagents and Antibodies
HCC44, H1993, H322, H1993, and H596 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% fetal bovine serum and penicillin/streptomycin. Cells were maintained in a humidified incubator at 37 °C in 5% CO2. 8-chloroadenosine was obtained from Santa Cruz Biotechnology. For immunoblot analyses, the following antibodies were used: pan-AKT (Cell Signaling Technologies #4691; 1:1000 dilution), phospho-AKT (S473) (Cell Signaling Technologies #4060; 1:2000 dilution), β-Actin (Cell Signaling Technologies #3700; 1:1000 dilution), and phospho-Tyrosine (Cell Signaling Technologies #9411; 1:2000 dilution). All antibody dilutions were made in Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA). Western membrane blocking was also accomplished with Odyssey Blocking Buffer. Immunoblots imaged with the Li-Cor Odyssey imaging system were incubated with the appropriate Li-Cor secondary antibodies at 1:10,000 before imaging. For experiments utilizing commercially sourced N6,N6-dimethyladenosine, the small molecule used was obtained from ChemBridge (San Diego, CA, USA).
4.6. In-Cell Western
For in-cell western experiments, cells were seeded in 96-well plates at 20,000 cells per well and treated with the appropriate drug 16 h after seeding. One hour after the start of treatment, the media was removed and cells were fixed with 4% paraformaldehyde (w/v) for 10 min at room temperature. Following the removal of the formaldehyde solution, permeabilization was accomplished by incubating the cells for 30 min with 0.1% Triton-X (v/v) in 1× PBS. The wells were blocked for one hour at room temperature with Odyssey Blocking Buffer, then incubated with the primary antibodies overnight at 4 °C. The antibodies used included pan-AKT (Cell Signaling Technologies #4691; 1:400 dilution) and phospho-AKT (S473) (Cell Signaling Technologies #4060; 1:200 dilution). Following the overnight incubation, wells were incubated with the appropriate Li-Cor secondary antibodies at 1:800 before imaging on the Li-Cor Odyssey imaging system.
4.7. Reverse Phase Protein Array
For reverse phase protein array (RPPA) experiments, HCC44 cells were seeded in duplicate in 6-well plates and treated with the appropriate compound for 2.5 h. Following the completion of treatment, cell lysates were collected and prepared per the MD Anderson RPPA Core Facility submission guidelines. Briefly, the media was removed from the treated cells, the wells were washed two times with ice cold 1× PBS, and ice cold lysis buffer was added to the plate (1% Triton X-100 (v/v), 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 100 mM NaF, 10 mM Na pyrophosphate, 1 mM Na3VO4, 10% glycerol (v/v), and freshly added protease and phosphatase inhibitors). The plate was incubated on ice for 20 min with the lysis buffer, shaking every 5 min. The lysates were transferred to a microcentrifuge tube and centrifuged for 10 min at 14,000 rpm at 4 °C. The supernatant was collected and protein concentration quantified. The lysates were combined with 4× sample buffer (40% Glycerol, 8% Sodium dodecyl sulfate (SDS), 0.25 M Tris-HCl, pH 6.8) and frozen at −80 °C. The RPPA experiment and subsequent data analysis was performed by MD Anderson RPPA Core Facility. Log2 transformed values were used for analysis and plotting; 2-way hierarchical cluster analysis was performed with R, version 3.2.3 using the ‘stats’ package and heatmap function.