A Methodology for Measuring the Heat Release Efficiency in Bubbling Fluidised Bed Combustors
Abstract
:1. Introduction
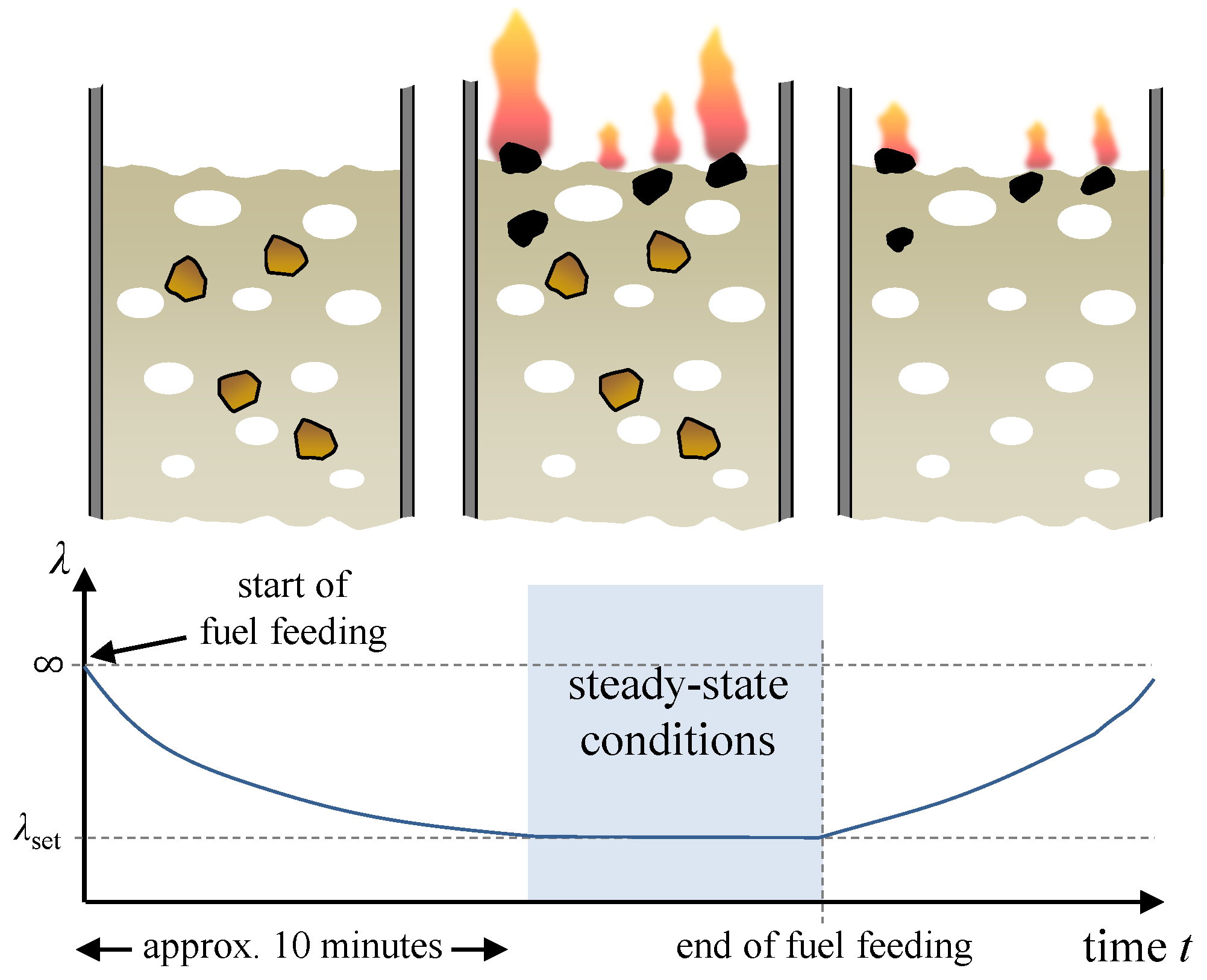
2. Mixing of Fuel Particles in Bubbling Fluidised Beds
3. Methodology
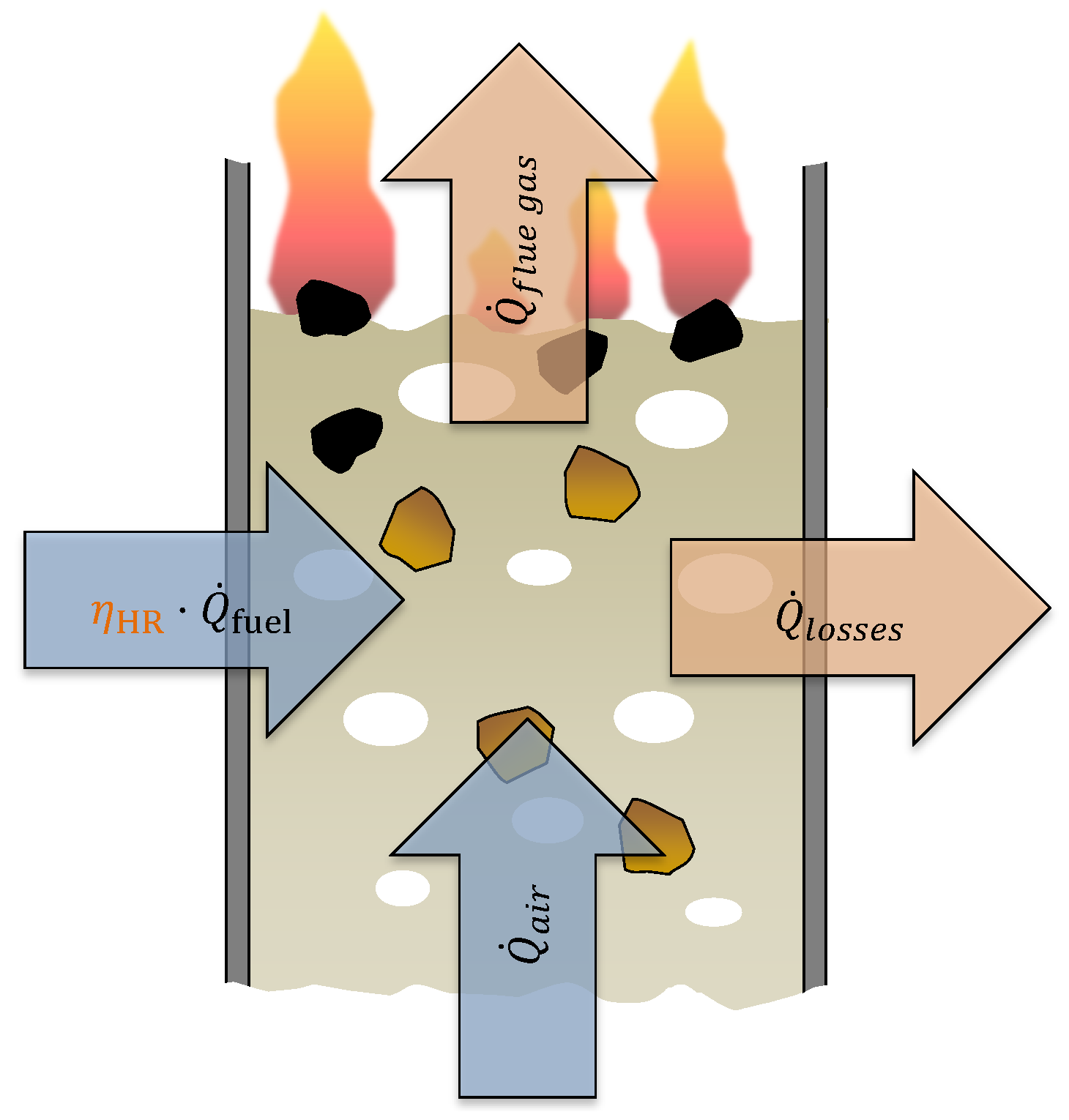
3.1. Mathematical Description
3.2. Experimental Implementation
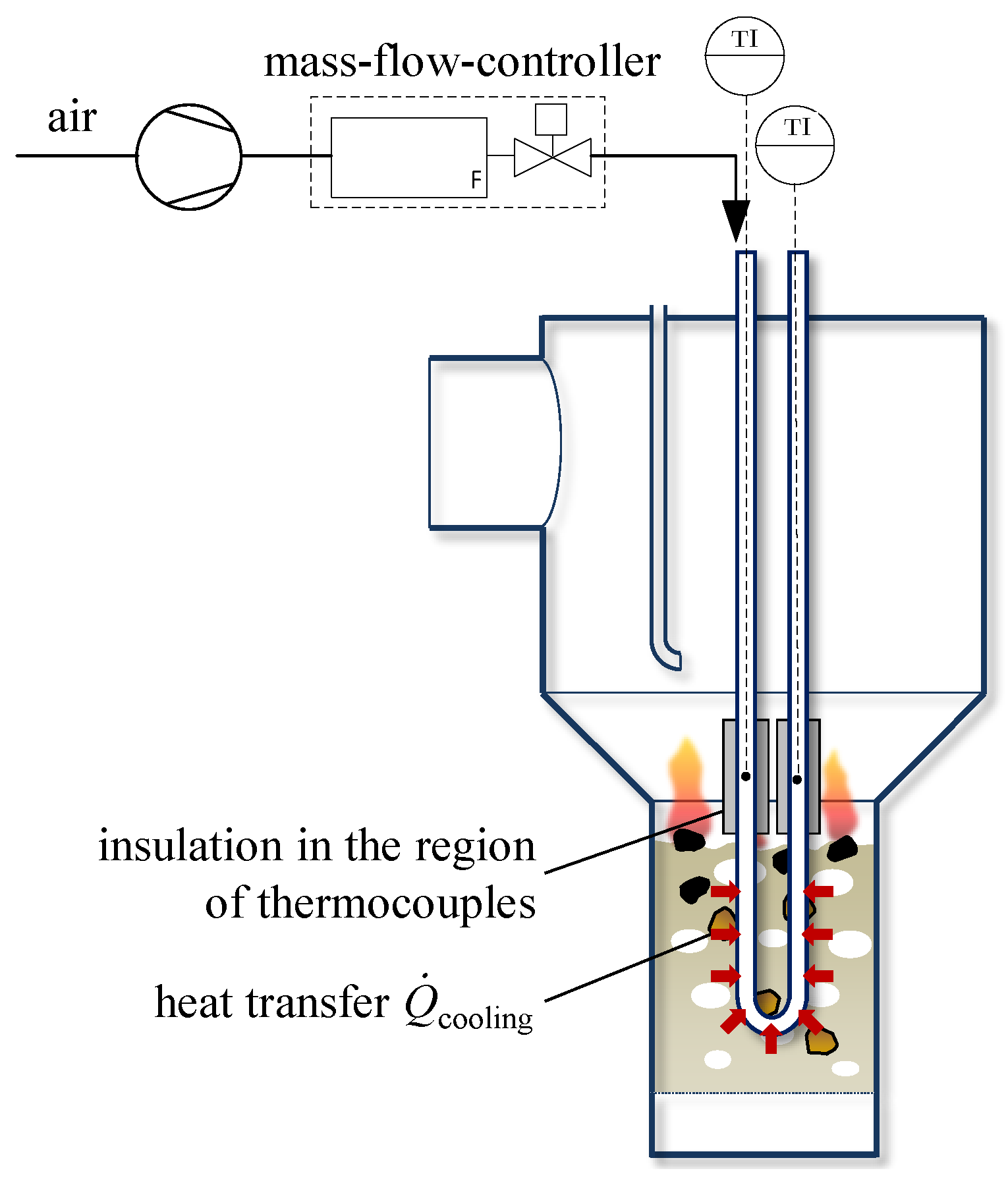
4. Experimental Setup
4.1. Plant Setup
4.2. Fuel Variation
4.3. Estimation of Thermal Losses
4.4. Error Estimation
5. Results and Discussion
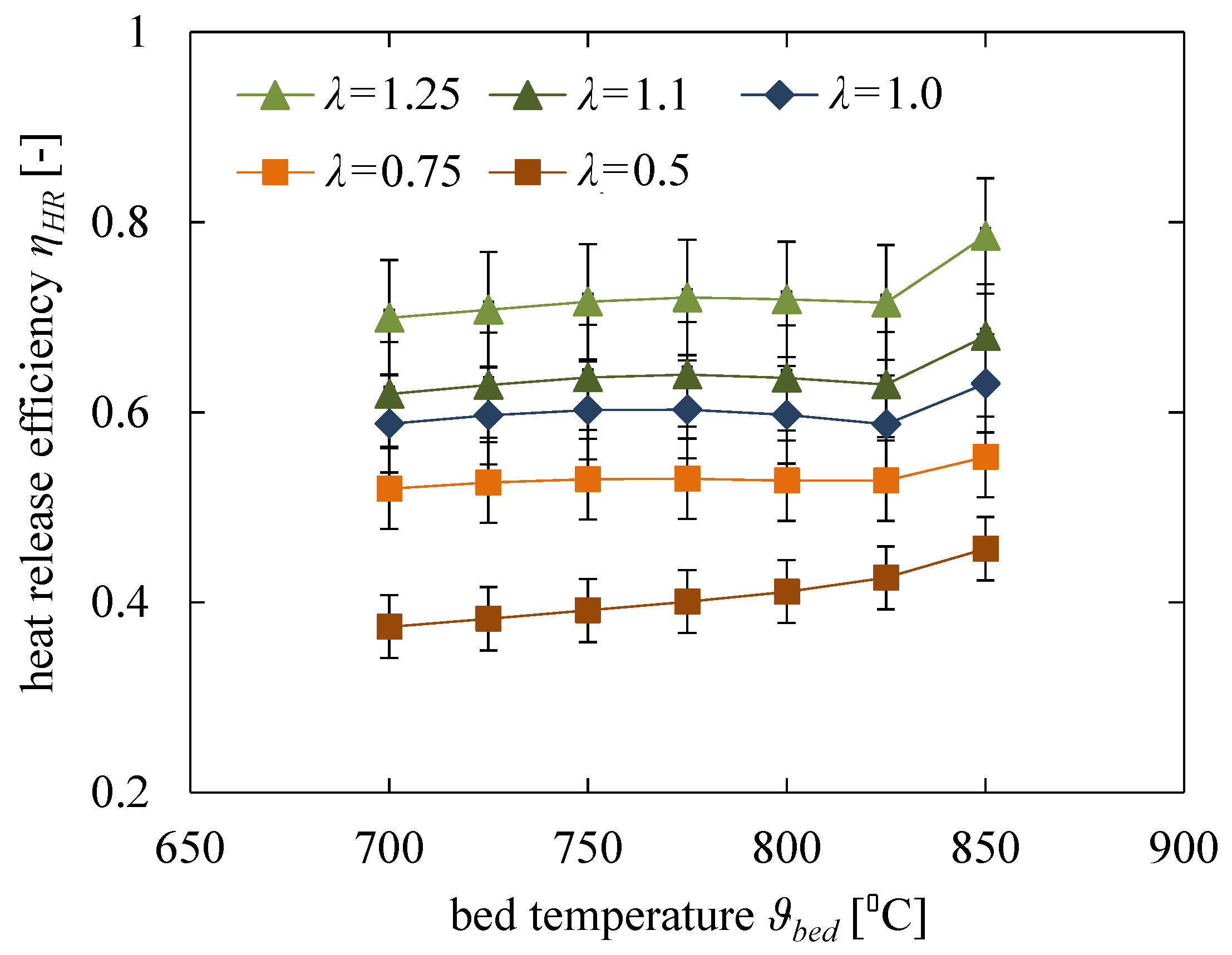
5.1. Influence of Fluidisation Number
5.2. Influence of Fluidised Bed Temperature
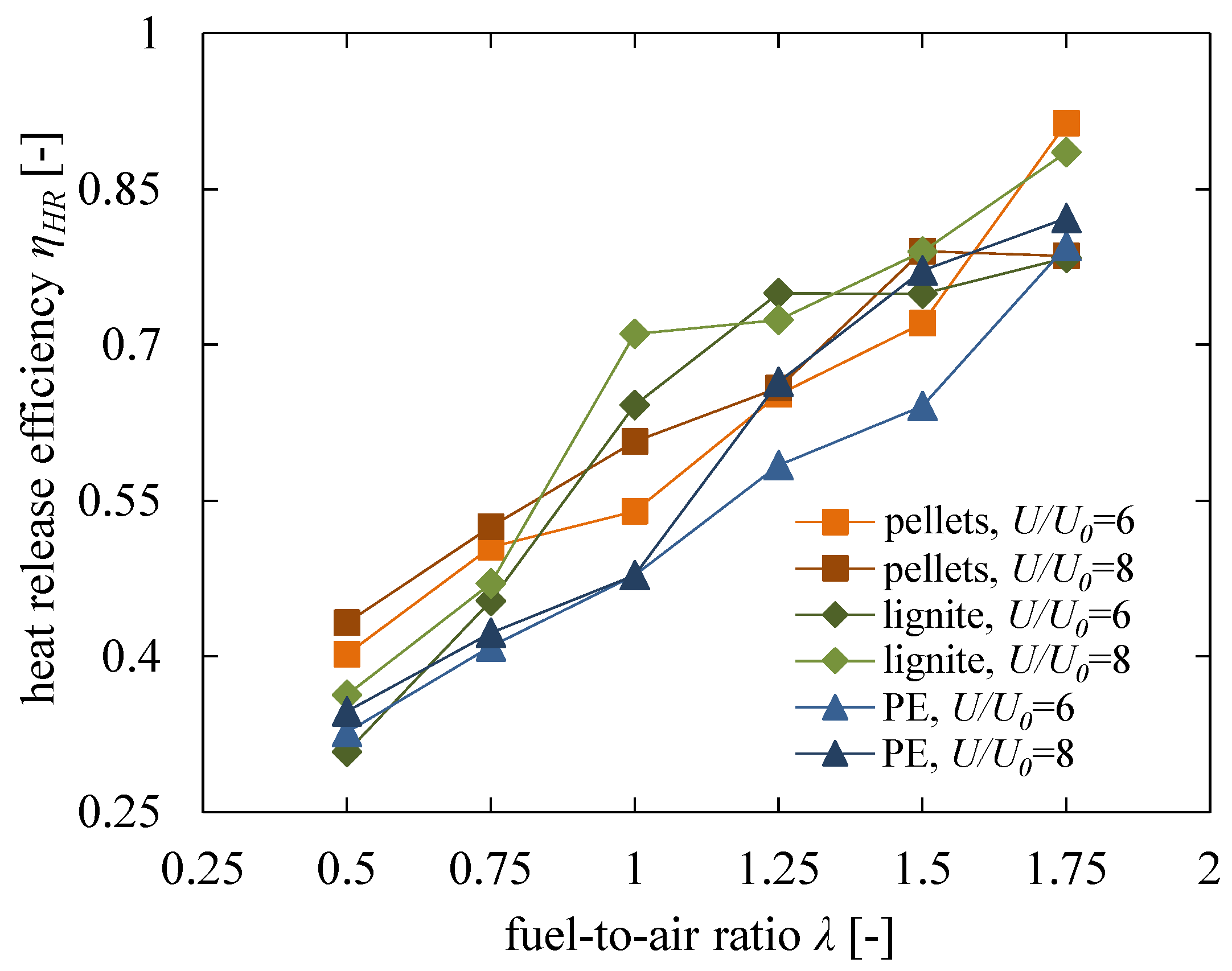
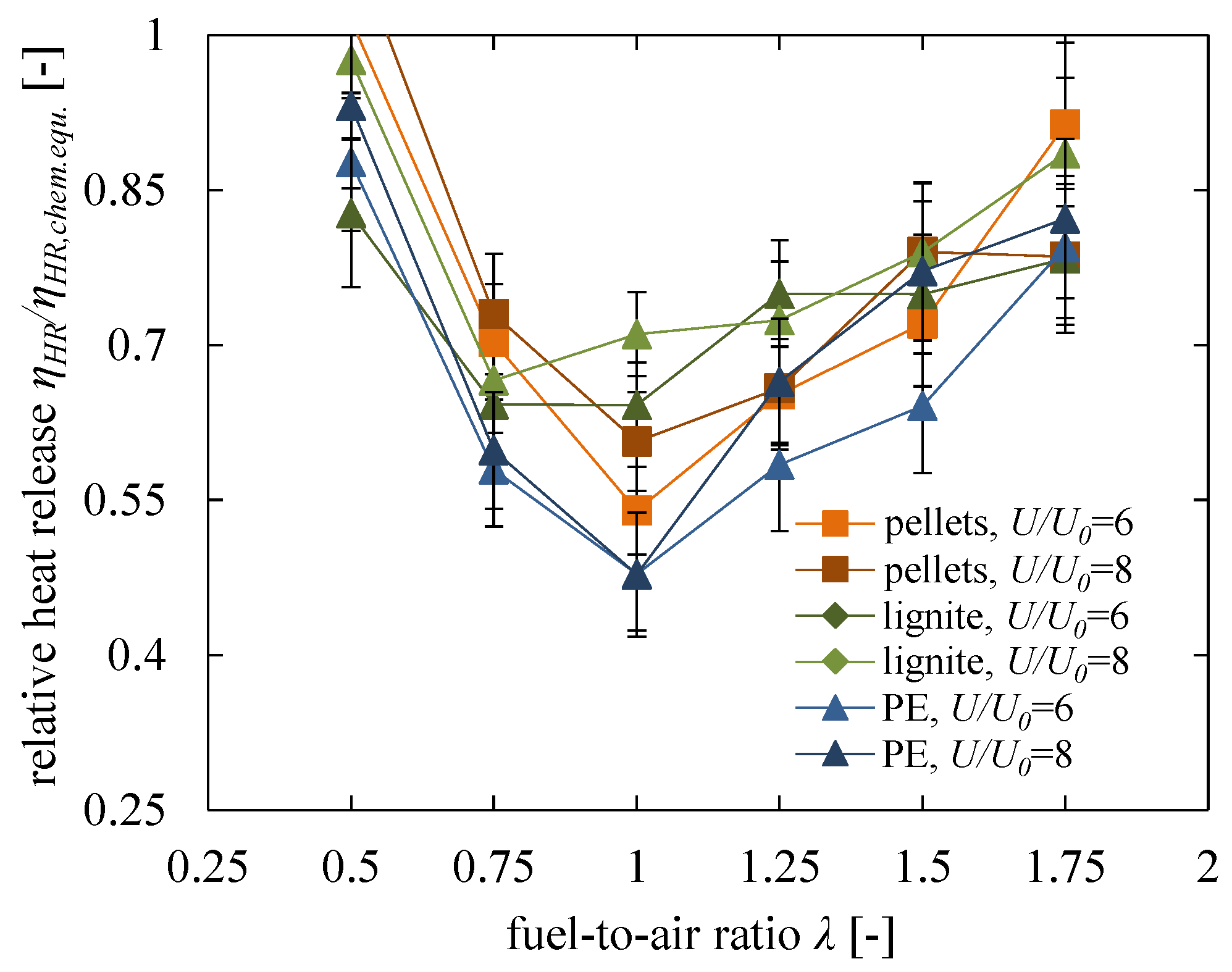
5.3. Influence of Air-to-Fuel Ratio and Fuel Properties
5.4. Comparison of Results with Scala’s Model
6. Conclusions
- The influence of the fluidised bed temperature has a minor effect on the heat release efficiency.
- Also, the trend to an increased heat release efficiency with an increasing fluidisation ratio is mainly in the scale of the measurement uncertainty. Unexpectedly, this does not fully confirm the common opinion, that the better mixing at higher fluidisation ratios will support the complete burnout of fuel particles inside the fluidised bed. However, the results so far do not provide enough information for a full understanding of this effect. Further tests with a detailed focus on the influence of fuel and especially the density of the bed material (e.g., olivine) will have to follow for a better understanding.
- A significant influence was found for the fuel-to-air ratio . As an operation at can of course not provide a heat release of 100%; a high fuel excess of is necessary to reach the maximum possible heat release according to the thermal equilibrium. A similar trend was found for , where only a high excess of air can ensure a nearly entire heat release within the fluidised bed. For stoichiometric conditions, a fuel-depending heat release efficiency of was obtained, which can be explained with non-ideal mixing of fuel and oxygen in the remaining residence time before leaving the dense fluidised bed.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BFB | bubbling fluidised bed |
| CFB | circulating fluidised bed |
| PE | polyethylene |
| Ar | Archimedes number [-] |
| specific heat capacity of the air [] | |
| specific heat capacity of the flue gas [] | |
| particle size [m] | |
| porosity [-] | |
| in-bed heat release efficiency of the BFB combustion [-] | |
| heating value of the fuel [] | |
| air-to-fuel ratio [-] | |
| fuel mass flow [] | |
| air mass flow [] | |
| flue gas mass flow [] | |
| heat capacity of the dense fluidised bed [] | |
| heat input in the dense fluidised bed [kW] | |
| fuel heat input [kW] | |
| heat losses [kW] | |
| electrical bed heater input [kW] | |
| electrical input [kW] | |
| Re | Reynolds number [-] |
| density of the dense fluidised bed phase [] | |
| density of solid phase [] | |
| density of gaseous phase [] | |
| t | time [s] |
| temperature of the fluidised bed [°C] | |
| temperature of the air [°C] | |
| temperature of the flue gas [°C] | |
| U | fluidisation velocity [] |
| minimum fluidisation velocity [] | |
| fluidisation number [-] |
References
- Koornneef, J.; Junginger, M.; Faaij, A. Development of fluidized bed combustion-An overview of trends, performance and cost. Prog. Energy Combust. Sci. 2007, 33, 19–55. [Google Scholar] [CrossRef] [Green Version]
- Werther, J.; Holighaus, R. Wirbelschicht-Technologie: Neuentwicklungen für Kraftwerkstechnik und Umweltschutz. Chem. Ing. Tech. 1978, 50, 662–669. [Google Scholar] [CrossRef]
- Leckner, B. Fluidized bed combustion: Mixing and pollutant limitation. Prog. Energy Combust. Sci. 1998, 24, 31–61. [Google Scholar] [CrossRef]
- Schu, R.; Leithner, R. Mehrstufige Dampfüberhitzung Effizienzsteigerung von EBS-, Biomasse-und Solarthermiekraftwerken. In Berliner Abfallwirtschafts-und Energiekonferenz; Thomé-Kozmiensky, K., Beckmann, M., Eds.; TK Verlag: Neuruppin, Germany, 2008. [Google Scholar]
- Winter, F.; Szentannai, P. Brennstoffmix in Wirbelschichtfeuerungen: Status und Entwicklungstendenzen. In Proceedings of the 6th Internationale Energiewirtschaftstagung an der TU Wien, Vienna, Austria, 11–13 February 2009. [Google Scholar]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Plankenbü, T.; Müller, D.; Karl, J. Slagging Prevention and Plant Optimisation by Means of Numerical Simulation. In Proceedings of the 25th European Biomass Conference & Exhibition, Stockholm, Sweden, 12–15 June 2017. [Google Scholar]
- Plankenbühler, T.; Müller, D.; Karl, J. Influence of Fine Fuel Particles on Ash Deposition in Industrial-Scale Biomass Combustion: Experiments and Computational Fluid Dynamics Modeling. Energy Fuels 2019, 33, 5911–5917. [Google Scholar] [CrossRef]
- Gatternig, B.; Karl, J. Investigations on the mechanisms of ash-induced agglomeration in fluidized-bed combustion of biomass. Energy Fuels 2015, 29, 931–941. [Google Scholar] [CrossRef]
- Gatternig, B.; Karl, J. The influence of particle size, fluidization velocity and fuel type on ash-induced agglomeration in biomass combustion. Front. Energy Res. 2014, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Karl, J. Biomass CHP with micro-fluidized-bed combustion. In Proceedings of the 21st European Biomass Conference & Exhibition, Copenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- Rowe, P.N.; Nienow, A.W. Particle mixing and segregation in gas fluidised beds. A review. Powder Technol. 1976, 15, 141–147. [Google Scholar] [CrossRef]
- Wu, S.Y.; Baeyens, J. Segregation by size difference in gas fluidized beds. Powder Technol. 1998, 98, 139–150. [Google Scholar] [CrossRef]
- dos Santos, F.; Goldstein, L. Experimental aspects of biomass fuels in a bubbling fluidized bed combustor. Chem. Eng. Process. Process Intensif. 2008, 47, 1541–1549. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Ollero, P.; Leckner, B. Optimization of char and tar conversion in fluidized bed biomass gasifiers. Fuel 2013, 103, 42–52. [Google Scholar] [CrossRef]
- Wirsum, M.; Fett, F.; Iwanowa, N.; Lukjanow, G. Particle mixing in bubbling fluidized beds of binary particle systems. Powder Technol. 2001, 120, 63–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, B.; Zhong, W. Experimental investigation on mixing and segregation behavior of biomass particle in fluidized bed. Chem. Eng. Process. Process Intensif. 2009, 48, 745–754. [Google Scholar] [CrossRef]
- Shen, L.; Xiao, J.; Niklasson, F.; Johnsson, F. Biomass mixing in a fluidized bed biomass gasifier for hydrogen production. Chem. Eng. Sci. 2007, 62, 636–643. [Google Scholar] [CrossRef]
- Bruni, G.; Solimene, R.; Marzocchella, A.; Salatino, P.; Yates, J.G.; Lettieri, P.; Fiorentino, M. Self-segregation of high-volatile fuel particles during devolatilization in a fluidized bed reactor. Powder Technol. 2002, 128, 11–21. [Google Scholar] [CrossRef]
- Cui, H.; Grace, J.R. Fluidization of biomass particles: A review of experimental multiphase flow aspects. Chem. Eng. Sci. 2007, 62, 45–55. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.; Yang, Z.; Zhao, C. Pressurized oxy-fuel combustion characteristics of single coal particle in a visualized fluidized bed combustor. Combust. Flame 2020, 211, 218–228. [Google Scholar] [CrossRef]
- Sette, E.; Berdugo Vilches, T.; Pallarès, D.; Johnsson, F. Measuring fuel mixing under industrial fluidized-bed conditions - A camera-probe based fuel tracking system. Appl. Energy 2016, 163, 304–312. [Google Scholar] [CrossRef]
- Köhler, A.; Pallarès, D.; Johnsson, F. Modeling Axial Mixing of Fuel Particles in the Dense Region of a Fluidized Bed. Energy Fuels 2020, 34, 3294–3304. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P. Modelling fluidized bed combustion of high-volatile solid fuels. Chem. Eng. Sci. 2002, 57, 1175–1196. [Google Scholar] [CrossRef]
- Scala, F.; Chirone, R. Fluidized bed combustion of alternative solid fuels. Exp. Therm. Fluid Sci. 2004, 28, 691–699. [Google Scholar] [CrossRef]
- Ottmann, M. Verbrennung Biogener Brennstoffe in Stationären Wirbelschichtfeuerungen. Ph.D. Thesis, Technische Universität München, München, Germany, 2007. [Google Scholar]
- Reschmeier, R.; Roveda, D.; Müller, D.; Karl, J. Pyrolysis kinetics of wood pellets in fluidized beds. J. Anal. Appl. Pyrolysis 2014, 108, 117–129. [Google Scholar] [CrossRef]
- Reschmeier, R.; Karl, J. Experimental study of wood char gasification kinetics in fluidized beds. Biomass Bioenergy 2016, 85, 288–299. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Perugini, F.; Ponte, M.; Arena, U. Fluidized bed pyrolysis of a recycled polyethylene. Polym. Degrad. Stab. 2002, 76, 479–487. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; García, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Baumhakl, C. Substitute Natural Gas Production with direct Conversion of Higher Hydrocarbons. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2014. [Google Scholar]
- Prüfung Fester Brennstoffe—Presslinge aus Naturbelassenem Holz—Anforderungen und Prüfung—Fassung DIN 51731; Beuth: Berlin, Germany, 1996.
- Annamalia, K.; Sweeten, J.M.; Ramalingam, S.C. Technical Notes: Estimation of Gross Heating Values of Biomass Fuels. Trans. ASAE 1987, 30, 1205–1208. [Google Scholar] [CrossRef]
- Paulauskas, R.; Džiugys, A.; Striugas, N. Experimental investigation of wood pellet swelling and shrinking during pyrolysis. Fuel 2015, 142, 145–151. [Google Scholar] [CrossRef]
- Michel, W. (Ed.) Wirbelschichttechnik in der Energiewirtschaft; Deutscher Verlag für Grundstoffindustrie GmbH: Leipzig, Germany, 1992. [Google Scholar]




| Property | Wood Pellets 1) | Lignite | Poly-Ethylene 2) |
|---|---|---|---|
| c [-%] | 46.8 | 53.6 | 85.6 |
| h [-%] | 5.7 | 3.9 | 14.4 |
| o [-%] | 40.1 | 19.2 | 0 |
| n [-%] | 0 | 0.6 | 0 |
| s [-%] | 0 | 0.4 | 0 |
| w [-%] | 6.9 | 19.0 | 0 |
| ash [-%] | 0.5 | 3.5 | 0 |
| volatile matter [-%] | 78.7 | 42.0 | 100 |
| fixed carbon [-%] | 14.0 | 35.5 | 0 |
| [] | 17.1 | 19.8 | 43.3 |
| particle size [] | 6 | 4–12 | 4.5 |
| density [] | 1000–1400 | n/a | 950 |
| Variable | (Relative) Uncertainty |
|---|---|
| fuel mass flow | 5% 1) |
| air mass flow | 0.6% 2) |
| heating value | 2% 3) |
| specific heat capacity flue gas | 3% |
| specific heat capacity air | 1% |
| fluidised bed temperature | 2.1% 4) |
| temperature gradients , and | 2% 5) |
| electrical bed heater | 10% 6) |
| Influence of | Variation of | Constant Parameters |
|---|---|---|
| fluidisation number | 800 °C, & , wood pellets | |
| bed temperature | 700 °C °C | , , wood pellets |
| fuel properties | wood pellets, lignite, polyethylene at | 800 °C, , |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, D.; Plankenbühler, T.; Karl, J. A Methodology for Measuring the Heat Release Efficiency in Bubbling Fluidised Bed Combustors. Energies 2020, 13, 2420. https://doi.org/10.3390/en13102420
Müller D, Plankenbühler T, Karl J. A Methodology for Measuring the Heat Release Efficiency in Bubbling Fluidised Bed Combustors. Energies. 2020; 13(10):2420. https://doi.org/10.3390/en13102420
Chicago/Turabian StyleMüller, Dominik, Thomas Plankenbühler, and Jürgen Karl. 2020. "A Methodology for Measuring the Heat Release Efficiency in Bubbling Fluidised Bed Combustors" Energies 13, no. 10: 2420. https://doi.org/10.3390/en13102420





