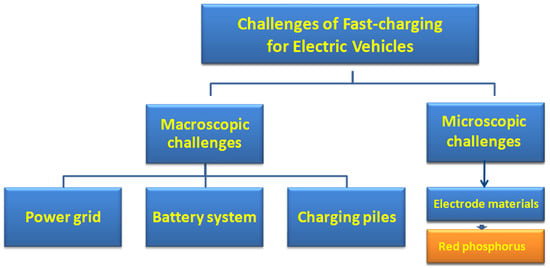
Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review
Abstract
1. Introduction
2. Macroscopic Challenges in EV Fast Charging
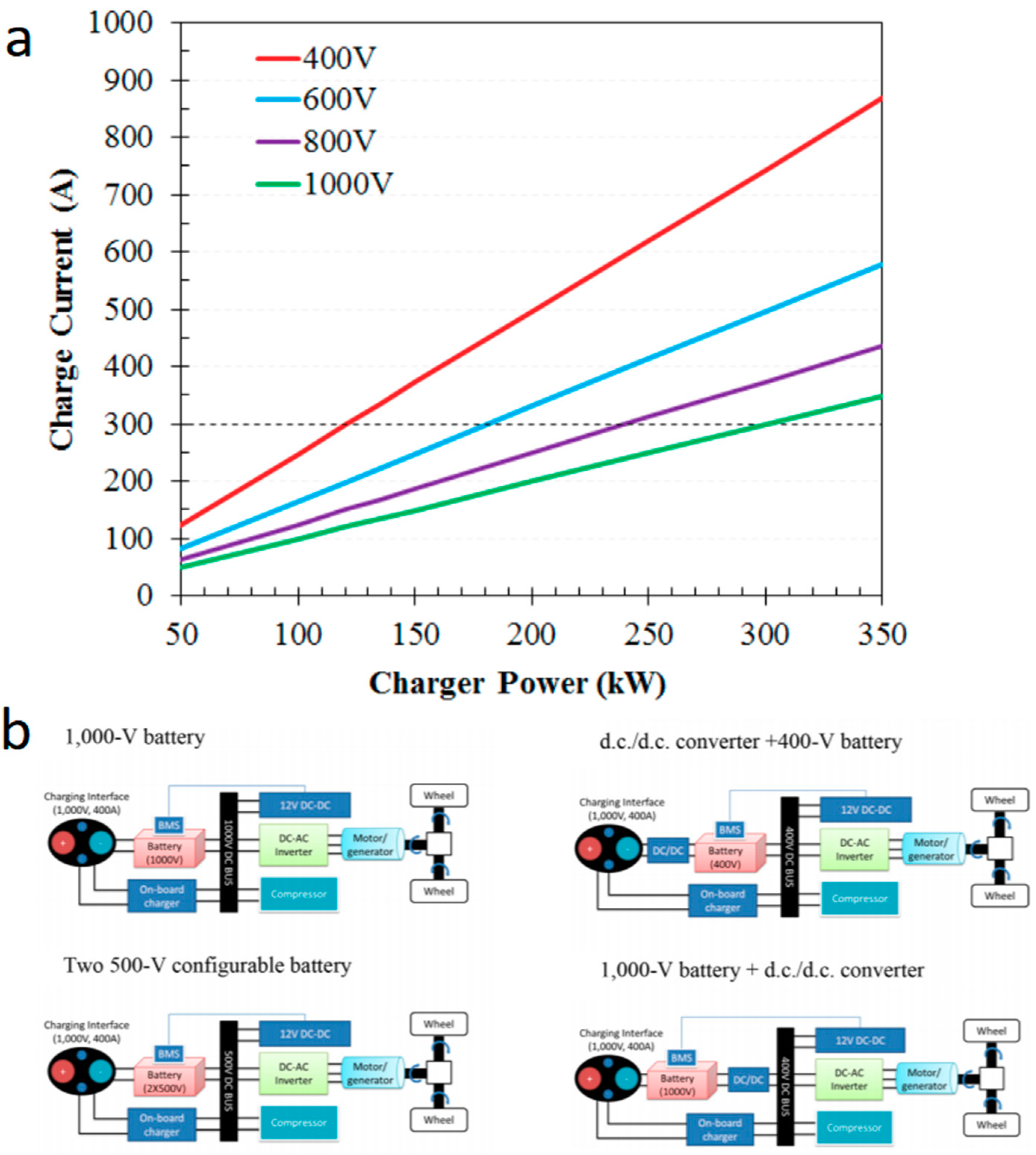
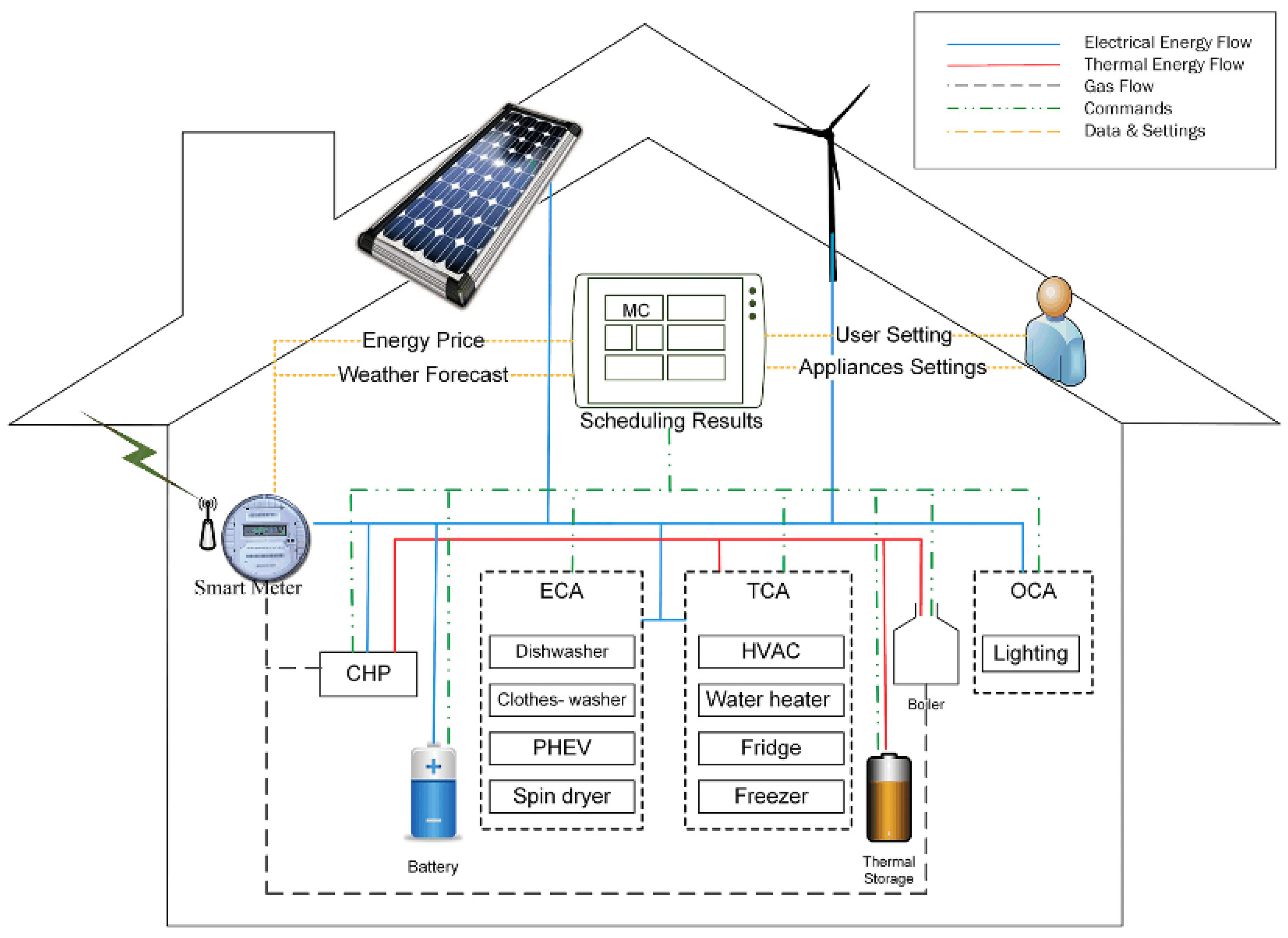
2.1. Consideration in the Battery Systems of EVs
2.1.1. Battery/Battery Pack Design
2.1.2. BMS Design
2.1.3. Charge Protocol
2.2. Consideration in Charging Pile
2.3. Considerations for the Power Grid
3. Microscopic Challenges in EV Fast Charging
3.1. Effect on Battery Cells
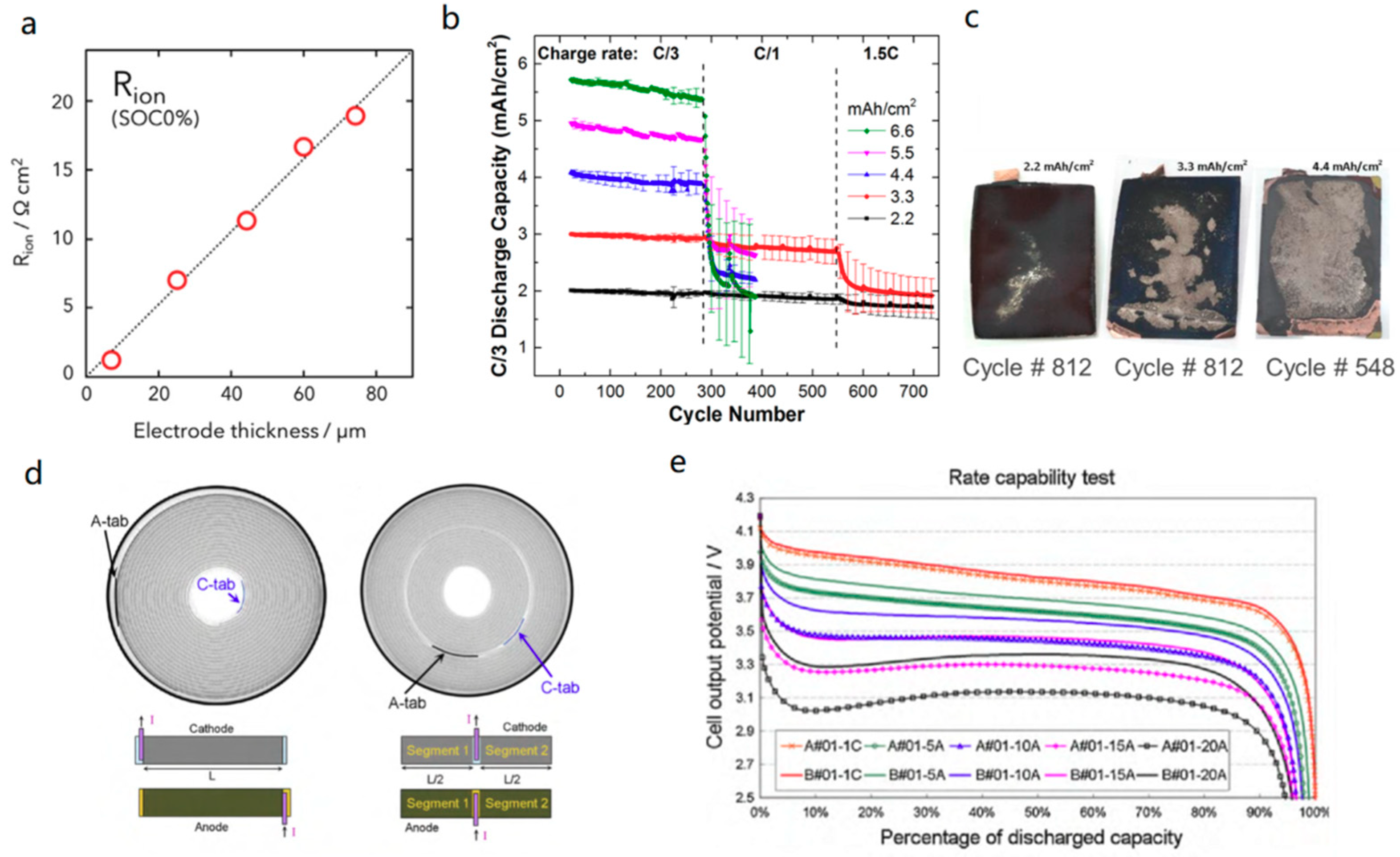
3.1.1. Anode
3.1.2. Cathode, Electrolyte, and Current Collector
3.2. Potential Solutions for Fast-Charging Battery Cells
3.2.1. Electrode Materials
3.2.2. Red Phosphorus Anode
4. Perspective
5. Further Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Michelbacher, C.; Ahmed, S.; Bloom, I.; Burnham, A.; Carlson, B.; Dias, F.; Dufek, E.J.; Jansen, A.N.; Keyser, M.; Markel, A. Enabling fast charging–Introduction and overview. J. Power Sources 2017, 367. [Google Scholar] [CrossRef]
- Meintz, A.; Zhang, J.; Vijayagopal, R.; Kreutzer, C.; Ahmed, S.; Bloom, I.; Burnham, A.; Carlson, R.B.; Dias, F.; Dufek, E.J. Enabling fast charging–Vehicle considerations. J. Power Sources 2017, 367, 216–227. [Google Scholar] [CrossRef]
- Ahmed, S.; Bloom, I.; Jansen, A.N.; Tanim, T.; Dufek, E.J.; Pesaran, A.; Burnham, A.; Carlson, R.B.; Dias, F.; Hardy, K. Enabling fast charging–A battery technology gap assessment. J. Power Sources 2017, 367, 250–262. [Google Scholar] [CrossRef]
- Keyser, M.; Pesaran, A.; Li, Q.; Santhanagopalan, S.; Smith, K.; Wood, E.; Ahmed, S.; Bloom, I.; Dufek, E.; Shirk, M. Enabling fast charging–Battery thermal considerations. J. Power Sources 2017, 367, 228–236. [Google Scholar] [CrossRef]
- Burnham, A.; Dufek, E.J.; Stephens, T.; Francfort, J.; Michelbacher, C.; Carlson, R.B.; Zhang, J.; Vijayagopal, R.; Dias, F.; Mohanpurkar, M. Enabling fast charging–Infrastructure and economic considerations. J. Power Sources 2017, 367, 237–249. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Hesse, H.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-ion battery storage for the grid—A review of stationary battery storage system design tailored for applications in modern power grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Zheng, M.; Zhong, W.H.; Cao, G. Strategies for Building Robust Traffic Networks in Advanced Energy Storage Devices: A Focus on Composite Electrodes. Adv. Mater. 2019, 31, 1804204. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. The mechanism of ultrafast supercapacitors. J. Mater. Chem. A 2018, 6, 2866–2876. [Google Scholar] [CrossRef]
- Wang, Q.; Shaffer, C.E.; Sinha, P.K. Controlling Factors of Cell Design on Large-Format Li-Ion Battery Safety during Nail Penetration. Front. Energy Res. 2015, 3. [Google Scholar] [CrossRef][Green Version]
- Colclasure, A.M.; Dunlop, A.R.; Trask, S.; Polzin, B.; Jansen, A.; Smith, K. Requirements for Enabling Extreme Fast Charging of High Energy Density Li-Ion Cells while Avoiding Lithium Plating. J. Electrochem. Soc. 2019, 166, A1412–A1424. [Google Scholar] [CrossRef]
- Kisu, K.; Aoyagi, S.; Nagatomo, H.; Iwama, E.; Reid, M.T.H.; Naoi, W.; Naoi, K. Internal resistance mapping preparation to optimize electrode thickness and density using symmetric cell for high-performance lithium-ion batteries and capacitors. J. Power Sources 2018, 396, 207–212. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, K.-H.; Hu, C.-C.; Cheng, T.-T. Performance comparisons and resistance modeling for multi-segment electrode designs of power-oriented lithium-ion batteries. Electrochim. Acta 2010, 55, 6433–6439. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, G.; Ge, S.; Xu, T.; Ji, Y.; Yang, X.-G.; Leng, Y. Lithium-ion battery structure that self-heats at low temperatures. Nature 2016, 529, 515. [Google Scholar] [CrossRef]
- Ye, Y.; Saw, L.H.; Shi, Y.; Somasundaram, K.; Tay, A.A. Effect of thermal contact resistances on fast charging of large format lithium ion batteries. Electrochim. Acta 2014, 134, 327–337. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- He, M.S.; Kollmeyer, P.J.; Haußmann, M.; Emadi, A. A Comparison of the Performance and Thermal Management Requirements of Lithium-Ion Batteries during Ultra-Fast Charging. In Proceedings of the 2018 IEEE Transportation Electrification Conference and Expo (ITEC), Long Beach, CA, USA, 13–15 June 2018; pp. 675–680. [Google Scholar]
- Schmidt, A.; Smith, A.; Ehrenberg, H. Power capability and cyclic aging of commercial, high power lithium ion battery cells with respect to different cell designs. J. Power Sources 2019, 425, 27–38. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, P.; Sun, Y. Thermal analyses of LiFePO4/graphite battery discharge processes. J. Power Sources 2013, 243, 181–194. [Google Scholar] [CrossRef]
- Tanim, T.R.; Shirk, M.G.; Bewley, R.L.; Dufek, E.J.; Liaw, B.Y. Fast charge implications: Pack and cell analysis and comparison. J. Power Sources 2018, 381, 56–65. [Google Scholar] [CrossRef]
- Malik, M.; Dincer, I.; Rosen, M.A.; Mathew, M.; Fowler, M. Thermal and electrical performance evaluations of series connected Li-ion batteries in a pack with liquid cooling. Appl. Therm. Eng. 2018, 129, 472–481. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Chen, H.; Wang, Q. Water cooling based strategy for lithium ion battery pack dynamic cycling for thermal management system. Appl. Therm. Eng. 2018, 132, 575–585. [Google Scholar] [CrossRef]
- Chung, Y.; Kim, M.S. Thermal analysis and pack level design of battery thermal management system with liquid cooling for electric vehicles. Energy Convers. Manag. 2019, 196, 105–116. [Google Scholar] [CrossRef]
- Lu, Z.; Meng, X.; Wei, L.; Hu, W.; Zhang, L.; Jin, L. Thermal management of densely-packed EV battery with forced air cooling strategies. Energy Procedia 2016, 88, 682–688. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An alternative cooling system to enhance the safety of Li-ion battery packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Amietszajew, T.; McTurk, E.; Fleming, J.; Bhagat, R. Understanding the limits of rapid charging using instrumented commercial 18650 high-energy Li-ion cells. Electrochim. Acta 2018, 263, 346–352. [Google Scholar] [CrossRef]
- Soltanimehr, M.; Afrand, M. Thermal conductivity enhancement of COOH-functionalized MWCNTs/ethylene glycol–water nanofluid for application in heating and cooling systems. Appl. Therm. Eng. 2016, 105, 716–723. [Google Scholar] [CrossRef]
- Nazari, M.; Karami, M.; Ashouri, M. Comparing the thermal performance of water, Ethylene Glycol, Alumina and CNT nanofluids in CPU cooling: Experimental study. Exp. Therm. Fluid Sci. 2014, 57, 371–377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Chen, Z.; Xie, J.; Zhang, X. A novel active equalization method for lithium-ion batteries in electric vehicles. Appl. Energy 2015, 145, 36–42. [Google Scholar] [CrossRef]
- Jarrett, A.; Kim, I.Y. Design optimization of electric vehicle battery cooling plates for thermal performance. J. Power Sources 2011, 196, 10359–10368. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Q.; Zhang, C.; Zhang, W. Evaluation of Acceptable Charging Current of Power Li-Ion Batteries Based on Polarization Characteristics. IEEE Trans. Ind. Electron. 2014, 61, 6844–6851. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, C.; Cui, N.; Guerrero, J.M. A cell-to-cell battery equalizer with zero-current switching and zero-voltage gap based on quasi-resonant LC converter and boost converter. IEEE Trans. Power Electron. 2014, 30, 3731–3747. [Google Scholar] [CrossRef]
- Hannan, M.; Hoque, M.; Ker, P.; Begum, R.; Mohamed, A. Charge equalization controller algorithm for series-connected lithium-ion battery storage systems: Modeling and applications. Energies 2017, 10, 1390. [Google Scholar] [CrossRef]
- Liu, K.; Hu, X.; Yang, Z.; Xie, Y.; Feng, S. Lithium-ion battery charging management considering economic costs of electrical energy loss and battery degradation. Energy Convers. Manag. 2019, 195, 167–179. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18650 high-power cells. J. Energy Storage 2016, 6, 125–141. [Google Scholar] [CrossRef]
- Ayoub, E.; Karami, N. Review on the charging techniques of a Li-Ion battery. In Proceedings of the 2015 Third International Conference on Technological Advances in Electrical, Electronics and Computer Engineering (TAEECE), Beirut, Lebanon, 29 April–1 May 2015; pp. 50–55. [Google Scholar]
- Guo, Z.; Liaw, B.Y.; Qiu, X.; Gao, L.; Zhang, C. Optimal charging method for lithium ion batteries using a universal voltage protocol accommodating aging. J. Power Sources 2015, 274, 957–964. [Google Scholar] [CrossRef]
- García, G.; Dieckhöfer, S.; Schuhmann, W.; Ventosa, E. Exceeding 6500 cycles for LiFePO4/Li metal batteries through understanding pulsed charging protocols. J. Mater. Chem. A 2018, 6, 4746–4751. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Zhang, D.; Liu, Z.; He, Y.; Wang, Z.L.; Sun, C. Effects of pulse charging on the performances of lithium-ion batteries. Nano Energy 2019, 56, 555–562. [Google Scholar] [CrossRef]
- Fleury, X.; Noh, M.H.; Geniès, S.; Thivel, P.X.; Lefrou, C.; Bultel, Y. Fast-charging of Lithium Iron Phosphate battery with ohmic-drop compensation method: Ageing study. J. Energy Storage 2018, 16, 21–36. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Reichman, B.; Wang, X. Modeling the effect of two-stage fast charging protocol on thermal behavior and charging energy efficiency of lithium-ion batteries. J. Energy Storage 2018, 20, 298–309. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, X.; Lu, L.; Li, J.; Han, X.; Ouyang, M. Non-destructive fast charging algorithm of lithium-ion batteries based on the control-oriented electrochemical model. Appl. Energy 2017, 204, 1240–1250. [Google Scholar] [CrossRef]
- Noh, M.; Thivel, P.; Lefrou, C.; Bultel, Y. Fast-charging of lithium iron phosphate battery with ohmic-drop compensation method. J. Energy Storage 2016, 8, 160–167. [Google Scholar] [CrossRef]
- Mussa, A.S.; Klett, M.; Behm, M.; Lindbergh, G.; Lindström, R.W. Fast-charging to a partial state of charge in lithium-ion batteries: A comparative ageing study. J. Energy Storage 2017, 13, 325–333. [Google Scholar] [CrossRef]
- Sun, X.-H.; Yamamoto, T.; Morikawa, T. Fast-charging station choice behavior among battery electric vehicle users. Transp. Res. Part D Transp. Environ. 2016, 46, 26–39. [Google Scholar] [CrossRef]
- Dong, X.; Mu, Y.; Xu, X.; Jia, H.; Wu, J.; Yu, X.; Qi, Y. A charging pricing strategy of electric vehicle fast charging stations for the voltage control of electricity distribution networks. Appl. Energy 2018, 225, 857–868. [Google Scholar] [CrossRef]
- Xie, F.; Liu, C.; Li, S.; Lin, Z.; Huang, Y. Long-term strategic planning of inter-city fast charging infrastructure for battery electric vehicles. Transp. Res. Part E Logist. Transp. Rev. 2018, 109, 261–276. [Google Scholar] [CrossRef]
- Michelbacher, C.J.; Ahmed, S.; Bloom, I.; Burnham, A.; Carlson, B.; Dias, F.; Dufek, E.J.; Jansen, A.N.; Keyser, M.; Markel, A. Enabling Fast Charging: A Technology Gap Assessment; Idaho National Lab.(INL): Idaho Falls, ID, USA, 2017. [Google Scholar]
- Funke, S.Á.; Plötz, P.; Wietschel, M. Invest in fast-charging infrastructure or in longer battery ranges? A cost-efficiency comparison for Germany. Appl. Energy 2019, 235, 888–899. [Google Scholar] [CrossRef]
- Blasius, E.; Wang, Z. Effects of charging battery electric vehicles on local grid regarding standardized load profile in administration sector. Appl. Energy 2018, 224, 330–339. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Meinrenken, C.J.; Ding, Y. Economic and environmental benefits of coordinating dispatch among distributed electricity storage. Appl. Energy 2018, 210, 842–855. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.; Chu, C.-C.; Gadh, R. Vehicle grid integration for demand response with mixture user model and decentralized optimization. Appl. Energy 2018, 231, 481–493. [Google Scholar] [CrossRef]
- Shirazi, E.; Jadid, S. Cost reduction and peak shaving through domestic load shifting and DERs. Energy 2017, 124, 146–159. [Google Scholar] [CrossRef]
- Pearre, N.S.; Ribberink, H. Review of research on V2X technologies, strategies, and operations. Renew. Sustain. Energy Rev. 2019, 105, 61–70. [Google Scholar] [CrossRef]
- Amini, M.H. A panorama of interdependent power systems and electrified transportation networks. In Sustainable Interdependent Networks II; Springer: Berlin, Germany, 2019; pp. 23–41. [Google Scholar]
- Burns, J.; Stevens, D.; Dahn, J. In-situ detection of lithium plating using high precision coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Yang, X.-G.; Ge, S.; Liu, T.; Leng, Y.; Wang, C.-Y. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources 2018, 395, 251–261. [Google Scholar] [CrossRef]
- Matasso, A.; Wetz, D.; Liu, F. The Effects of Internal Pressure Evolution on the Aging of Commercial Li-Ion Cells. J. Electrochem. Soc. 2015, 162, A92–A97. [Google Scholar] [CrossRef]
- Schindler, S.; Bauer, M.; Petzl, M.; Danzer, M.A. Voltage relaxation and impedance spectroscopy as in-operando methods for the detection of lithium plating on graphitic anodes in commercial lithium-ion cells. J. Power Sources 2016, 304, 170–180. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kim, H.-M.; Oh, S.-T.; Sol-Nip, L. Detection Method of LI Plating, Method and Apparatus for Charging Secondary Battery and Secondary Battery System Using the Same; LG Chem Ltd: Seoul, Korean, 2018. [Google Scholar]
- Bitzer, B.; Gruhle, A. A new method for detecting lithium plating by measuring the cell thickness. J. Power Sources 2014, 262, 297–302. [Google Scholar] [CrossRef]
- Fonseca Rodrigues, M.-T.; Maroni, V.A.; Gosztola, D.J.; Yao, K.P.C.; Kalaga, K.; Shkrob, I.A.; Abraham, D.P. Lithium Acetylide: A Spectroscopic Marker for Lithium Deposition During Fast Charging of Li-Ion Cells. ACS Appl. Energy Mater. 2019, 2, 873–881. [Google Scholar] [CrossRef]
- Xu, R.; de Vasconcelos, L.; Shi, J.; Li, J.; Zhao, K. Disintegration of meatball electrodes for LiNix MnyCozO2 cathode materials. Exp. Mech. 2018, 58, 549–559. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Huang, Z.; Yang, C.; Zuo, Z.; Zhou, H. Understanding the accumulated cycle capacity fade caused by the secondary particle fracture of LiNi1-xy CoxMnyO2 cathode for lithium ion batteries. J. Solid State Electrochem. 2017, 21, 673–682. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1−x−yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Keyser, M.; Smith, K.; Santhanagopalan, S.; Usseglio-Viretta, F.; Colclasure, A. Extreme Fast Charging (National Renewable Energy Laboratory). X-CEL: eXtreme. Fast Charg. Cell Eval. Lithium-Ion. Batter. 2018, 163, 10. [Google Scholar]
- Logan, E.; Tonita, E.M.; Gering, K.; Li, J.; Ma, X.; Beaulieu, L.; Dahn, J. A study of the physical properties of Li-Ion battery electrolytes containing esters. J. Electrochem. Soc. 2018, 165, A21–A30. [Google Scholar] [CrossRef]
- Ma, X.; Arumugam, R.S.; Ma, L.; Logan, E.; Tonita, E.; Xia, J.; Petibon, R.; Kohn, S.; Dahn, J. A study of three ester co-solvents in lithium-ion cells. J. Electrochem. Soc. 2017, 164, A3556–A3562. [Google Scholar] [CrossRef]
- Poetz, S.; Fuchsbichler, B.; Schmuck, M.; Koller, S. Development of a 3d current collector for the positive electrode in lithium-ion batteries. J. Appl. Electrochem. 2014, 44, 989–994. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2018. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Yurkiv, V.; Foroozan, T.; Ragone, M.; Shahbazian-Yassar, R.; Mashayek, F. Lithium Diffusion Mechanism through Solid-Electrolyte Interphase (SEI) In Rechargeable Lithium Batteries. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Barsoukov, E. Impedance spectroscopy: Theory, experiment, and applications. History 2005, 1, 1–13. [Google Scholar]
- Zheng, K.; Świerczek, K. Possibility of determination of transport coefficients D and k from relaxation experiments for sphere-shaped powder samples. Solid State Ion. 2018, 323, 157–165. [Google Scholar] [CrossRef]
- Maiza, M.; Mammeri, Y.; Nguyen, D.A.; Legrand, N.; Desprez, P.; Franco, A.A. Evaluating the impact of transport inertia on the electrochemical response of lithium ion battery single particle models. J. Power Sources 2019, 423, 263–270. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Huang, X.; Chen, L. Determination of chemical diffusion coefficient of lithium ion in graphitized mesocarbon microbeads with potential relaxation technique. J. Electrochem. Soc. 2001, 148, A737–A741. [Google Scholar] [CrossRef]
- Yang, H.; Bang, H.J.; Prakash, J. Evaluation of electrochemical interface area and lithium diffusion coefficient for a composite graphite anode. J. Electrochem. Soc. 2004, 151, A1247–A1250. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, Y.; Wang, J.; Chen, C.; Chen, D.; Wang, C.; Ciucci, F. Egg yolk-derived phosphorus and nitrogen dual doped nano carbon capsules for high-performance lithium ion batteries. Mater. Lett. 2016, 167, 93–97. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.-X.; Yang, W.-J.; Jiang, S.; Hou, X.-W.; Guo, R.; Liu, W.; Huang, P.; Lu, J.; Gu, H.-T. Enhanced Rate Performance of Li4Ti5O12 Anode for Advanced Lithium Batteries. J. Electrochem. Soc. 2019, 166, A5014–A5018. [Google Scholar] [CrossRef]
- Yang, N.-H.; Wu, Y.-S.; Chou, J.; Wu, H.-C.; Wu, N.-L. Silicon oxide-on-graphite planar composite synthesized using a microwave-assisted coating method for use as a fast-charging lithium-ion battery anode. J. Power Sources 2015, 296, 314–317. [Google Scholar] [CrossRef]
- Cui, J.; Yao, S.; Kim, J.-K. Recent progress in rational design of anode materials for high-performance Na-ion batteries. Energy Storage Mater. 2017, 7, 64–114. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Kim, G.-O.; Yun, S.-R.; Ryu, K.-S. NiO nanoparticles with plate structure grown on graphene as fast charge–discharge anode material for lithium ion batteries. Electrochim. Acta 2012, 78, 406–411. [Google Scholar] [CrossRef]
- Saadat, S.; Zhu, J.; Sim, D.H.; Hng, H.H.; Yazami, R.; Yan, Q. Coaxial Fe3O4/CuO hybrid nanowires as ultra fast charge/discharge lithium-ion battery anodes. J. Mater. Chem. A 2013, 1, 8672–8678. [Google Scholar] [CrossRef]
- Kim, N.Y.; Lee, G.; Choi, J. Fast-Charging and High Volumetric Capacity Anode Based on Co3O4/CuO@ TiO2 Composites for Lithium-Ion Batteries. Chem. A Eur. J. 2018, 24, 19045–19052. [Google Scholar] [CrossRef]
- Wang, L.; Ding, K.; Wei, B.; Li, C.; Shi, X.; Zhang, Y.; He, X. Leaf-like α-Fe2O3 micron-particle: Preparation and its usage as anode materials for lithium ion batteries. Mater. Chem. Phys. 2018, 207, 58–66. [Google Scholar] [CrossRef]
- Lin, C.; Deng, S.; Kautz, D.J.; Xu, Z.; Liu, T.; Li, J.; Wang, N.; Lin, F. Intercalating Ti2Nb14O39 Anode Materials for Fast-Charging, High-Capacity and Safe Lithium–Ion Batteries. Small 2017, 13, 1702903. [Google Scholar] [CrossRef]
- Park, H.; Wu, H.B.; Song, T.; Lou, X.W.; Paik, U. Porosity-controlled TiNb2O7 microspheres with partial nitridation as a practical negative electrode for high-power lithium-ion batteries. Adv. Energy Mater. 2015, 5, 1401945. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z. Inward lithium-ion breathing of hierarchically porous silicon anodes. Nat. Commun. 2015, 6, 8844. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Ali, I.; Tippabhotla, S.K.; Radchenko, I.; Al-Obeidi, A.; Stan, C.V.; Tamura, N.; Budiman, A.S. Probing Stress States in Silicon Nanowires During Electrochemical Lithiation Using In Situ Synchrotron X-Ray Microdiffraction. Front. Energy Res. 2018, 6. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Z.-J.; Yang, L.; Cheng, S.; Liu, M. A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J. Mater. Chem. A 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- Ryu, W.-H.; Nam, D.-H.; Ko, Y.-S.; Kim, R.-H.; Kwon, H.-S. Electrochemical performance of a smooth and highly ordered TiO2 nanotube electrode for Li-ion batteries. Electrochim. Acta 2012, 61, 19–24. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Sun, X.; Li, X.; He, D. Size-controllable porous NiO electrodes for high-performance lithium ion battery anodes. Mater. Res. Bull. 2017, 96, 533–537. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Ren, S.; Zhang, B.; Yang, S.; Cao, B. Construction of hollow Co3O4 cubes as a high-performance anode for lithium ion batteries. New J. Chem. 2017, 41, 7960–7965. [Google Scholar] [CrossRef]
- Zhu, G.L.; Zhao, C.Z.; Huang, J.Q.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Small 2019, 1805389. [Google Scholar] [CrossRef]
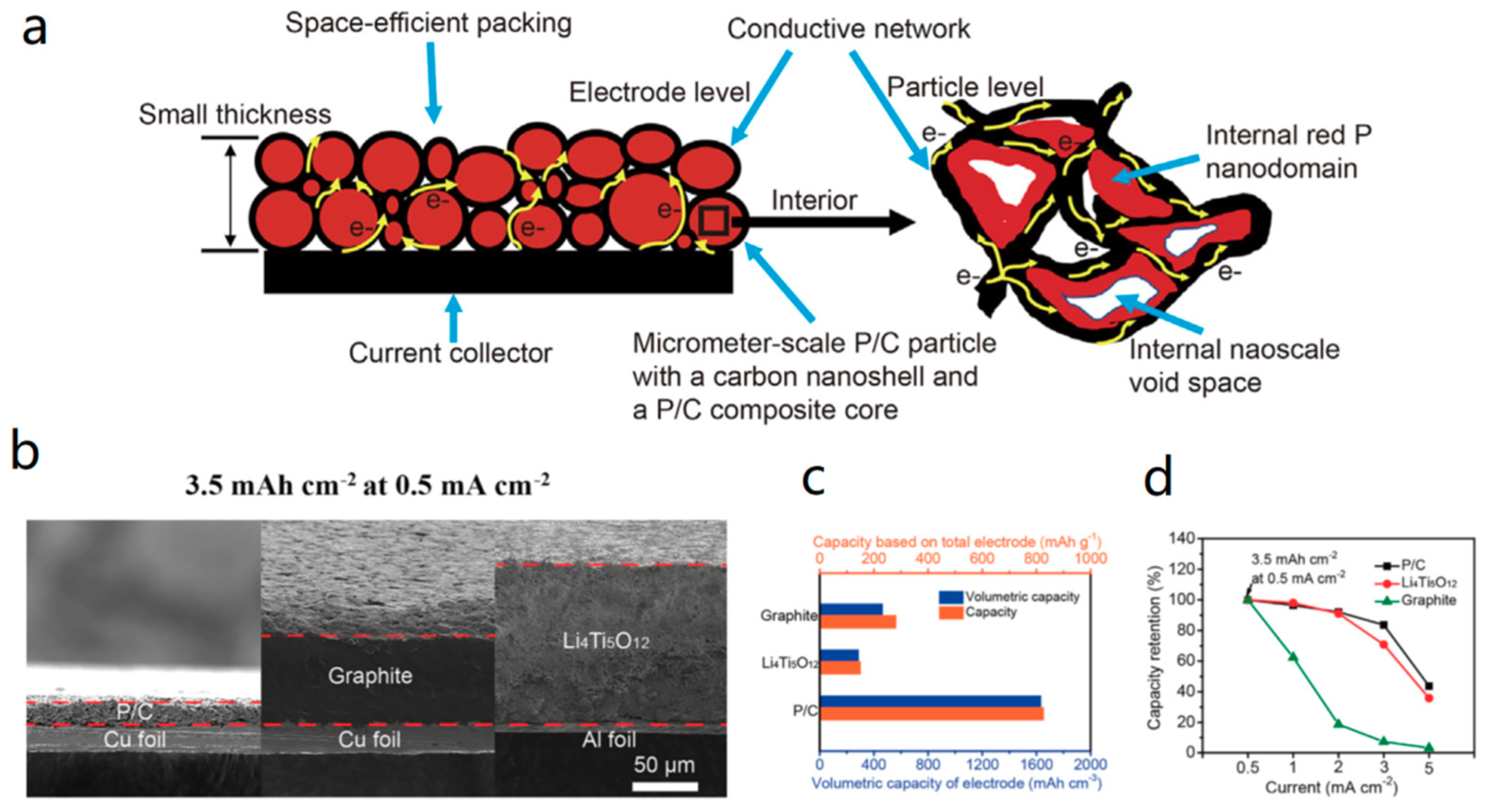
- Sun, Y.; Wang, L.; Li, Y.; Li, Y.; Lee, H.R.; Pei, A.; He, X.; Cui, Y. Design of Red Phosphorus Nanostructured Electrode for Fast-Charging Lithium-Ion Batteries with High Energy Density. Joule 2019. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Shen, C.; Liu, Q.; Cao, X.; Ma, Y.; Chen, L.; Lau, C.; Chen, T.-C.; Wei, F. Red phosphorus nanodots on reduced graphene oxide as a flexible and ultra-fast anode for sodium-ion batteries. ACS Nano 2017, 11, 5530–5537. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S.; Xu, R.; Wang, J.; Peng, Z.; Zhang, Q.; Yu, Y. Boosting potassium-ion battery performance by encapsulating red phosphorus in free-standing nitrogen-doped porous hollow carbon nanofibers. Nano Lett. 2019, 19, 1351–1358. [Google Scholar] [CrossRef]
- Khalid, M.; Varela, H. A general potentiodynamic approach for red phosphorus and sulfur nanodot incorporation on reduced graphene oxide sheets: Metal-free and binder-free electrodes for supercapacitor and hydrogen evolution activities. J. Mater. Chem. A 2018, 6, 3141–3150. [Google Scholar] [CrossRef]
- Sibari, A.; Marjaoui, A.; Lakhal, M.; Kerrami, Z.; Kara, A.; Benaissa, M.; Ennaoui, A.; Hamedoun, M.; Benyoussef, A.; Mounkachi, O. Phosphorene as a promising anode material for (Li/Na/Mg)-ion batteries: A first-principle study. Sol. Energy Mater. Sol. Cells 2018, 180, 253–257. [Google Scholar] [CrossRef]
- Chang, W.-C.; Tseng, K.-W.; Tuan, H.-Y. Solution synthesis of iodine-doped red phosphorus nanoparticles for lithium-ion battery anodes. Nano Lett. 2017, 17, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Si, H.; Zhang, Y.; Liu, J.; Liu, J.; Zhang, Y. TiO2-modified red phosphorus nanosheets entangled in carbon nanotubes for high performance lithium ion batteries. Electrochim. Acta 2019, 297, 319–327. [Google Scholar] [CrossRef]
- Yuan, D.; Cheng, J.; Qu, G.; Li, X.; Ni, W.; Wang, B.; Liu, H. Amorphous red phosphorous embedded in carbon nanotubes scaffold as promising anode materials for lithium-ion batteries. J. Power Sources 2016, 301, 131–137. [Google Scholar] [CrossRef]
- Yu, Z.; Song, J.; Gordin, M.L.; Yi, R.; Tang, D.; Wang, D. Phosphorus-graphene nanosheet hybrids as lithium-ion anode with exceptional high-temperature cycling stability. Adv. Sci. 2015, 2, 1400020. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-Structured Phosphorus Composite as High-Capacity Anode Materials for Lithium Batteries. Angew. Chem. 2012, 124, 9168–9171. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Bai, A.; Wang, L.; Li, J.; He, X.; Wang, J.; Wang, J. Composite of graphite/phosphorus as anode for lithium-ion batteries. J. Power Sources 2015, 289, 100–104. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; He, X.; Wang, J. Effect of pore size distribution of carbon matrix on the performance of phosphorus@ carbon material as anode for lithium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 4217–4223. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Li, J.; He, X. Red phosphorus composite anodes for Li-ion batteries. Ionics 2018, 24, 303–308. [Google Scholar] [CrossRef]




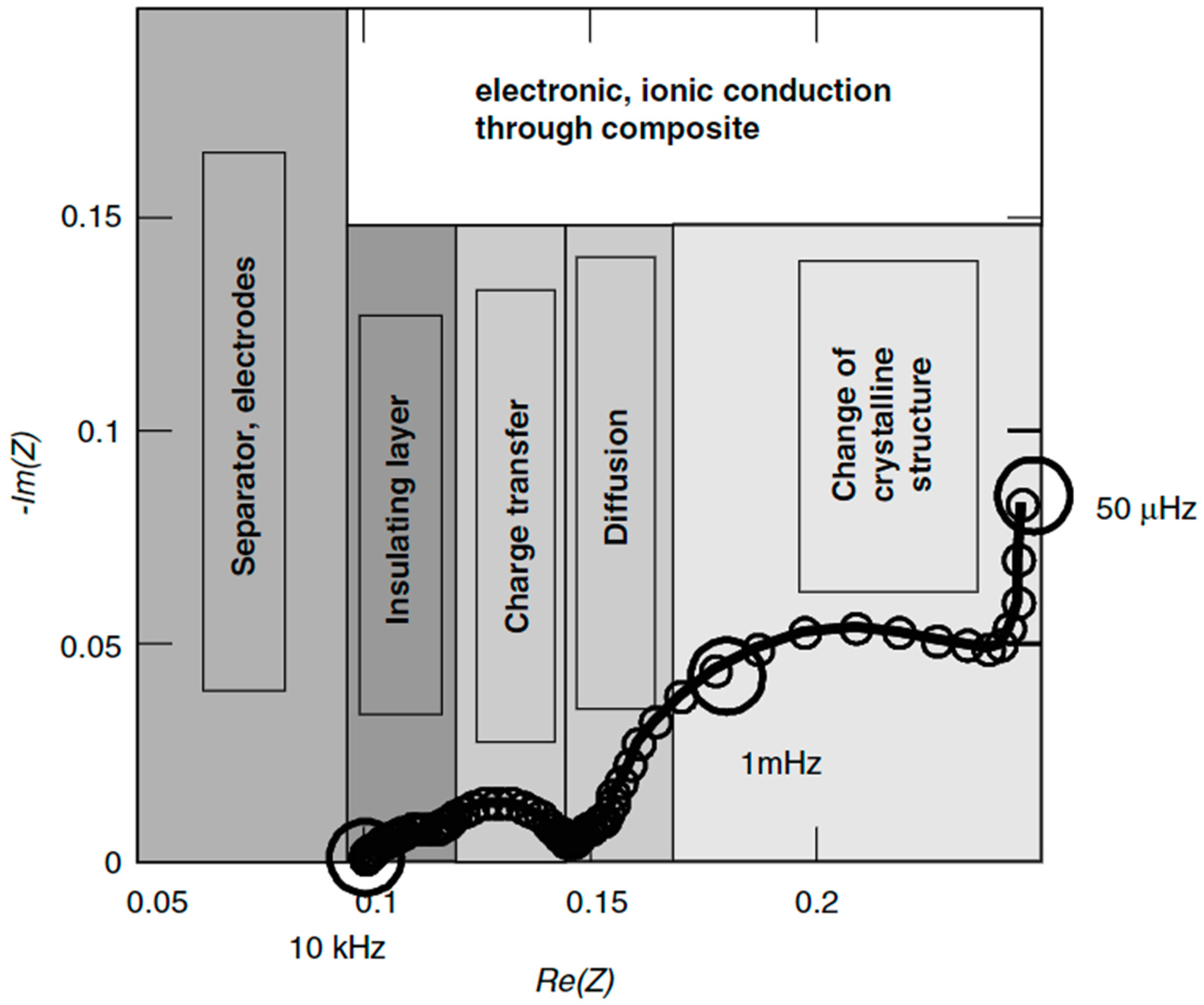



| Charging Time, SOC = 80%, minutes | 8 | 10 | 23 | 47 | 53 | 61 |
| Charging Time, SOC = 60%, minutes | 5 | 7 | 15 | 30 | 34 | 39 |
| Charger Power, needed, kW | 601 | 461 | 199 | 100 | 88 | 77 |
| Anode Thickness, um | 14 | 19 | 43 | 87 | 98 | 103 |
| Heat Generated during Charge, kWh, per pack | 2.35 | 2.20 | 1.89 | 1.77 | 1.75 | 1.45 |
| Post-Charge Cell, SOC = 80%, Temperature, °C | 22.4 | 24.4 | 25.9 | 26.4 | 26.4 | 19.4 |
| Cell Mass, kg | 2.75 | 2.40 | 1.74 | 1.49 | 1.46 | 1.45 |
| Cell Cost to Original Equipment Manufacturer, $per kWh | $229 | $196 | $132 | $107 | $104 | $103 |
| Cost Difference, $per kWh | $126 | $93 | $30 | $4 | $1 | $0 |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Energy density (Wh kg−1 ) | 175 | 300 | 300 | 175 |
| Number of cells (cells) | 484 | 282 | 282 | 484 |
| Cell efficiency (%) | 70 | 90 | 70 | 90 |
| Pack heat removed (Kw) | 2 | 15 | 2 | 15 |
| Heat generation per cell (W) | 239.9 | 138.3 | 411.3 | 80.7 |
| Cooling provider per cell (W) | 4.14 | 53.2 | 7.1 | 31 |
| Heat transfer coefficient (W m−2k−1) | 10 | 100 | 10 | 100 |
| Charging Model | Level 1 (110 V, 1.4 kW) | Level 2 (220 V, 7.2 kW) | DC Fast Charger (480 V, 50 kW) | Tesla Super Charger (480 V, 140 kW) | XFC (800 V, 1400 kW) |
|---|---|---|---|---|---|
| Range per minute of charge (miles) | 0.082 | 0.42 | 2.92 | 8.17 | 23.3 |
| Time to charge for 200 miles (minutes) | 2,143 | 417 | 60 | 21 | 7.5 |
| Today | Future | ||||
|---|---|---|---|---|---|
| Charge Voltage | 400 V | 400–1000 V | |||
| Charge Inlet | CHAdeMO, SAE J1772 CCS, Tesla | XFC Designed Inlet(s) for 1000 V@400 A | |||
| Vehicle | 400 V, 125 A, 50 kW 400 V, 350 A, 140 kW | 400–800 V, 400 A, 240–320 kW 1000 V, 210–280 A, 210–280 kW | 1000 V, 400 A, 400 kW | ||
| Battery | 1.5–2 C | 2.0–3.3 C | 3.3–4.6 C | 4.6–6 C | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Wang, L.; Chen, Z.; He, X. Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review. Energies 2019, 12, 3897. https://doi.org/10.3390/en12203897
Zhao H, Wang L, Chen Z, He X. Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review. Energies. 2019; 12(20):3897. https://doi.org/10.3390/en12203897
Chicago/Turabian StyleZhao, Hong, Li Wang, Zonghai Chen, and Xiangming He. 2019. "Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review" Energies 12, no. 20: 3897. https://doi.org/10.3390/en12203897
APA StyleZhao, H., Wang, L., Chen, Z., & He, X. (2019). Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review. Energies, 12(20), 3897. https://doi.org/10.3390/en12203897