Experimental Study on the Evaporation and Condensation Heat Transfer Characteristics of a Vapor Chamber
Abstract
:1. Introduction
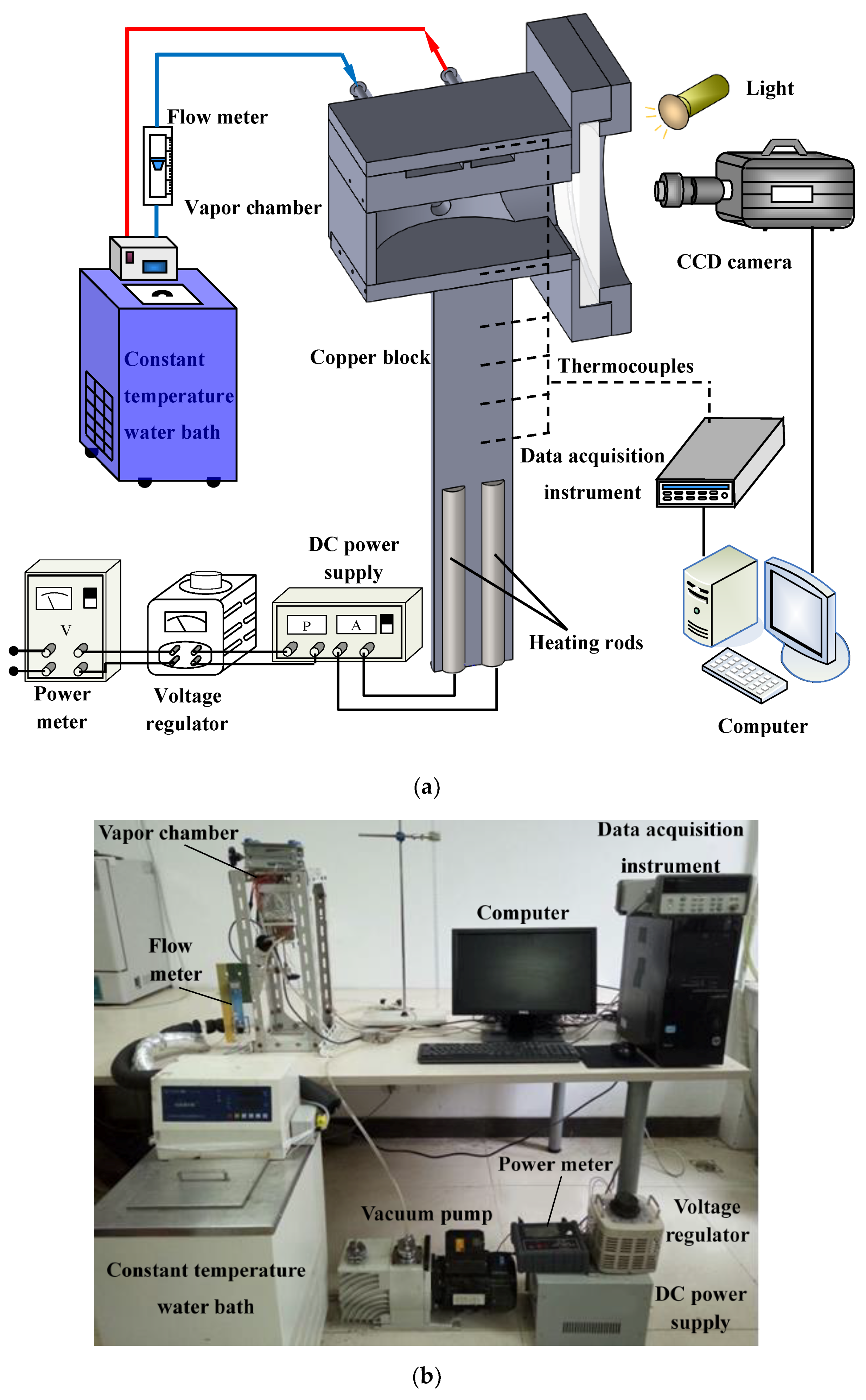
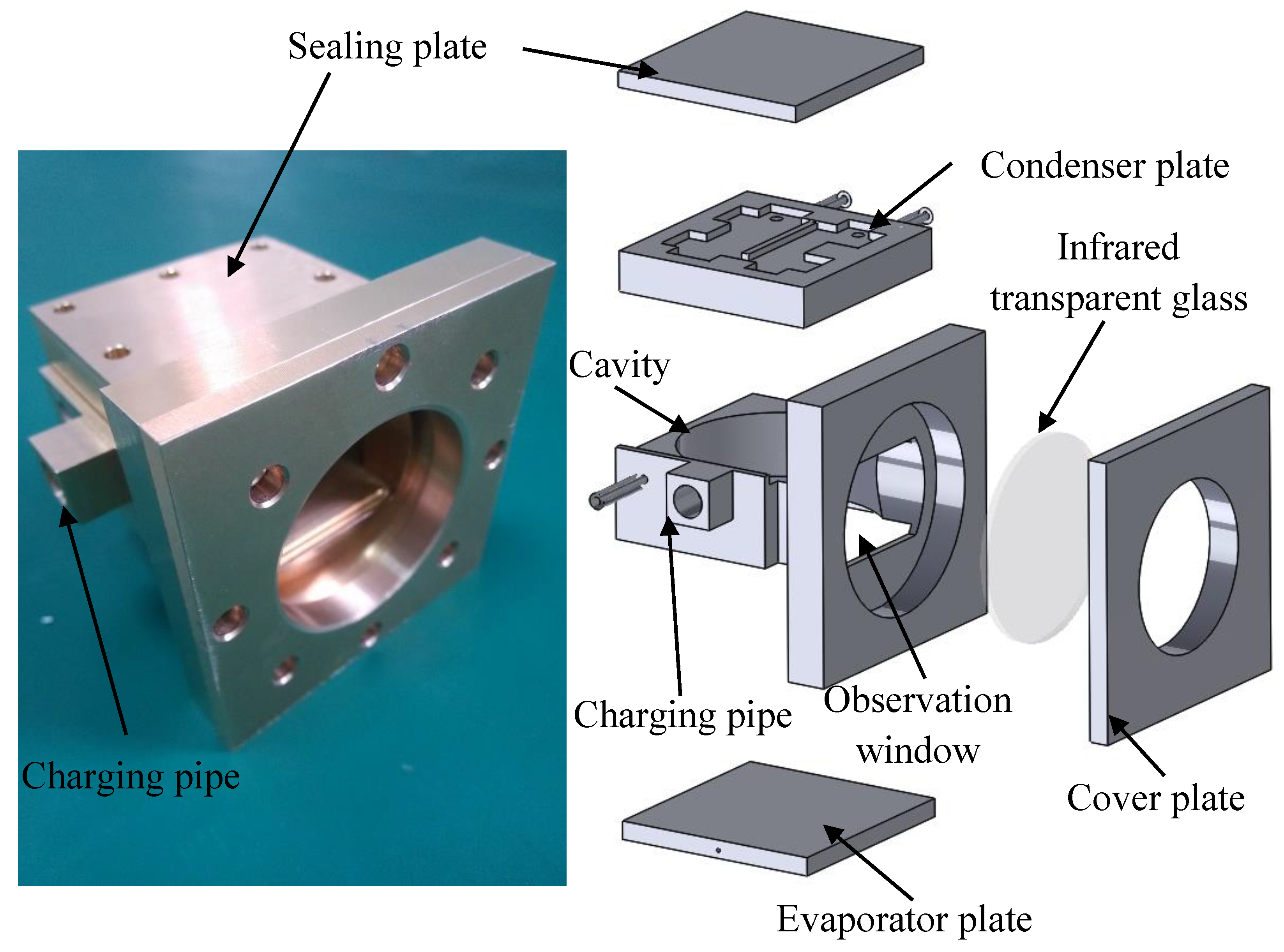
2. Experiment Methodology
2.1. Experimental System
2.2. Evaluation Index
3. Results and Discussion
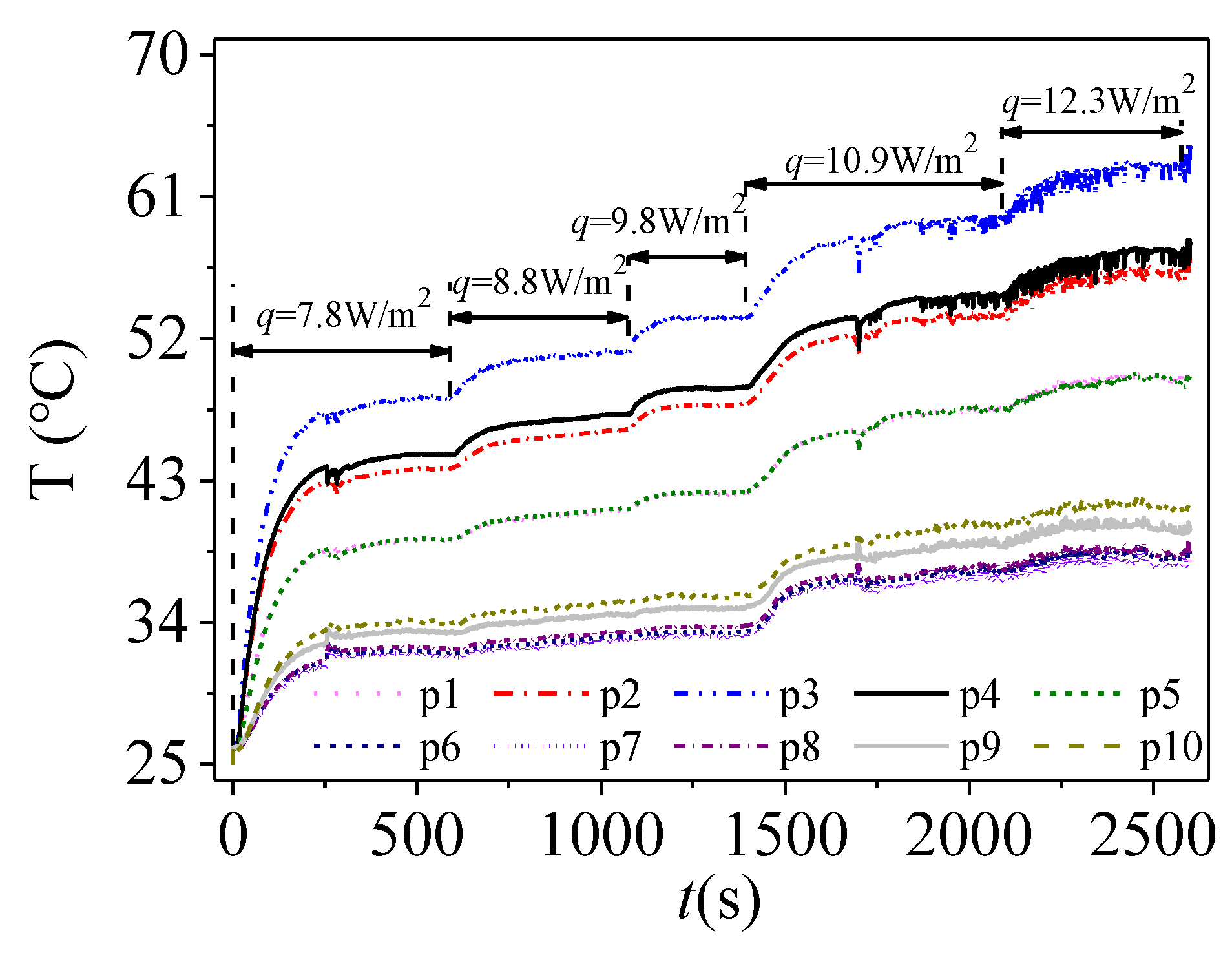
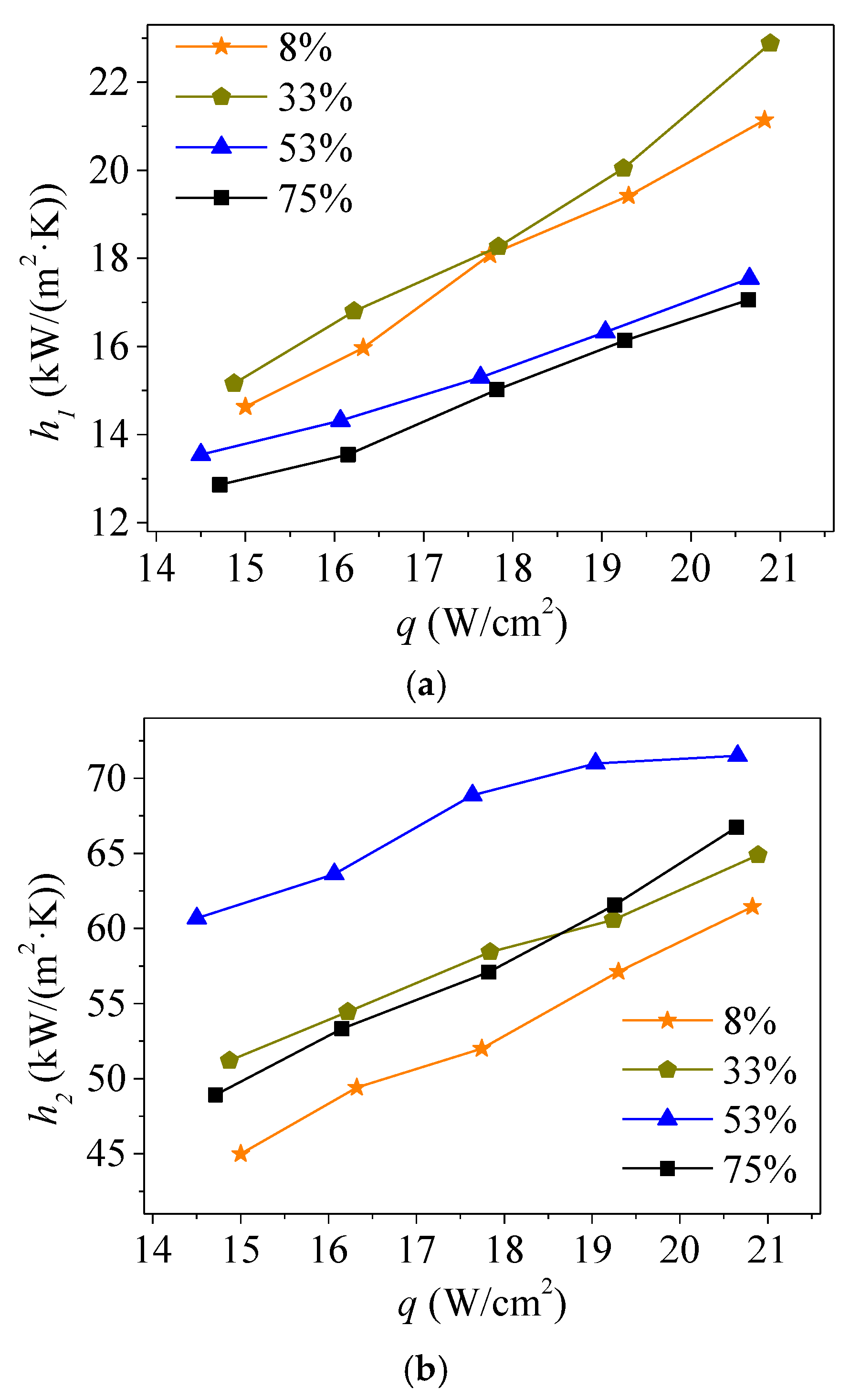
3.1. Thermal Performance Analysis
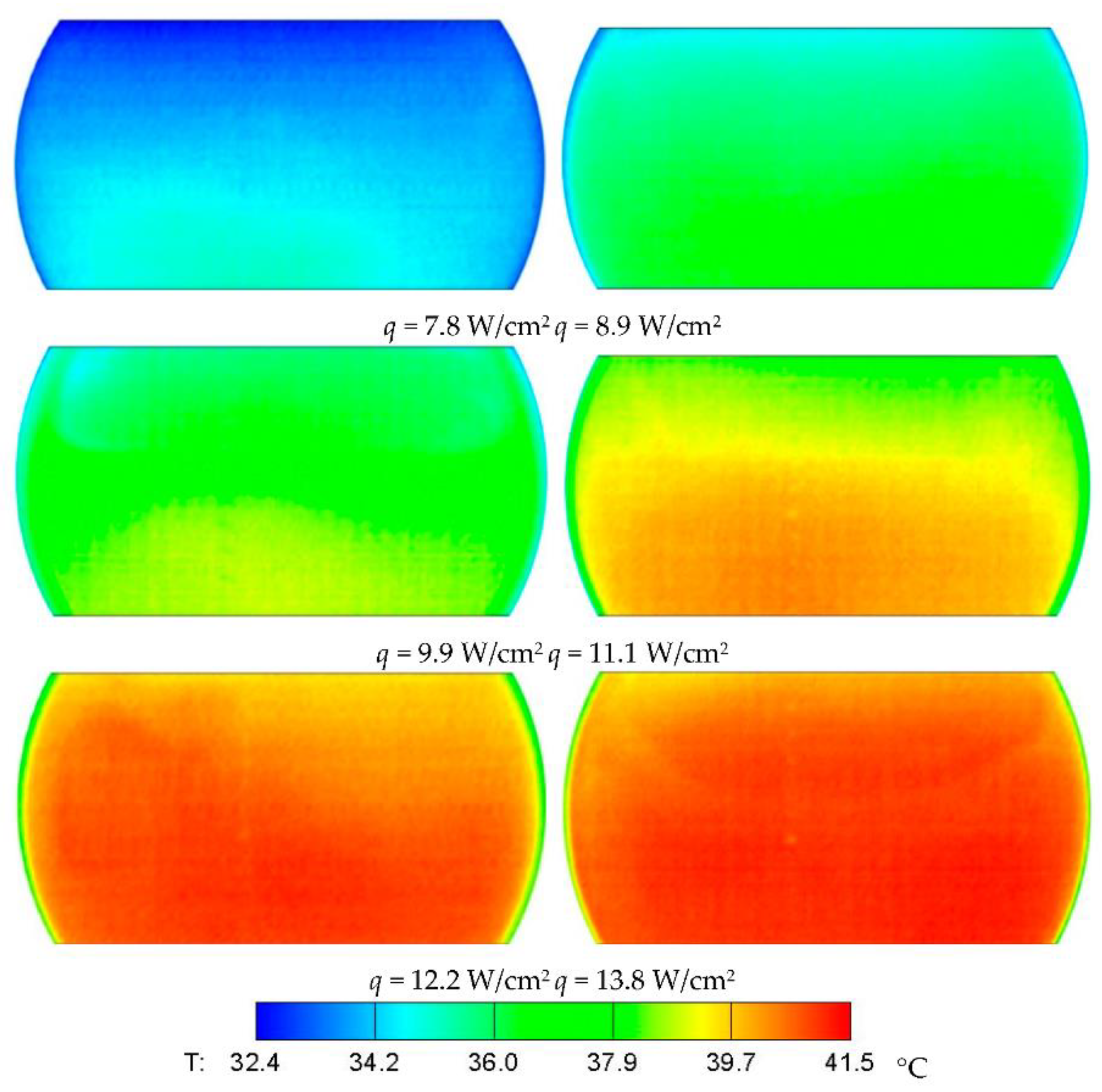
3.2. Temperature Distribution in Cavity
4. Conclusions
- (1)
- The optimum filling rates for the evaporation phase change and condensation phase change were different: 33% for evaporation and 52% for condensation. The condensation heat transfer coefficient was nearly triple that of evaporation under the same liquid filling rate, leading to better thermal performance of the vapor chamber with a liquid filling rate of 33%.
- (2)
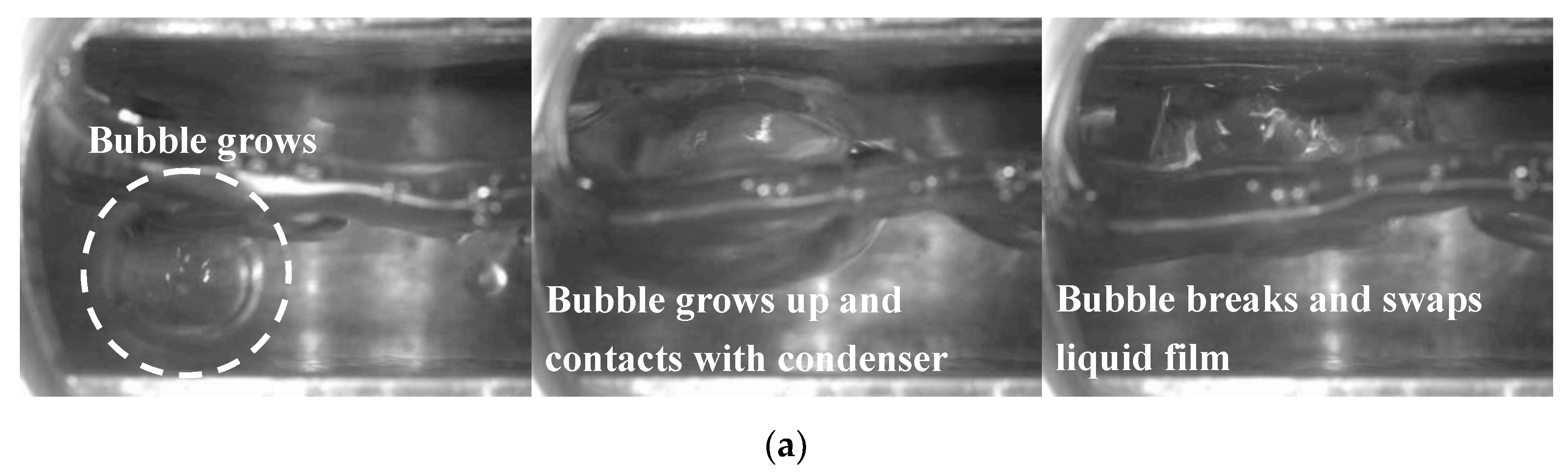
- The evaporation/boiling heat transfer could be improved by promoting the detachment of the condensate from the condenser surface using the interference of easily broken bubbles and boiling liquid. In addition, a rise in heat input significantly improved the temperature distribution due to the intensified heat transfer on the evaporator surface.
- (3)
- A minimal thermal resistance value appeared as the liquid filling rate fluctuated between 8% and 75%, indicating that within a certain range of liquid filling rate, an optimal coupling degree between evaporation/boiling and condensation can be reached.
- (4)
- The evaporation/boiling heat transfer can be enhanced through the interference of easily broken bubbles and boiling liquid. According to the infrared thermal images, as the heat input increased, more uniform temperature distribution was observed due to intensified heat transfer on the evaporator surface.
Author Contributions
Funding
Conflicts of Interest
References
- Green, C.; Kottke, P.; Han, X.; Woodrum, C.; Sarvey, T.; Asrar, P.; Zhang, X.; Joshi, Y.; Fedorov, A.; Sitaraman, S.; et al. A review of two-phase forced cooling in three-dimensional stacked electronics: Technology integration. J. Electron. Packag. 2015, 137, 040802. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zhang, C.B.; Shi, M.H.; Wu, J.F.; Peterson, G.P. Study on flow and heat transfer characteristics of heat pipe with axial “omega”-shaped microgrooves. Int. J. Heat Mass Transf. 2009, 52, 636–643. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.-J.; Megaridis, C.M. Surface engineering for phase change heat transfer: A review. MRS Energy Sustain. 2014, 1, E4. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Shi, M. Hydrodynamics of double emulsion droplet in shear flow. Appl. Phys. Lett. 2013, 102, 051609. [Google Scholar] [CrossRef]
- Katzoff, S. Heat pipes and vapor chambers for thermal control of spacecraft. In Thermophysics of Spacecraft and Planetary Bodies; Heller, G.B., Ed.; Academic Press: New York, NY, USA, 1967; pp. 761–818. [Google Scholar]
- Deng, Z.; Liu, X.; Zhang, C.; Huang, Y.; Chen, Y. Melting behaviors of pcm in porous metal foam characterized by fractal geometry. Int. J. Heat Mass Transf. 2017, 113, 1031–1042. [Google Scholar] [CrossRef]
- Zhang, C.B.; Gao, W.; Zhao, Y.J.; Chen, Y.P. Microfluidic generation of self-contained multicomponent microcapsules for self-healing materials. Appl. Phys. Lett. 2018, 113, 203702. [Google Scholar] [CrossRef]
- Chen, Y.P.; Deng, Z.L. Hydrodynamics of a droplet passing through a microfluidic t-junction. J. Fluid Mech. 2017, 819, 401–434. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Zou, M.; Gao, W.; Liu, C.; Shang, L.; Gu, Z.; Zhao, Y. Bioinspired shape-memory graphene film with tunable wettability. Sci. Adv. 2017, 3, e1700004. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zhang, H.; Zou, M.H.; Chen, Y.P.; Zhao, Y.J. Programmable wettability on photocontrolled graphene film. Sci. Adv. 2018, 4, eaat7392. [Google Scholar] [CrossRef]
- Weibel, J.A.; Garimella, S.V. Chapter four—Recent advances in vapor chamber transport characterization for high-heat-flux applications. In Advances in Heat Transfer, 1st ed.; Sparrow, E.M., Cho, Y.I., Abraham, J.P., Gorman, J.M., Eds.; Elsevier: New York, NY, USA, 2013; Volume 45, pp. 209–301. [Google Scholar]
- Jouhara, H.; Chauhan, A.; Nannou, T.; Almahmoud, S.; Delpech, B.; Wrobel, L.C. Heat pipe based systems—Advances and applications. Energy 2017, 128, 729–754. [Google Scholar] [CrossRef]
- Deng, Z.; Zheng, Y.; Liu, X.; Zhu, B.; Chen, Y. Experimental study on thermal performance of an anti-gravity pulsating heat pipe and its application on heat recovery utilization. Appl. Therm. Eng. 2017, 125, 1368–1378. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Shi, M. Dynamic performance analysis on start-up of closed-loop pulsating heat pipes (clphps). Int. J. Therm. Sci. 2013, 65, 224–233. [Google Scholar] [CrossRef]
- Naphon, P.; Wiriyasart, S.; Wongwises, S. Thermal cooling enhancement techniques for electronic components. Int. Commun. Heat Mass Transf. 2015, 61, 140–145. [Google Scholar] [CrossRef]
- Ranjan, R.; Patel, A.; Garimella, S.V.; Murthy, J.Y. Wicking and thermal characteristics of micropillared structures for use in passive heat spreaders. Int. J. Heat Mass Transf. 2011, 55, 586–596. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Z.; Chen, Y. Temperature jump at rough gas–solid interface in couette flow with a rough surface described by cantor fractal. Int. J. Heat Mass Transf. 2014, 70, 322–329. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Deng, Z.; Shi, M. Role of rough surface topography on gas slip flow in microchannels. Phys. Rev. E 2012, 86, 016319. [Google Scholar] [CrossRef] [PubMed]
- Wiriyasart, S.; Naphon, P. Thermal performance enhancement of vapor chamber by coating mini-channel heat sink with porous sintering media. Int. J. Heat Mass Transf. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Zhang, C.B.; Yu, F.W.; Li, X.J.; Chen, Y.P. Gravity–capillary evaporation regimes in micro-grooves. AIChE J. 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Shi, M.; Yang, Y. Thermal and hydrodynamic characteristics of constructal tree-shaped minichannel heat sink. AIChE J. 2010, 56, 2018–2029. [Google Scholar] [CrossRef]
- Liu, W.Y.; Peng, Y.; Luo, T.; Luo, Y.Q.; Huang, K.D. The performance of the vapor chamber based on the plant leaf. Int. J. Heat Mass Transf. 2016, 98, 746–757. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zheng, F.W.; Li, Y.X. Enhancing boiling and condensation co-existing heat transfer in a small and closed space by copper foam inserts. Int. J. Heat Mass Transf. 2017, 108, 961–971. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, D.; Lu, L.; Wang, Z. A multi-artery vapor chamber and its performance. Appl. Therm. Eng. 2013, 60, 15–23. [Google Scholar] [CrossRef]
- Ju, Y.S.; Kaviany, M.; Nam, Y.; Sharratt, S.; Hwang, G.S.; Catton, I.; Fleming, E.; Dussinger, P. Planar vapor chamber with hybrid evaporator wicks for the thermal management of high-heat-flux and high-power optoelectronic devices. Int. J. Heat Mass Transf. 2013, 60, 163–169. [Google Scholar] [CrossRef]
- Ryu, S.; Han, J.; Kim, J.; Lee, C.; Nam, Y. Enhanced heat transfer using metal foam liquid supply layers for micro heat spreaders. Int. J. Heat Mass Transf. 2017, 108, 2338–2345. [Google Scholar] [CrossRef]
- Wong, S.-C.; Hsieh, K.-C.; Wu, J.-D.; Han, W.-L. A novel vapor chamber and its performance. Int. J. Heat Mass Transf. 2010, 53, 2377–2384. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Liu, B.; Liu, J.; Huang, K.; Wang, L.; Chen, W. The performance of the novel vapor chamber based on the leaf vein system. Int. J. Heat Mass Transf. 2015, 86, 656–666. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Wang, N.; Tian, Y.; Chen, X. A novel wick structure of vapor chamber based on the fractal architecture of leaf vein. Int. J. Heat Mass Transf. 2013, 63, 120–133. [Google Scholar] [CrossRef]
- Yao, F.; Miao, S.; Zhang, M.; Chen, Y. An experimental study of an anti-gravity vapor chamber with a tree-shaped evaporator. Appl. Therm. Eng. 2018, 141, 1000–1008. [Google Scholar] [CrossRef]
- Ranjan, R.; Murthy, J.Y.; Garimella, S.V.; Vadakkan, U. A numerical model for transport in flat heat pipes considering wick microstructure effects. Int. J. Heat Mass Transf. 2011, 54, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, Y.; Wu, R.; Shi, M. Flow boiling in constructal tree-shaped minichannel network. Int. J. Heat Mass Transf. 2011, 54, 202–209. [Google Scholar] [CrossRef]
- Patankar, G.; Weibel, J.A.; Garimella, S.V. A validated time-stepping analytical model for 3d transient vapor chamber transport. Int. J. Heat Mass Transf. 2018, 119, 867–879. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Shi, M. Effects of roughness elements on laminar flow and heat transfer in microchannels. Chem. Eng. Process. 2010, 49, 1188–1192. [Google Scholar] [CrossRef]
- Patankar, G.; Weibel, J.A.; Garimella, S.V. Patterning the condenser-side wick in ultra-thin vapor chamber heat spreaders to improve skin temperature uniformity of mobile devices. Int. J. Heat Mass Transf. 2016, 101, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, Y.; Wu, L.; Shi, M. Thermal response of brick wall filled with phase change materials(PCM) under fluctuating outdoor temperatures. Energy Build. 2011, 43, 3514–3520. [Google Scholar] [CrossRef]
- Hsieh, S.-S.; Lee, R.-Y.; Shyu, J.-C.; Chen, S.-W. Thermal performance of flat vapor chamber heat spreader. Energ. Convers. Manag. 2008, 49, 1774–1784. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Wang, C. An experimental study of boiling and condensation co-existing phase change heat transfer in small confined space. Int. J. Heat Mass Transf. 2013, 64, 1082–1090. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Wang, C. A visualization study of the influences of liquid levels on boiling and condensation co-existing phase change heat transfer phenomenon in small confined spaces. Int. J. Heat Mass Transf. 2014, 73, 415–423. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Wu, S.; Zhang, M.; Yang, W.; Tang, F. Visualization study of startup modes and operating states of a flat two-phase micro thermosyphon. Energies 2018, 11, 2291. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, X.; Shen, C.; Yao, F.; Zhang, M. Experimental Study on the Evaporation and Condensation Heat Transfer Characteristics of a Vapor Chamber. Energies 2019, 12, 11. https://doi.org/10.3390/en12010011
Liu Y, Han X, Shen C, Yao F, Zhang M. Experimental Study on the Evaporation and Condensation Heat Transfer Characteristics of a Vapor Chamber. Energies. 2019; 12(1):11. https://doi.org/10.3390/en12010011
Chicago/Turabian StyleLiu, Yanfei, Xiaotian Han, Chaoqun Shen, Feng Yao, and Mengchen Zhang. 2019. "Experimental Study on the Evaporation and Condensation Heat Transfer Characteristics of a Vapor Chamber" Energies 12, no. 1: 11. https://doi.org/10.3390/en12010011



